Key Points
Question
Does acupuncture reduce joint pain related to aromatase inhibitors among postmenopausal women with early-stage breast cancer?
Findings
In this multicenter randomized clinical trial of 226 women with early-stage breast cancer, patients in the acupuncture group, compared with those in the sham acupuncture group or the waitlist control group, had significant reductions in changes in joint pain scores from baseline to 6 weeks (between-group difference vs sham acupuncture, 0.92 points and 0.96 points vs the waitlist control group [0- to 10-point scale]).
Meaning
Acupuncture was associated with statistically significant reductions in aromatase inhibitor–related joint pain at 6 weeks, although the magnitude of the improvement was of uncertain clinical importance.
Abstract
Importance
Musculoskeletal symptoms are the most common adverse effects of aromatase inhibitors and often result in therapy discontinuation. Small studies suggest that acupuncture may decrease aromatase inhibitor–related joint symptoms.
Objective
To determine the effect of acupuncture in reducing aromatase inhibitor–related joint pain.
Design, Setting, and Patients
Randomized clinical trial conducted at 11 academic centers and clinical sites in the United States from March 2012 to February 2017 (final date of follow-up, September 5, 2017). Eligible patients were postmenopausal women with early-stage breast cancer who were taking an aromatase inhibitor and scored at least 3 on the Brief Pain Inventory Worst Pain (BPI-WP) item (score range, 0-10; higher scores indicate greater pain).
Interventions
Patients were randomized 2:1:1 to the true acupuncture (n = 110), sham acupuncture (n = 59), or waitlist control (n = 57) group. True acupuncture and sham acupuncture protocols consisted of 12 acupuncture sessions over 6 weeks (2 sessions per week), followed by 1 session per week for 6 weeks. The waitlist control group did not receive any intervention. All participants were offered 10 acupuncture sessions to be used between weeks 24 and 52.
Main Outcomes and Measures
The primary end point was the 6-week BPI-WP score. Mean 6-week BPI-WP scores were compared by study group using linear regression, adjusted for baseline pain and stratification factors (clinically meaningful difference specified as 2 points).
Results
Among 226 randomized patients (mean [SD] age, 60.7 [8.6] years; 88% white; mean [SD] baseline BPI-WP score, 6.6 [1.5]), 206 (91.1%) completed the trial. From baseline to 6 weeks, the mean observed BPI-WP score decreased by 2.05 points (reduced pain) in the true acupuncture group, by 1.07 points in the sham acupuncture group, and by 0.99 points in the waitlist control group. The adjusted difference for true acupuncture vs sham acupuncture was 0.92 points (95% CI, 0.20-1.65; P = .01) and for true acupuncture vs waitlist control was 0.96 points (95% CI, 0.24-1.67; P = .01). Patients in the true acupuncture group experienced more grade 1 bruising compared with patients in the sham acupuncture group (47% vs 25%; P = .01).
Conclusions and Relevance
Among postmenopausal women with early-stage breast cancer and aromatase inhibitor–related arthralgias, true acupuncture compared with sham acupuncture or with waitlist control resulted in a statistically significant reduction in joint pain at 6 weeks, although the observed improvement was of uncertain clinical importance.
Trial Registration
ClinicalTrials.gov Identifier: NCT01535066
This randomized clinical trial compares true acupuncture with sham acupuncture and with waitlist control on the reduction of joint pain related to aromatase inhibitor use in postmenopausal women with early-stage breast cancer.
Introduction
Aromatase inhibitors have proven efficacy for the treatment of hormone-sensitive breast cancer.1 However, many patients experience adverse effects, including aromatase inhibitor–related arthralgias (pain and stiffness), which occur in approximately 50% of patients2,3,4 and contribute to nonadherence with therapy.5,6 Data from clinical trials suggest that nonadherence to endocrine therapy is associated with reduced disease-free survival.7
Acupuncture is a traditional Chinese therapy that involves insertion of fine, single-use, sterile needles in acupoints according to a system of channels and meridians that were developed by early practitioners of Traditional Chinese Medicine.8 The needles are stimulated by manual manipulation, electrical stimulation, or heat.8 Acupuncture is a popular nonpharmacological modality used for treating a variety of conditions and may result in reduced opioid use.9 Several studies have evaluated acupuncture as a treatment for aromatase inhibitor–related arthralgias.10,11,12,13 However, these studies provided uncertain interpretations because of small sample size, ineffective blinding, and implementation at single centers. Concerns were also raised that the sham control intervention may have had positive physiological effects and therefore could be an active control.
This multicenter randomized blinded sham- and waitlist-controlled clinical trial was conducted to evaluate the effect of acupuncture on joint pain related to aromatase inhibitors among women with early-stage breast cancer.
