Key Points
Question
Does intravenous alteplase benefit patients with ischemic stroke presenting with minor neurologic deficits that are judged not clearly disabling?
Findings
In this randomized clinical trial that included 313 of a planned 948 patients with acute ischemic stroke, there was no significant difference in the adjusted percentage with favorable functional outcome at 90 days for those treated with alteplase vs aspirin (78% vs 81%).
Meaning
Although the study did not demonstrate a significant benefit of alteplase for patients with minor nondisabling acute ischemic stroke, the very early study termination precludes any definitive conclusions.
Abstract
Importance
More than half of patients with acute ischemic stroke have minor neurologic deficits (National Institutes of Health Stroke Scale [NIHSS] score of 0-5) at presentation. Although prior major trials of alteplase included patients with low NIHSS scores, few without clearly disabling deficits were enrolled.
Objective
To evaluate the efficacy and safety of alteplase in patients with NIHSS scores of 0 to 5 whose deficits are not clearly disabling.
Design, Setting, and Participants
The PRISMS trial was designed as a 948-patient, phase 3b, double-blind, double-placebo, multicenter randomized clinical trial of alteplase compared with aspirin for emergent stroke at 75 stroke hospital networks in the United States. Patients with acute ischemic stroke whose deficits were scored as 0 to 5 on the NIHSS and judged not clearly disabling and in whom study treatment could be initiated within 3 hours of onset were eligible and enrolled from May 30, 2014, to December 20, 2016, with final follow-up on March 22, 2017.
Interventions
Participants were randomized to receive intravenous alteplase at the standard dose (0.9 mg/kg) with oral placebo (n = 156) or oral aspirin, 325 mg, with intravenous placebo (n = 157).
Main Outcomes and Measures
The primary outcome was the difference in favorable functional outcome, defined as a modified Rankin Scale score of 0 or 1 at 90 days via Cochran-Mantel-Haenszel test stratified by pretreatment NIHSS score, age, and time from onset to treatment. Because of early termination of the trial, prior to unblinding or interim analyses, the plan was revised to examine the risk difference of the primary outcome by a linear model adjusted for the same factors. The primary safety end point was symptomatic intracranial hemorrhage (sICH) within 36 hours of intravenous study treatment.
Results
Among 313 patients enrolled at 53 stroke networks (mean age, 62 [SD, 13] years; 144 [46%] women; median NIHSS score, 2 [interquartile range {IQR}, 1-3]; median time to treatment, 2.7 hours [IQR, 2.1-2.9]), 281 (89.8%) completed the trial. At 90 days, 122 patients (78.2%) in the alteplase group vs 128 (81.5%) in the aspirin group achieved a favorable outcome (adjusted risk difference, −1.1%; 95% CI, −9.4% to 7.3%). Five alteplase-treated patients (3.2%) vs 0 aspirin-treated patients had sICH (risk difference, 3.3%; 95% CI, 0.8%-7.4%).
Conclusions and Relevance
Among patients with minor nondisabling acute ischemic stroke, treatment with alteplase vs aspirin did not increase the likelihood of favorable functional outcome at 90 days. However, the very early study termination precludes any definitive conclusions, and additional research may be warranted.
Trial Registration
ClinicalTrials.gov Identifier: NCT02072226
This randomized trial compares the effects of intravenous alteplase vs oral aspirin treatment on functional outcomes in patients with minor nondisabling acute ischemic stroke.
Introduction
Mild stroke is the most commonly cited reason for nonuse of intravenous alteplase among patients with ischemic stroke who present to the hospital within the guideline-based eligible treatment window of 4.5 hours.1 Yet prospective data suggest that 30% of such patients have functional disability at 90 days after stroke.2,3 Reasons for the development of disability might include underappreciated deficits, stroke progression, or medical comorbidities leading to additional medical events, including recurrent stroke. Vessel reperfusion may avert disability because of the first 2 of these reasons. Clinical use of alteplase for ischemic stroke with low National Institutes of Health Stroke Scale (NIHSS) scores has increased in recent years, presumably based on concern for this substantial poststroke disability.4,5
Although alteplase is the standard of care for patients with ischemic stroke and disabling deficits regardless of severity judged by NIHSS scores,6,7 the optimal management of patients with not clearly disabling deficits is unclear. Most major trials of alteplase (NINDS Parts 1 and 2; ECASS 1, 2, and 3; Atlantis Parts A and B; and EPITHET) explicitly excluded varying subsets of patients with the mildest deficits (see eTable 1 in Supplement 1 for exclusion criteria). The IST 3 trial permitted enrollment of patients with minor deficits, but only when the local enrolling physician had personal equipoise regarding benefit and without collecting data to distinguish which enrolled patients had disabling vs nondisabling deficits at presentation.8 In the absence of definitive evidence, current US clinical guidelines indicate uncertainty regarding use of alteplase in patients with low NIHSS scores and nondisabling deficits (strength of recommendation class IIb [weak]; quality of evidence level C-LD [limited data]).7
The purpose of this study was to test the efficacy and safety of alteplase administered within 3 hours of onset of ischemic stroke symptoms among patients presenting with minor deficits (NIHSS score of 0-5) judged not clearly disabling at presentation.
Methods
Trial Design
The study was approved by institutional review boards for all study sites, and all enrolled patients provided written informed consent. The protocol, protocol amendment, and statistical analysis plan are provided in Supplement 2, Supplement 3, and Supplement 4, and detailed methods have been published.9
The Potential of rtPA for Ischemic Strokes With Mild Symptoms (PRISMS) trial was designed as a phase 3b, randomized, double-blind clinical trial testing the safety and efficacy of intravenous alteplase administered within 3 hours of symptom onset (time from witnessed onset or time from last known well time if unwitnessed). The time window of 3 hours was chosen to harmonize with the US Food and Drug Administration–labeled time window for use of alteplase in patients with disabling ischemic stroke.
Patient Selection and Randomization
Inclusion criteria included clinical diagnosis of acute ischemic stroke, age 18 years or older, NIHSS score of 0 to 5, and deficits judged to not be clearly disabling at presentation. A clearly disabling deficit was operationally defined as a deficit that, if unchanged, would prevent the patient from performing basic activities of daily living (ie, bathing, ambulating, toileting, hygiene, and eating) or returning to work. Local clinicians made this determination in consultation with patients and available family. Intensive study efforts to facilitate uniform application of enrollment criteria, including web-based and smartphone-based patient selection tools, pocket laminated cards, and tutorials, were implemented. Local investigators were also advised to ensure that patients could walk unassisted. Exclusion criteria included prestroke disability (modified Rankin Scale score of 2-6), dysphagia, intracranial hemorrhage on acute neuroimaging, and other standard contraindications to intravenous alteplase as reflected in the clinical guidelines that were current during the trial.10
Participants were randomized in a 1:1 ratio stratified by site. A step-forward randomization procedure was used to ensure that the treatment assignment was available prior to the arrival of each eligible patient, allowing for rapid initiation of study treatment.11 A “use next” drug kit was assigned by a dynamic randomization algorithm managed by an interactive web response system. Balance within site was controlled via the urn method, which drew the first treatment assignment from a metaphorical active urn containing a complete block of treatment assignments and then placed it into an inactive urn; a minimally balanced set of assignments was returned to facilitate balance.12 The overall allocation was balanced using a biased coin method in which treatments were allocated with unequal but not deterministic probability when imbalance achieved preset criteria.13 A patient was considered enrolled when the study treatment bolus was administered.
Study Intervention
Participants were randomized to receive either intravenous alteplase at standard dosing for ischemic stroke (0.9 mg/kg) with a placebo oral aspirin or oral aspirin, 325 mg, with placebo intravenous alteplase. The placebos were identical in appearance to active study drugs to maintain treatment blinding of patients and investigators. Intravenous study treatment was to be administered within 3 hours of stroke onset. The same was encouraged for the oral study treatment; however, it could be administered up to 24 hours after symptom onset at institutions that required formal swallow evaluation. Clinical management after study treatment administration was to be in accordance with institutional protocols and clinical guidelines for care after alteplase administration. The study mandated follow-up neuroimaging (computed tomography or magnetic resonance imaging per institutional standard of care) within 22 to 36 hours after intravenous study treatment bolus.
Study Outcomes
The primary outcome end point was a modified Rankin Scale (mRS) score of 0 or 1 (total range, 0 [symptom free] to 6 [dead]), reflecting favorable functional outcome, evaluated at 90 days after enrollment and adjusted for age, time from symptom onset to treatment, and baseline NIHSS score.
Secondary outcome measures of efficacy, also assessed at 90 days, consisted of level of disability (assessed by the 6-level ordinal mRS score, collapsing levels 5 and 6) and global favorable recovery, which was defined as an mRS score of 0 or 1, NIHSS score of 0 or 1, Barthel Index of 95 or 100 (total range, 0 [totally dependent] to 100 [patient performs self-care and mobility without assistance]), and Glasgow Outcome Scale score of 1 (total range, 1 [good recovery] to 5 [death]).
Exploratory outcome measures of efficacy included ambulatory speed (10-Meter Walk Test), which assesses comfortable walking speed over 6 m; stroke-related quality of life (Stroke Impact Scale 16) score, which ranges from 0 to 100, with higher scores indicating better physical performance (strength, hand function, physical and instrumental activities of daily living, and mobility); and general health-related quality of life (EuroQoL Group EQ-5D) score, which ranges from 0 (death) to 1 (perfect health) at 90 days. Cognitive outcomes and a brief depression assessment were also assessed (results not presented in this article).
The primary safety end point was symptomatic intracranial hemorrhage (sICH), defined as any neurologic decline within 36 hours attributed to ICH by local investigators; this definition was modified from the NINDS-tPA trials’ definition requiring only a temporal association of decline with ICH.14 Other safety measures included an alternate definition of sICH (a deterioration in NIHSS score of ≥4 with a radiological parenchymal hemorrhage type 2),15 any radiologic ICH within 36 hours, all-cause mortality within 90 days, stroke-related and neurologic deaths within 90 days, and the severity and spectrum of all adverse events.
Data Collection and Monitoring
Key clinical and radiological data were collected, including (1) baseline neuroimaging and NIHSS score; (2) 22- to 36-hour repeat neuroimaging and NIHSS score; (3) 5-day (or discharge if sooner) NIHSS score, stroke etiology, and discharge location; (4) 30-day mRS score by telephone follow-up; and (5) final 90-day mRS score, along with secondary and exploratory outcomes, assessed in person when possible. Ethnicity and race were self-reported (fixed categories) and were collected to consider the generalizability of the results. All adverse events, regardless of relationship to study treatment, were reported until 30 days after study treatment administration. After 30 days, only data on serious adverse events, nonserious adverse events of special interest (consisting of sICH events not otherwise reported, stroke recurrence, or suspected transmission of an infectious agent via a medicinal product), and adverse events resulting in withdrawal from the study were collected. Concomitant medication use was recorded at all visits.
Study monitors performed ongoing source data verification. An independent data monitoring committee, composed of 1 stroke neurologist, 1 emergency physician, 1 neuroradiologist, and 1 statistician, provided ongoing review of accumulating adverse events. The independent data monitoring committee was also charged with conducting the interim futility analysis, planned to take place after 50% of participants had completed follow-up. Two independent, blinded neuroradiologists at the central imaging core interpreted relevant imaging data.
Determination of presenting events as either ischemic cerebral events (stroke or transient ischemic attack) or neurovascular mimic was carried out by site investigators. For patients with neuroimaging evidence of new infarction, site investigators’ diagnoses of ischemic stroke were accepted without further review. For patients without neuroimaging evidence of new infarction, central review was performed by steering committee neurologists (P.K. and J.G.R.) prior to database lock and without unblinding, independently rendering diagnoses of ischemic cerebral event or neurovascular-mimicking condition. When assessments were discordant, discussions were held between the site investigator and central reviewer to ensure that all relevant data were considered in the final determination. The site principal investigators then made a final diagnostic determination.
Statistical Analysis
The trial was designed to detect a 9% absolute difference in the proportion of participants with favorable outcome with 80% power, using a 1-sided type I error rate of .025 to test the superiority hypothesis, under the assumption that 65% of participants randomized to receive aspirin would experience a favorable outcome, and allowing for 1 interim futility analysis. The sample size calculation was 1-sided to reflect the objective of establishing the superiority of alteplase over standard medical care. Accounting for these assumptions, the necessary sample size was calculated to be 856 participants. The sample size was adjusted to 948 participants to account for dilution of the treatment effect associated with nonadherence parameters (loss to follow-up, treatment crossovers, and neurovascular mimics [stroke and transient ischemic attack]). The planned 9% effect size was derived from a previously published post hoc analysis of the IST-3 trial subset of patients that met the PRISMS trial’s eligibility criteria (with the exception that qualifying deficits could not be ascertained as not clearly disabling).16 The steering committee expected that treatment effects of less than 9% were still likely to be clinically meaningful. It was anticipated that a higher rate of favorable outcome in participants randomized to aspirin and a lower rate of nonadherence parameters would allow detection of a lower absolute treatment effect with the same 80% power.