Methods
Study Oversight
The study was conducted at 11 academic and community sites and was approved by appropriate individual institutional review boards. Sites were required to have 2 trained acupuncturists for the duration of the trial. Study participants were informed of the investigational nature of the study and provided written informed consent. Race and ethnicity, which were self-reported by patients and recorded by site investigators, were reported to aid in the determination of the generalizability of the findings.14 The trial protocol is reported in Supplement 1.
Patient Characteristics
Eligible study participants were women with histologically confirmed (stages I-III) primary invasive estrogen receptor–positive carcinoma of the breast, progesterone receptor–positive carcinoma of the breast, or both. Patients were required to be postmenopausal or premenopausal with use of a gonadotropin-releasing hormone agonist, to be taking a third-generation aromatase inhibitor for more than 30 days prior to registration with plans to continue for at least 1 additional year, to have recovered from the effects of surgery or chemotherapy, and to have a Zubrod performance status of 0 to 1.15 Inclusion criteria included a score of greater than 3 (range, 0-10; higher scores indicate greater pain) on the worst pain item of the Brief Pain Inventory-Short Form (BPI-SF) that (by patient report) started or increased since starting aromatase inhibitor therapy.16
Exclusion criteria included prior acupuncture treatment for joint symptoms at any time, although patients were allowed to have received 2 or fewer acupuncture treatments within 12 months prior to registration for other reasons. Study participants must not have received opioid analgesics, topical analgesics, oral corticosteroids, intramuscular corticosteroids, or intra-articular steroids, or any other medical therapy, alternative therapy, or physical therapy for the treatment of joint pain or joint stiffness within 28 days prior to registration. Patients must not have had a history of bone fracture or surgery of the affected knees, hands, or both within 6 months prior to enrollment. Patients with a severe bleeding disorder or a latex allergy were excluded.
Study Intervention
Study participants were randomized 2:1:1 to the true acupuncture group, the sham acupuncture group, or the waitlist control group, with randomization dynamically balanced by study site to account for potential differences in acupuncture administration.12 The acupuncture study interventions were developed by a consensus of acupuncture experts based on previous studies of acupuncture for aromatase inhibitor–related arthralgias with adherence to the Standards for Reporting of Controlled Trials in Acupuncture (STRICTA) recommendations.13 The details of the acupuncture point protocol (ie, body site of needle placement) and extensive training and standardization methods (in-person training, study manuals, monthly phone calls, and remote quality assurance monitoring) have been previously described.17
Briefly, both true acupuncture and sham acupuncture consisted of twelve 30- to 45- minute sessions administered over a period of 6 weeks (2 per week) followed by 1 session per week for 6 weeks. For true acupuncture, stainless steel, single-use, sterile and disposable needles were used and inserted at traditional depths and angles. The joint-specific protocol was tailored to as many as 3 of the patient’s most painful joint areas.18 Needles were restimulated manually once during each session. The sham acupuncture consisted of a core standardized prescription of minimally invasive, shallow needle insertion using thin and short needles at nonacupuncture points. The sham acupuncture protocol also included joint-specific treatments and an auricular sham based on the application of adhesives to nonacupuncture points on the ear. The waitlist control group received no acupuncture treatments and received no other intervention for 24 weeks after randomization. At 24 weeks, all patients received vouchers for 10 true acupuncture bonus sessions to be used prior to the 52-week visit.
Patient-Reported Outcome Measures
Primary End Point
There is no consensus on the best measure for aromatase inhibitor–induced arthralgias. The BPI-SF was used in several prior interventional trials of aromatase inhibitor arthralgias.10,19,20 This 14-item questionnaire asks individuals to rate joint pain over the prior week and the degree to which the pain interferes with activities using a 0- to 10-point scale (0 [no pain] to 10 [pain as bad as you can imagine]).16 For this study, the primary end point was the BPI Worst Pain Item (BPI-WP) score at 6 weeks of treatment. A reduction of 2 points on the BPI-WP has been identified as a clinically meaningful change for a patient.21
Prespecified Secondary End Points
Prespecified secondary end points were measured and evaluated using several instruments. The BPI-SF was used to measure worst pain, worst stiffness, pain severity, and pain-related interference scores (range from no symptoms to worst, 0-10); administered at 6, 12, 16, 20, 24, and 52 weeks to assess the duration of effect following completion of the intervention.