The trial initially intended to test the primary end point via a Cochran-Mantel-Haenszel hypothesis test, stratified by age (<65 vs ≥65 years), time from symptom onset to treatment (0-2 vs >2 hours), and pretreatment NIHSS score (0-2 vs 3-5). Because the trial was terminated early by the sponsor for enrollment below target, it was recognized that the study would be underpowered to formally test the hypothesis. Therefore, the statistical analysis plan was finalized, prior to database lock and unblinding, to evaluate the size of an effect (and its associated uncertainty). The updated plan was to evaluate the hypothesis by examining the adjusted risk difference (point values and 95% CIs) in the rate of the primary outcome (mRS score of 0 or 1) using a linear model, which included treatment assignment, age, time from symptom onset to study treatment, and baseline NIHSS score as continuous covariates.
A sensitivity analysis confined to patients with final diagnoses of acute cerebral ischemia was also planned. Additional exploratory analyses of the primary outcome were prespecified to test the consistency of the findings: (1) unadjusted risk difference; (2) logistic regression, adjusting for pretreatment NIHSS score, age, and time to treatment; (3) risk difference adjusted for additional potential covariate imbalances using a propensity score; and (4) repeated-measures model (in which mRS score outcomes at day 30 and day 90 were modeled jointly by a logistic regression).
Missing primary outcome data (90-day mRS scores) were prespecified for imputation with the 30-day mRS score result, when available, and otherwise imputed via hot-deck method, which randomly selected the outcome value from a pool of patients with observed mRS scores matched by treatment group, age, pretreatment NIHSS score, and time of symptom onset.
The secondary outcome of ordinal analysis was achieved by fitting a proportional odds model with mRS score at day 90 as the dependent variable and treatment group, pretreatment NIHSS score, age, and time from symptom onset to treatment as the continuous predictors. Quadratic terms for the continuous covariates would be added to the model, if the Wald P value for the quadratic term was less than 0.1. The proportional odds assumption was tested by the score test.
The secondary outcome of global favorable recovery was derived from a generalized linear model with logit-link function, computed with generalized estimating equations. This global statistic simultaneously evaluated the effect in all 4 outcome measures (mRS score of 0 or 1, NIHSS score of 0 or 1, Barthel Index of 95 or 100, and Glasgow Outcome Scale score of 1).
Analyses for heterogeneity of treatment effect for the primary outcome were prespecified for subgroups of age, baseline NIHSS score, and time from symptom onset to treatment. Odds ratios (ORs) were estimated using multivariable logistic regressions. P values were estimated by adding the interaction of each subgroup and treatment variable to the logistic regression model. A possible interaction was prespecified as P < .10.
A post hoc analysis evaluated the likelihood of alteplase benefit, given study findings, for clinical interpretation and future trial planning purposes. Using an uninformative prior on the treatment-specific unadjusted outcome proportions, the posterior distribution of the risk difference was constructed via simulation to calculate the probability of alteplase benefit. This uninformative prior β[1, 1] reflects a uniform distribution on the interval [0, 1]; this prior was used to construct the posterior distribution of the risk difference via simulation to calculate the probability of alteplase benefit. The analysis was not adjusted for differences in age, time to treatment, or baseline NIHSS score, as with the primary outcome. The trial steering committee clinicians were surveyed in December 2015 (prior to study termination and without unblinding) to propose effect sizes that would change clinical practice toward treatment in the context of various associated sICH risk rates of alteplase. They suggested that an alteplase effect size as low as 6% might still change clinical practice toward treatment in the context of a 3% increased sICH risk. Therefore, the post hoc analysis considered the likelihood of (1) any benefit of alteplase and (2) an absolute benefit of alteplase of more than 6%.
For statistical analyses, SAS version 9.2 or higher (SAS Institute Inc) was executed on the SunOS version 5.10 (SUN 64) platform. The post hoc Bayesian analysis was executed using WinBUGS14.
Early Study Termination
Study enrollment was terminated on December 20, 2016, by the sponsor, prior to database lock and without unblinding, because of patient recruitment below target. This was a financial decision based on the fact that the trial could not be completed within the allotted funds in the specified time frame. The interim futility analysis was not completed for consideration of this decision because less than 50% of patients had completed follow-up. Neither the sponsor, the independent data monitoring committee, nor the steering committee evaluated study data as part of the decision to terminate the trial. The academic members of the steering committee recommended against termination but accepted this financial decision by the sponsor. The sponsor assured that the accrued data would be fully analyzed and disseminated to maximize the contributions of the enrolled patients and thereby maintain ethical responsibilities. The independent data monitoring committee had 2 reviews of aggregate data and recruitment rates but did not participate in the decision to terminate enrollment; the committee was informed of the decision after it was made. After enrollment was halted, all enrolled patients were to complete protocol-specified procedures through 90-day follow-up. As a result of the early termination of the study, the plan for analysis of the primary outcome was revised as detailed above.
Results
Study Population
From May 1, 2014, to December 20, 2016, 313 patients were enrolled, with a mean age of 62 years (SD, 13 years), 144 (46%) women, a median NIHSS score of 2 (interquartile range, 1-3), and a median time from symptom onset to start of study treatment of 2.7 hours (interquartile range, 2.1-2.9). Of the 313 enrolled patients, 281 (89.8%) completed the trial’s primary outcome assessment and data for the remainder were imputed as prespecified. Study randomization, enrollment, and follow-up are shown in Figure 1 and patient characteristics in Table 1. Study groups were generally well balanced, with the most common medical risk factors being hypertension (80.1%) and hyperlipidemia (72.3%), and atrial fibrillation in only 12.8%. The most prevalent neurologic deficits at enrollment were sensory (46%), facial palsy (39%), and dysarthria (28%), as shown in the context of associated deficits in eFigure 1 in Supplement 1.
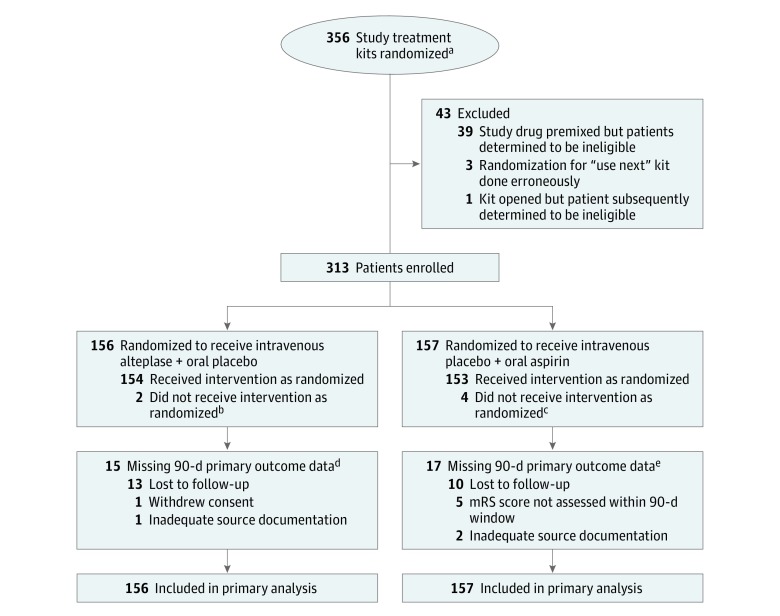
Figure 1. Flow of Patients Through the PRISMS Trial.
aThe total number of patients assessed for eligibility is not available because screening log data were not available. The total number randomized does not reflect the enrolled population because in step-forward randomization, a “use next” drug kit was assigned prior to a potential trial candidate’s arrival at an emergency department. Many patients who arrived at an emergency department after a kit was designated were not eligible and were not enrolled in the trial. A patient was considered enrolled when the study treatment bolus was administered.
bThese 2 patients received intravenous placebo instead of intravenous alteplase because of pharmacy error.
cTwo patients received oral placebo instead of oral aspirin because of pharmacy error; 1 patient was determined to have an aspirin allergy after enrollment; and 1 patient had neurologic worsening and became eligible for intravenous alteplase treatment as standard of care.
dMissing data were imputed using the 30-day modified Rankin Scale (mRS) score for 12 patients and hot-deck imputation for 3 patients.
eMissing data were imputed using the 30-day mRS score for 13 patients and hot-deck imputation for 4 patients.
Table 1. Baseline Patient Characteristics.
| Characteristics | Intravenous Alteplase + Oral Placebo (n=156) | Intravenous Placebo + Oral Aspirin (n = 157) |
|---|---|---|
| Age, mean (SD), y | 62 (14) | 61 (13) |
| Male sex, No. (%) | 77 (49) | 92 (59) |
| Race, No. (%)a | ||
| White | 117 (75.0) | 126 (80.3) |
| Black/African American | 35 (22.4) | 27 (17.2) |
| American Indian or Alaska Native | 1 (0.6) | 3 (1.9) |
| Asian | 0 | 1 (0.6) |
| ≥2 races | 1 (0.6) | 0 |
| Unknown | 2 (1.3) | 0 |
| Hispanic or Latino ethnicity, No. (%)a | 14 (9.0) | 18 (11.5) |
| Medical history, No. (%) | ||
| Hypertension | 126 (81.3) | 124 (79.0) |
| Hyperlipidemia | 114 (73.1) | 114 (72.6) |
| Diabetes mellitus | 57 (36.5) | 44 (28.0) |
| Previous stroke | 28 (17.9) | 24 (15.3) |
| Atrial fibrillation | 23 (14.7) | 17 (10.8) |
| Medications prior to onset, No. (%) | ||
| Antiplatelet agents | 64 (41.0) | 59 (37.6) |
| Anticoagulant agents | 1 (0.6) | 1 (0.6) |
| Time from symptom onset to intravenous study treatment bolus, h | ||
| No. (%) | ||
| 0 to 2 | 25 (16.0) | 36 (22.9) |
| >2 to 3 | 126 (80.8) | 119 (75.8) |
| >3b | 5 (3.2) | 2 (1.3) |
| Median (IQR) | 2.7 (2.2-2.9) | 2.6 (2.1-2.9) |
| Time from onset to oral study treatment, median (IQR), h | 2.9 (2.5-3.1) | 2.8 (2.4-3.1) |
| Baseline NIHSS scorec | ||
| No. (%) | ||
| 0 | 7 (4.5) | 7 (4.5) |
| 1 | 38 (24.4) | 50 (31.8) |
| 2 | 52 (33.3) | 50 (31.8) |
| 3 | 32 (20.5) | 30 (19.1) |
| 4 | 21 (13.5) | 16 (10.2) |
| 5 | 6 (3.8) | 4 (2.5) |
| Mean (SD) | 2.3 (1.2) | 2.0 (1.2) |
| Glucose level, mean (SD), mg/dL | 141.2 (72.9) | 132.1 (61.8) |
| Systolic blood pressure >140 mm Hg, No. (%) | 83 (53.2) | 112 (71.3) |
| Diastolic blood pressure >90 mm Hg, No. (%) | 31 (19.9) | 44 (28.0) |
| Baseline international normalized ratio >1.1, No. (%) | 42 (26.9) | 52 (33.1) |
| Baseline ASPECTS, median (range)d | 10 (7-10) | 10 (7-10) |
Abbreviation: IQR, interquartile range.
SI conversion: To convert glucose to mmol/L, multiply by 0.0555.
Race and ethnicity were self-reported as categories of Hispanic/Latino (yes/no) and either American Indian or Alaska Native, Asian, black or African American, Native Hawaiian or other Pacific Islander, white, or other.
Six of the 7 patients received the study bolus within 3 hours and 15 minutes.
The National Institutes of Health Stroke Scale (NIHSS) score classifies neurologic deficit from 0 (no deficit) to 42 (most severe).
Alberta Stroke Program Early Computed Tomography Score (ASPECTS) semiquantitatively measures the extent of acute early ischemic changes in the middle cerebral artery distribution on a computed tomography scan, with a score range of 0 (maximum changes) to 10 (no ischemic changes).