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) version 3.1, a validated measure for assessing osteoarthritis of the knees or hips, evaluated patients using 24 questions related to 3 subscales (pain [0-500], stiffness [0-200], and physical function [0-1700])22; administered at 6, 12, 24, and 52 weeks. The Modified Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands (M-SACRAH) was used to assess hand pain, stiffness, and functional status (range, 0-100)23; administered at 6, 12, 24, and 52 weeks. For both instruments, higher scores indicate greater symptom severity.
The Functional Assessment of Cancer Therapy-Endocrine Symptoms (FACT-ES) measured physical and functional well-being and endocrine symptoms (score range, 0-128); administered at 6, 12, 24, and 52 weeks. The PROMIS Pain Impact-Short Form (PROMIS PI-SF; score range, 6-30)24,25 was administered at 6, 12, 24, and 52 weeks. Both insturments have 5 response levels (low scores [not at all] to high scores [very much]), and higher scores reflect better symptoms. Additional prespecified secondary end points were analgesic and opioid use and safety and tolerability of acupuncture. Other outcomes included an assessment of masking among patients enrolled in the true acupuncture and sham acupuncture study groups.
Statistical Analyses
The primary hypothesis was that true acupuncture would decrease joint pain associated with the use of aromatase inhibitors (according to the BPI-SF worst pain item [BPI-WP]) compared with both sham acupuncture or waitlist control at 6 weeks. For a 2-point difference between groups and an assumed 3.0-point standard deviation at 6 weeks, 208 eligible patients were required for 82% power (using 2-sided tests; α = .025) for true acupuncture vs sham acupuncture and true acupuncture vs waitlist control. The design further specified an estimated 5% nonadherence and 10% dropout rate at the primary end point evaluation time of 6 weeks. In addition, the design incorporated a 10% contamination rate. The primary outcome was analyzed using the intention-to-treat principle (ie, as randomized), using a complete-case approach given limited missing data (<10%), without using imputation.
The primary outcome at week 6 and the secondary outcomes at weeks 6 and 12 were examined using multivariable linear regression and adjusting for the baseline score, indicator variables for study sites, and 2 indicator variables for intervention group. The baseline score was not included as a component of the outcome because it was obtained prior to intervention administration.
Longitudinal measures through week 24 (12 weeks following completion of the intervention) were analyzed as secondary outcomes using linear mixed models with a random effect for patient and an a priori unstructured covariance matrix.26 Fixed-effect factors associated with the outcomes of interest included intervention assignment using separate indicator variables (with true acupuncture as the reference category), continuous time (both as a linear variable and quadratic variable), an intervention × time interaction (both linear and quadratic time), the baseline score, and indicator variables for study sites.
In a post hoc analysis, we reported the proportion of patients reporting an individual-level clinically meaningful reduction (2 points from baseline on a scale of 0-10 or [calculated separately] a relative reduction of 30% ), based on current guidelines for clinical trials evaluating interventions to reduce pain.27,28 Risk differences (RDs) and relative risks (RRs) were estimated using Poisson regression with robust standard errors.29
Statistical analyses were conducted using SAS version 9.44 (SAS Institute), including SAS proc mixed for linear mixed models.30 For the primary end point, Bonferroni adjustment was used for multiple comparisons (setting α = .025) for each independent comparison of true acupuncture vs sham acupuncture and true acupuncture vs waitlist control. No adjustments for multiple comparisons were made for all secondary or post hoc analyses, which are considered exploratory.
Results
Patient Characteristics
From March 2012 to February 2017, a total of 226 patients were randomly assigned to the true acupuncture (n = 110), sham acupuncture (n = 59), or waitlist control (n = 57) group (Figure 1). Patient baseline characteristics are shown in Table 1. Median age was lower in the sham acupuncture group (57.0 years) than in the waitlist control (60.6 years) or true acupuncture (60.8 years) groups. Fewer Hispanic patients were randomized to the waitlist control group, and more Asian patients were randomized to the true acupuncture group. The overall median time for receiving aromatase inhibitor therapy prior to enrollment was 1.1 years. The patient characteristics were well balanced by study site and clinical factors.
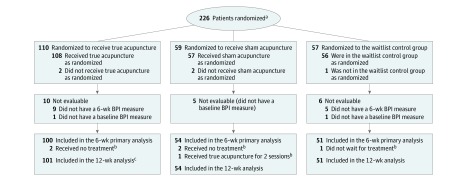
Figure 1. Flow of Randomized Patients for the Effect of True Acupuncture vs Sham Acupuncture and True Acupuncture vs Waitlist Control on Joint Pain Using the 6-Week Brief Pain Inventory-Short Form Worst Pain Score.