Features of the presenting event are shown in Table 2. Final diagnosis was acute cerebral ischemia in 87.0% of patients and neurovascular mimic in 13.0%. Among patients with acute cerebral ischemia, the most common stroke mechanisms were small vessel disease (36.6%) and undetermined mechanism (31.5%), with cardioembolism (13.6%) and large artery atherosclerosis (11.0%) less frequent.
Table 2. Presenting Event Characteristics.
| Characteristics | No. (%) | |
|---|---|---|
| Intravenous Alteplase + Oral Placebo (n = 156) | Intravenous Placebo + Oral Aspirin (n = 157) | |
| Rapid improvement of symptoms prior to study treatment administration | 8 (5.1) | 7 (4.5) |
| Localization of presenting deficita | ||
| Right hemisphere | 75 (48.1) | 67 (42.7) |
| Left hemisphere | 59 (37.8) | 62 (39.5) |
| Unknown | 19 (12.2) | 21 (13.4) |
| Brainstem/cerebellum | 9 (5.8) | 18 (11.5) |
| Final diagnosis | ||
| Acute cerebral ischemia | 136 (88.3) | 131 (85.6) |
| Neurovascular mimicb | 18 (11.7) | 22 (14.4) |
| Ischemic cerebral event etiologyc | n=138 | n=135 |
| Small vessel disease | 48 (34.8) | 52 (38.5) |
| Undetermined etiology | 40 (29.0) | 46 (34.1) |
| Cardioembolism | 20 (14.5) | 17 (12.6) |
| Large artery atherosclerosis | 20 (14.5) | 10 (7.4) |
| Other determined etiologyd | 10 (7.2) | 10 (7.4) |
Localization of presenting deficits was assigned by the local investigator at the time of hospital discharge based on clinical examination and/or neuroimaging. A single patient may have had a stroke in more than 1 location.
Leading etiologies of neurovascular mimics were seizure and/or postictal (without concurrent stroke): alteplase, n = 1, aspirin, n = 2; psychogenic: alteplase, n = 3, aspirin, n = 7; complicated migraine: alteplase, n = 5, aspirin, n = 6; recrudescence of prior stroke: alteplase, n = 2, aspirin, n = 0; and other reasons: alteplase, n = 7, aspirin, n = 7.
Assigned by the treating physician.
Precise other etiologies not available.
Primary Outcome
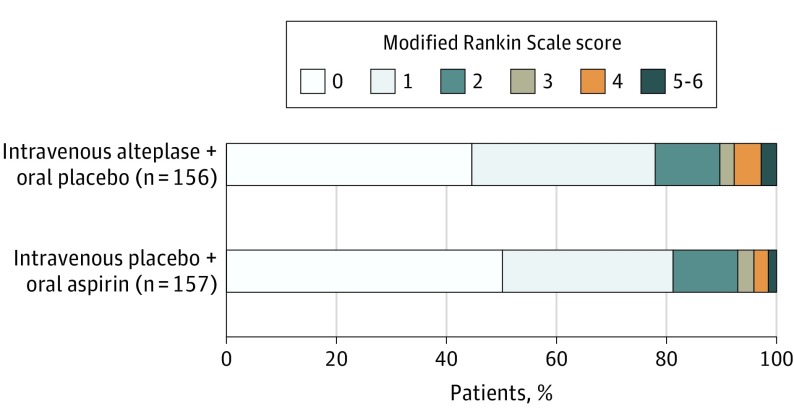
In the primary analysis, 122 patients (78.2%) randomized to alteplase had favorable outcomes at 90 days compared with 128 patients (81.5%) randomized to aspirin (adjusted absolute risk difference, −1.1%; 95% CI, −9.4% to 7.3%). The full distribution of levels of disability on the mRS score at 90 days are shown in Figure 2 and eTable 2 in Supplement 1, and distributions without missing data imputation are shown in eFigure 2 in Supplement 1.
Figure 2. Modified Rankin Scale Score Distributions at 90 Days by Treatment Group.
These distributions, which were used for the primary outcome analysis, included imputation for missing 90-day scores.
Secondary Outcomes
Secondary outcomes, including the ordinal analysis of mRS scores (OR, 0.81; 95% CI, 0.5-1.2) and global favorable recovery (OR, 0.86; 95% CI, 0.5-1.4), did not significantly favor either group (Table 3).
Table 3. 90-Day Efficacy Results.
| Outcomes | No. (%) | Effect Estimate, Risk Difference or OR (95% CI)a | |
|---|---|---|---|
| Intravenous Alteplase + Oral Placebo (n = 156) | Intravenous Placebo + Oral Aspirin (n = 157) | ||
| Primary Outcome | |||
| mRS score of 0 or 1, adjustedb | −1.1 (−9.4 to 7.3) | ||
| Secondary Outcomes | |||
| mRS score distribution at 90 dc | OR, 0.81 (0.5 to 1.2) | ||
| 0 | 70 (44.9) | 79 (50.3) | |
| 1 | 52 (33.3) | 49 (31.2) | |
| 2 | 18 (11.5) | 18 (11.5) | |
| 3 | 4 (2.6) | 5 (3.2) | |
| 4 | 8 (5.1) | 4 (2.5) | |
| 5-6 | 4 (2.6) | 2 (1.3) | |
| Global favorable recoveryd | OR, 0.86 (0.5 to 1.4) | ||
| Exploratory Outcomes | |||
| mRS score of 0 or 1, unadjustede | 122 (78.2) | 128 (81.5) | −3.3 (−12.2 to 5.6) |
| mRS score of 0, adjustedb | 70 (44.9) | 79 (50.3) | −3.6 (−14.2 to 7.1) |
| NIHSS score of 0 or 1, adjustedb | 108 (85.7) | 98 (81.7) | OR, 1.3 (0.65 to 2.6) |
| NIHSS score, mean (SD) | 1.2 (3.75) | 0.8 (2.01) | 0.4 (−0.4 to 1.1) |
| Barthel Index of 95 or 100, adjustedb,f | 107 (79.3) | 118 (88.7) | OR, 0.5 (0.3 to 1.1) |
| Glasgow Outcome Scale score of 1, adjustedb,g | 110 (81.5) | 113 (85.6) | OR, 0.8 (0.4 to 1.6) |
| Ambulatory performance, mean (SD), m/se,h | 0.95 (0.34) | 0.98 (0.44) | −0.03 (−0.13 to 0.08) |
| EuroQoL Group EQ-5D quality-of-life score, mean (SD)e,i | 0.81 (0.21) | 0.83 (0.20) | −0.02 (−0.07 to 0.03) |
| Stroke Impact Scale 16 score, mean (SD)e,j | 85.1 (21.0) | 86.3 (21.4) | −1.1 (−6.2 to 4.0) |
| Recurrent ischemic stroke | 5 (3.2) | 6 (4.0) | |
| Exploratory Analyses for the Primary Outcome | |||
| Acute cerebral ischemia cases only (mimics excluded)b | 107/138 (77.5) | 109/135 (80.7) | −1.4 (−10.5 to 7.7) |
| mRS score of 0 or 1, nonimputed | 110/141 (78.0) | 114/140 (81.4) | −2.3 (−11.1 to 6.6) |
| Propensity score–adjusted modelk | −2.4 (−11.2 to 6.4) | ||
| Logistic regression modell | OR, 0.9 (0.5 to 1.7) | ||
| Repeated-measures modelm | OR, 0.9 (0.5 to 1.4) | ||
Abbreviations: NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; OR, odds ratio.
Positive values for risk differences and ORs greater than 1 favor alteplase.
Adjusted risk difference values were calculated from a linear regression with treatment, age, time from symptom onset to treatment, and baseline NIHSS score. Quadratic terms for age and baseline NIHSS score were also added to the model if the Wald P value for the quadratic term was P < .10. Modified Rankin Scale scores range from 0 (symptom free) to 6 (dead).
The proportional odds assumption was met (score test P = .50); therefore, the common OR was calculated by fitting a proportional odds model with mRS scores at day 90 as the dependent variable and treatment group, pretreatment NIHSS score, age, and time from symptom onset to treatment as the continuous predictors. Quadratic terms for the continuous covariates were added to the model if the Wald P value for the quadratic term was less than 0.1.
The global favorable recovery statistic was derived from a generalized linear model with logit-link function computed using generalized estimating equations. This global statistic simultaneously evaluated the effect in all 4 outcome measures (mRS score of 0 or 1, NIHSS score of 0 or 1, Barthel Index of 95 or 100, and Glasgow Outcome Scale score of 1).
Confidence interval was computed using the normal approximation method.
The Barthel Index ranges from 0 (totally dependent) to 100 (patient performs self-care and mobility without assistance).
The Glasgow Outcome Scale score ranges from 1 (good recovery) to 5 (death).
Ambulatory performance assesses comfortable walking speed for 6 meters.
The EuroQoL Group EQ-5D score ranges from 0 (death) to 1 (perfect health).
The Stroke Impact Scale 16 score ranges from 0 to 100, with higher scores indicating better physical performance (strength, hand function, physical and instrumental activities of daily living, and mobility).
Propensity scores were derived from the logistic regression for treatment group with the following covariates: sex, race, ethnicity, smoking, history of hypertension, history of diabetes, history of atrial fibrillation, history of stroke, prior antiplatelet drug use, prior anticoagulant drug use, baseline glucose level, baseline systolic blood pressure, and baseline international normalized ratio.
Adjusted OR was obtained from a logistic regression adjusted for pretreatment NIHSS score, age, and time from symptom onset to treatment as continuous covariates and quadratic terms for the continuous covariates if the Wald P value for the quadratic term was <0.1.
Using all available mRS score responses at both day 30 and day 90. Odds ratios are adjusted for age, time from symptom onset to treatment, baseline NIHSS score, and quadratic terms for age (logistic) and age and baseline NIHSS score (repeated measures).
Adverse Events
Adverse event results are shown in Table 4. The primary adverse event, sICH within 36 hours, occurred in 5 patients, all treated with alteplase (3.2% vs 0; absolute risk difference, 3.3%; 95% CI, 0.8%-7.4%) (eFigure 3 in Supplement 1). Four sICH events were parenchymal hematoma type 2 (hematomas exceeding 30% of the infarction area with significant space-occupying effect) and 1 was a remote parenchymal hematoma type 1 (small petechial hemorrhage outside of the infarction bed). Symptomatic ICH volumes in each of the 5 patients were 0.4, 10, 18, 29, and 70 cm3; mRS scores at 90 days were 1, 2, 2, 3, and 4; and none of the patients with sICH died. Serious adverse events occurred in 40 patients (26.0%) treated with alteplase compared with 20 (13.1%) treated with aspirin; all adverse events are summarized in eTable 3 in Supplement 1. One patient who was treated with alteplase died at 90 days of volvulus, adjudicated as related to a history of bowel obstruction and resection and unrelated to study treatment.
Table 4. Adverse Event Results (As-Treated Analysis).
| Adverse Events | No. (%) | Risk Difference, % (95% CI) | |
|---|---|---|---|
| Intravenous Alteplase + Oral Placebo (n = 156) | Intravenous Placebo + Oral Aspirin (n = 157) | ||
| Primary Adverse Event Assessment | |||
| Symptomatic intracranial hemorrhage within 36 h | 5 (3.2) | 0 | 3.3 (0.8 to 7.4) |
| Secondary Adverse Event Assessments | |||
| Symptomatic intracranial hemorrhage within 36 h, SITS-MOST definitiona | 2 (1.3) | 0 | 1.3 (−1.2 to 4.6) |
| Any radiologic intracranial hemorrhage within 36 h by central reader | 11 (7.1) | 5 (3.3) | 3.9 (−1.2 to 9.5) |
| By radiological subtypeb | |||
| Hemorrhagic infarction type 1 | 2 | 3 | |
| Hemorrhagic infarction type 2 | 2 | 1 | |
| Parenchymal hematoma type 1 | 1 | 0 | |
| Parenchymal hematoma type 2 | 4 | 0 | |
| Remote parenchymal hematoma type 1 | 2 | 0 | |
| Intraventricular hemorrhage | 2c | 0 | |
| Subarachnoid hemorrhage | 3c | 1 | |
| Mortality within 90 d | 1 (0.6) | 0 | |
| Stroke-related and neurologic deaths within 90 d | 0 | 0 | |
| Patients with serious adverse eventsd | 40 (26.0) | 20 (13.1) | 12.9 (4.1 to 21.7)e |
| Patients with any adverse events | 119 (77.3) | 104 (68.0) | |
Abbreviation: SITS-MOST, Safe Implementation of Thrombolysis in Stroke–Monitoring Study.
The SITS MOST definition of symptomatic intracranial hemorrhage consisted of a deterioration in NIHSS score of ≥4 with a radiological parenchymal hemorrhage type 2 (described in footnote below).