BPI indicates Brief Pain Inventory.
aData were not collected to report the number of patients screened for eligibility prior to randomization.
bPatients’ status as major deviations began immediately in the course of follow-up and was applied to both the 6-week and 12-week analyses.
cOne patient who was not available for 6-week evaluation was evaluated at 12 weeks.
Table 1. Baseline Characteristics for Patients in the True Acupuncture, Sham Acupuncture, or Waitlist Control Group.
| No. (%)a | |||
|---|---|---|---|
| True Acupuncture (n = 110) |
Sham Acupuncture (n = 59) |
Waitlist Control (n = 57) |
|
| Age, median (range), yb | 60.8 (34.1-80.6) | 57.0 (40.6-77.5) | 60.6 (27.1-76.0) |
| Hispanic | |||
| Yes | 11 (10) | 7 (12) | 3 (5) |
| Unknown | 0 | 1 | 0 |
| Race | |||
| White | 88 (83) | 54 (93) | 51 (91) |
| Black | 6 (6) | 2 (3) | 2 (4) |
| Asian | 11 (10) | 2 (3) | 2 (4) |
| Pacific Islander | 0 | 0 | 1 (2) |
| American Indian | 1 (1) | 0 | 0 |
| Unknown | 4 | 1 | 1 |
| Study sitec | |||
| Columbia University Minority Underserved NCORP | 14 (13) | 8 (14) | 7 (12) |
| Kaiser Permanente Medical Center | 27 (25) | 14 (24) | 15 (26) |
| Spectrum Health Medical Group | 34 (31) | 17 (29) | 17 (30) |
| Other (8) | 35 (32) | 20 (34) | 18 (32) |
| Breast cancer stage | |||
| I | 41 (39) | 28 (47) | 28 (49) |
| II | 53 (50) | 23 (39) | 23 (40) |
| III | 12 (11) | 8 (14) | 6 (11) |
| Prior chemotherapy | 56 (51) | 31 (53) | 24 (42) |
| Prior tamoxifen | 18 (16) | 15 (25) | 10 (18) |
| Current or prior aromatase inhibitor therapyd | |||
| Anastrozole | 80 (73) | 44 (75) | 40 (70) |
| Currently using | 64 (58) | 37 (63) | 37 (65) |
| Not currently using | 15 (14) | 7 (12) | 3 (5) |
| Not reported | 1 (1) | 0 | 0 |
| Letrozole | 36 (33) | 17 (29) | 17 (30) |
| Currently using | 29 (26) | 13 (22) | 11 (19) |
| Not currently using | 7 (6) | 4 (7) | 6 (11) |
| Exemestane | 21 (19) | 10 (17) | 10 (18) |
| Currently using | 15 (14) | 9 (15) | 8 (14) |
| Not currently using | 6 (5) | 1 (2) | 2 (4) |
| Time receiving aromatase inhibitors, median (range), y | 1.0 (0.1-8.0) | 1.1 (0.1-9.0) | 1.1 (0.1-3.1) |
| Prior acupuncture | 19 (17) | 13 (22) | 12 (21) |
| Pain medications (any use)d | |||
| Acetaminophen/paracetamol | 26 (24) | 12 (20) | 8 (14) |
| Ibuprofen | 31 (28) | 20 (34) | 22 (39) |
| Other NSAID | 15 (14) | 8 (14) | 8 (14) |
| Other pain medication | 6 (5) | 3 (5) | 4 (7) |
| ≥1 medication | 66 (60) | 36 (61) | 33 (58) |
Abbreviations: NCORP, National Cancer Institute Community Oncology Research Program; NSAID, nonsteroidal anti-inflammatory drug.
Values are reported as No. (%) unless otherwise indicated.
Younger women were either surgically or chemically menopausal.
See eTable 5 in Supplement 2 for specific site names and locations.
Indicates the number of patients who answered yes. More than 1 subcategory type is allowed. Therefore, items may not sum.
Major protocol deviations were reported for 6 patients: 1 patient randomized to the sham acupuncture group received true acupuncture for 2 sessions, 4 patients randomized to acupuncture groups (true, 2; sham, 2) received no treatment, and 1 patient randomized to the waitlist control group was in too much pain and declined to participate (but did complete the patient-reported questionnaires). Twenty-one patients were not evaluable (Figure 1). No patients reported receiving acupuncture outside of the trial during the 12-week intervention period.
Worst Pain Scores
In total, 91% (100/110) of patients in the true acupuncture group, 92% (54/59) in the sham acupuncture group, and 89% (51/57) in the waitlist control group had both baseline and 6-week BPI-WP scores for analysis (Figure 1). Reporting rates at weeks 6 and 12 were similar. There was no statistically significant difference by study group in the amount of available data at follow-up (P = .93). Mean baseline BPI-WP score was 6.84 in the true acupuncture group, 6.55 in the sham acupuncture group, and 6.48 in the waitlist control group.