Radiological subtypes of intracerebral hemorrhage consisted of hemorrhagic infarction type 1, defined as a small petechial hemorrhage along the margin of infarction; hemorrhagic infarction type 2, defined as more confluent petechial hemorrhage within the infarcted area but without space-occupying effect; parenchymal hematoma type 1, defined as hematomal hemorrhage in at least 30% of the infarcted area with some slight space-occupying effect; and parenchymal hematoma type 2, defined as dense hematomal hemorrhage in more than 30% of the infarcted area with substantial space-occupying effect or any hemorrhagic area outside the infarcted area; remote parenchymal hematoma type 1, defined as small or medium-sized blood clots located remote from the actual infarction; and remote parenchymal hematoma type 2, defined as large, confluent, dense blood clots in an area remote from the actual infarction (substantial space-occupying effect may be present).
These patients (with intraventricular or subarachnoid hemorrhages) also had intracerebral hemorrhage.
Serious adverse events of increased frequency in the alteplase-treated group that were deemed by the local investigator to be treatment related consisted of vitreous hemorrhage (n = 1), hemorrhagic transformation of stroke (n = 2), cerebral hemorrhage (n = 5), and intracranial hemorrhage (n = 3).
This is a post hoc calculation.
Exploratory Outcomes and Analyses
All prespecified exploratory outcomes and sensitivity analyses for the primary outcome showed no statistically significant differences between groups (Table 3). Changes in NIHSS scores over time are shown in eFigure 4 in Supplement 1. Heterogeneity of treatment effect was not observed for subgroups based on age (P = .92 for interaction), baseline NIHSS score (P = .10 for interaction), or time from symptom onset to treatment (P = .70 for interaction) (eFigure 5 in Supplement 1).
Post Hoc Analysis
In a post hoc Bayesian analysis, when the primary efficacy end point results of the current study were added to an uninformative prior, the posterior probability that alteplase therapy would improve favorable outcomes (mRS score of 0 or 1 at 90 days) to any degree was 23%, and the probability that alteplase therapy would improve favorable outcomes by more than an absolute 6% was 1.9%. The 95% credible interval of the unadjusted risk difference was −12.2% to 5.5%.
Discussion
Among patients with minor, nondisabling acute ischemic stroke, treatment with alteplase compared with aspirin did not increase the likelihood of favorable functional outcome at 90 days. Treatment benefit with alteplase was also not demonstrated for secondary and exploratory end points at 90 days. The study results raise the hypothesis that even a 6% treatment effect might be unlikely. However, the very early study termination precludes any definitive conclusions.
The trial tested alteplase in a unique ischemic stroke subpopulation. The operational definition of not clearly disabling deficits at presentation was intended to capture patients for whom treatment evidence was lacking and community equipoise for treatment benefit was present. Single-center clinical data without this type of explicit operationalization has suggested that 30% of these types of patients would have disability (mRS score of 2-6) at 90 days,2,3 higher than the observed 19% rate. Better outcomes may have been observed in the current study because of the formal definition of the population and other trial entry criteria selecting for healthier patients. The functional outcomes of patients enrolled in the trial may have been improved by earlier administration of aspirin (75% within 3.1 hours of onset) compared with general practice recommendations (within 24-48 hours).8 Additionally, prior observational cohorts not undergoing thrombolysis may have included more severely affected patients, given recent trends toward increased use of alteplase for patients with minor stroke in clinical practice.4,5
An increase in sICH was associated with alteplase in this population (risk difference, 3.3%; 95% CI, 0.8%-7.4%) without an associated increase in mortality. In a nationwide US registry, among 5910 patients with NIHSS scores of 0 to 5 treated with alteplase in routine practice, a 1.8% (95% CI, 1.5%-2.2%) absolute risk of sICH was observed.17 The sICH risk associated with alteplase was anticipated to be even lower in the current trial, which consisted of patients who had both low NIHSS scores and deficits judged to not be clearly disabling. The point estimate for the absolute risk of sICH of 3.2% in the current trial was higher than this 1.8% rate but lower than the 5% to 6% risk of sICH associated with alteplase in patients with higher NIHSS scores; however, wide confidence intervals included both of these comparators.14,18 The sICH events in this trial had no associated mortality compared with the sICH events in the NINDS trials associated with 75% mortality.19
The findings from the current trial cannot be extrapolated to all patients with lower stroke severity based on an NIHSS score of 0 to 5. Those with disabling deficits, such as isolated leg weakness causing inability to walk and isolated aphasia preventing communication, were excluded from the current study. Furthermore, clinical guidelines and scientific statements regarding alteplase recommend “no exclusion for patients with mild but nonetheless disabling stroke symptoms….”7,20 Additionally, patients with mild deficits who have large vessel occlusions (a minority in this population) may be more prone to stroke progression and thus may require further study.21,22,23
Limitations
This study has several limitations. First, the trial’s early termination leads to significant uncertainty of all observations. Second, within the bounds of formal trial entry criteria, some sites may have selectively recruited patients with even milder, rather than more severe, deficits despite efforts to minimize this selection bias. Third, the definition of “not clearly disabling” was subjective and required interpretation by individual clinicians. Although efforts were made to encourage consistency in assessment of eligible patients, this cannot be confirmed. Fourth, the loss to follow-up at day 90 was relatively high. However, mRS scores from day 30 (available for 78% of these patients) are known to permit robust imputation.24
Conclusions
Among patients with minor nondisabling acute ischemic stroke, treatment with alteplase compared with aspirin did not increase the likelihood of favorable functional outcome at 90 days. However, the very early study termination precludes any definitive conclusions, and additional research may be warranted.
eTable 1. Exclusion Criteria for Mild Deficits in Previous Randomized, Intravenous Thrombolysis Trials
eTable 2. Modified Rankin Scale Score Distributions at Day 90
eTable 3. Summary of All Adverse Events by System Organ Class
eFigure 1. NIHSS Item Profiles for All Enrolled Patients
eFigure 2. mRS Distributions as Observed (Without 90-Day Imputation)
eFigure 3. Representative Images of Each Symptomatic Intracranial Hemorrhage (n=5)
eFigure 4. NIHSS Change Over Time According to NIHSS Baseline Score and Treatment Arm
eFigure 5. Subgroup Analysis of Primary Outcome (mRS 0-1 at 90 Days)
Final Protocol
Protocol Amendment
Statistical Analysis Plan
References
- 1.Messé SR, Khatri P, Reeves MJ, et al. Why are acute ischemic stroke patients not receiving IV tPA? results from a national registry. Neurology. 2016;87(15):1565-1574. doi: 10.1212/WNL.0000000000003198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer U, Baumgartner A, Arnold M, et al. What is a minor stroke? Stroke. 2010;41(4):661-666. doi: 10.1161/STROKEAHA.109.572883 [DOI] [PubMed] [Google Scholar]
- 3.Khatri P, Conaway MR, Johnston KC; Acute Stroke Accurate Prediction Study Investigators . Ninety-day outcome rates of a prospective cohort of consecutive patients with mild ischemic stroke. Stroke. 2012;43(2):560-562. doi: 10.1161/STROKEAHA.110.593897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines–Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549. doi: 10.1161/CIRCOUTCOMES.111.000095 [DOI] [PubMed] [Google Scholar]
- 5.Asdaghi N, Wang K, Ciliberti-Vargas MA, et al. ; FL-PR CReSD Investigators and Collaborators . Predictors of thrombolysis administration in mild stroke: Florida-Puerto Rico Collaboration to Reduce Stroke Disparities. Stroke. 2018;49(3):638-645. doi: 10.1161/STROKEAHA.117.019341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council . 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 8.Sandercock P, Wardlaw JM, Lindley RI, et al. ; IST-3 Collaborative Group . The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the Third International Stroke Trial [IST-3]): a randomised controlled trial. Lancet. 2012;379(9834):2352-2363. doi: 10.1016/S0140-6736(12)60768-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeatts SD, Broderick JP, Chatterjee A, et al. Alteplase for the treatment of acute ischemic stroke in patients with low National Institutes of Health Stroke Scale and not clearly disabling deficits (Potential of rtPA for Ischemic Strokes with Mild Symptoms PRISMS): rationale and design [published online January 1, 2018]. Int J Stroke. doi: 10.1177/1747493018765269 [DOI] [PubMed] [Google Scholar]
- 10.Jauch EC, Saver JL, Adams HP Jr, et al. ; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology . Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi: 10.1161/STR.0b013e318284056a [DOI] [PubMed] [Google Scholar]
- 11.Zhao W, Ciolino J, Palesch Y. Step-forward randomization in multicenter emergency treatment clinical trials. Acad Emerg Med. 2010;17(6):659-665. doi: 10.1111/j.1553-2712.2010.00746.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao W, Weng Y. Block urn design—a new randomization algorithm for sequential trials with two or more treatments and balanced or unbalanced allocation. Contemp Clin Trials. 2011;32(6):953-961. doi: 10.1016/j.cct.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei LJ. The adaptive biased coin design for sequential experiments. Ann Stat. 1978;6(1):92-100. doi: 10.1214/aos/1176344068 [DOI] [Google Scholar]
- 14.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 15.Wahlgren N, Ahmed N, Dávalos A, et al. ; SITS-MOST Investigators . Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke–Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275-282. doi: 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 16.Khatri P, Tayama D, Cohen G, et al. ; PRISMS and IST-3 Collaborative Groups . Effect of intravenous recombinant tissue-type plasminogen activator in patients with mild stroke in the Third International Stroke Trial-3: post hoc analysis. Stroke. 2015;46(8):2325-2327. doi: 10.1161/STROKEAHA.115.009951 [DOI] [PubMed] [Google Scholar]
- 17.Romano JG, Smith EE, Liang L, et al. Outcomes in mild acute ischemic stroke treated with intravenous thrombolysis: a retrospective analysis of the Get With the Guidelines–Stroke registry. JAMA Neurol. 2015;72(4):423-431. doi: 10.1001/jamaneurol.2014.4354 [DOI] [PubMed] [Google Scholar]
- 18.Mehta RH, Cox M, Smith EE, et al. ; Get With the Guidelines–Stroke Program . Race/ethnic differences in the risk of hemorrhagic complications among patients with ischemic stroke receiving thrombolytic therapy. Stroke. 2014;45(8):2263-2269. doi: 10.1161/STROKEAHA.114.005019 [DOI] [PubMed] [Google Scholar]
- 19.NINDS t-PA Stroke Study Group Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28(11):2109-2118. doi: 10.1161/01.STR.28.11.2109 [DOI] [PubMed] [Google Scholar]
- 20.Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. ; American Heart Association Stroke Council and Council on Epidemiology and Prevention . Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(2):581-641. doi: 10.1161/STR.0000000000000086 [DOI] [PubMed] [Google Scholar]
- 21.Rajajee V, Kidwell C, Starkman S, et al. Early MRI and outcomes of untreated patients with mild or improving ischemic stroke. Neurology. 2006;67(6):980-984. doi: 10.1212/01.wnl.0000237520.88777.71 [DOI] [PubMed] [Google Scholar]
- 22.Nedeltchev K, Schwegler B, Haefeli T, et al. Outcome of stroke with mild or rapidly improving symptoms. Stroke. 2007;38(9):2531-2535. doi: 10.1161/STROKEAHA.107.482554 [DOI] [PubMed] [Google Scholar]
- 23.Smith EE, Fonarow GC, Reeves MJ, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: findings from Get With the Guidelines–Stroke. Stroke. 2011;42(11):3110-3115. doi: 10.1161/STROKEAHA.111.613208 [DOI] [PubMed] [Google Scholar]
- 24.Ovbiagele B, Lyden PD, Saver JL; VISTA Collaborators . Disability status at 1 month is a reliable proxy for final ischemic stroke outcome. Neurology. 2010;75(8):688-692. doi: 10.1212/WNL.0b013e3181eee426 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Exclusion Criteria for Mild Deficits in Previous Randomized, Intravenous Thrombolysis Trials
eTable 2. Modified Rankin Scale Score Distributions at Day 90
eTable 3. Summary of All Adverse Events by System Organ Class
eFigure 1. NIHSS Item Profiles for All Enrolled Patients
eFigure 2. mRS Distributions as Observed (Without 90-Day Imputation)
eFigure 3. Representative Images of Each Symptomatic Intracranial Hemorrhage (n=5)
eFigure 4. NIHSS Change Over Time According to NIHSS Baseline Score and Treatment Arm
eFigure 5. Subgroup Analysis of Primary Outcome (mRS 0-1 at 90 Days)
Final Protocol
Protocol Amendment
Statistical Analysis Plan