Primary Outcome
Compared with baseline, the mean observed BPI-WP score was 2.05 points lower (reduced pain) at 6 weeks in the true acupuncture group, 1.07 points lower in the sham acupuncture group, and 0.99 points lower for the waitlist control group, with differences in adjusted 6-week mean BPI-WP scores between true acupuncture vs sham acupuncture of 0.92 points (95% CI, 0.20-1.65; P = .01) and between true acupuncture vs waitlist control of 0.96 points (95% CI, 0.24-1.67; P = .01) (Table 2). Thus, the study results rejected the null hypothesis that true acupuncture generated the same outcomes as sham acupuncture and waitlist control, although the magnitude of the effect did not achieve the prespecified difference of 2 points.
Table 2. Observed and Fitted Group Mean Results for Brief Pain Inventory-Short Form Scores at Weeks 6 and 12 in Each Group.
| Analysis by Group | No. of Patients | Group Mean Differencea | Proportion With >30% Improvement | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) | Fitted Difference (95% CI) | % | Risk Difference, % (95% CI) | Relative Risk (95% CI)b | P Value | |||||
| Baselinec | Follow-up | True vs Sham True vs Waitlist |
P Value | True vs Sham True vs Waitlist |
P Value | |||||
| Worst Paind | ||||||||||
| Week 6e | ||||||||||
| True | 100 | 6.84 (6.55 to 7.13) | 4.79 (4.36 to 5.22) | 49.0 | 1 [Reference] | |||||
| Sham | 54 | 6.55 (6.14 to 6.98) | 5.48 (4.78 to 6.18) | 0.92 (0.20 to 1.65) | .01 | 24.1 | 24.9 (9.9 to 40.0) | .001 | 1.95 (1.16 to 3.28) | .01 |
| Waitlist | 51 | 6.48 (6.07 to 6.89) | 5.49 (4.81 to 6.17) | 0.96 (0.24 to 1.67) | .01 | 23.5 | 25.5 (10.3 to 40.7) | .001 | 2.04 (1.19 to 3.48) | .009 |
| Week 12 | ||||||||||
| True | 101 | 6.84 (6.55 to 7.13) | 4.53 (4.05 to 5.01) | 51.5 | 1 [Reference] | |||||
| Sham | 54 | 6.55 (6.14 to 6.98) | 5.04 (4.33 to 5.74) | 0.73 (−0.07 to 1.53) | .08 | 46.3 | 5.2 (−11.3 to 21.7) | .54 | 1.09 (0.77 to 1.54) | .62 |
| Waitlist | 51 | 6.48 (6.07 to 6.89) | 6.29 (5.73 to 6.86) | 1.95 (1.19 to 2.70) | <.001 | 15.7 | 35.8 (21.9 to 49.8) | <.001 | 3.24 (1.66 to 6.34) | <.001 |
| Average Pain | ||||||||||
| Week 6 | ||||||||||
| True | 100 | 4.95 (4.64 to 5.27) | 3.50 (3.21 to 3.88) | 43.0 | 1 [Reference] | |||||
| Sham | 54 | 4.69 (4.22 to 5.17) | 3.93 (3.40 to 4.45) | 0.60 (0.03 to 1.17) | .04 | 25.9 | 17.1 (1.9 to 32.3) | .03 | 1.60 (0.97 to 2.63) | .07 |
| Waitlist | 51 | 4.95 (4.52 to 5.37) | 4.14 (3.65 to 4.63) | 0.71 (0.15 to 1.28) | .01 | 17.7 | 25.4 (11.1 to 39.6) | .0005 | 2.44 (1.28 to 4.62) | .006 |
| Week 12 | ||||||||||
| True | 101 | 4.95 (4.64 to 5.27) | 3.00 (2.62 to 3.38) | 60.4 | 1 [Reference] | |||||
| Sham | 53 | 4.69 (4.22 to 5.17) | 3.62 (3.06 to 4.18) | 0.79 (0.16 to 1.42) | .02 | 45.3 | 15.1 (−1.3 to 31.6) | .07 | 1.31 (0.94 to 1.83) | .11 |
| Waitlist | 51 | 4.95 (4.52 to 5.37) | 4.33 (3.80 to 4.87) | 1.38 (0.76 to 2.00) | <.001 | 29.4 | 31.0 (15.3 to 46.7) | .0001 | 2.04 (1.30 to 3.21) | .002 |
| Pain Interference | ||||||||||
| Week 6 | ||||||||||
| True | 100 | 4.24 (3.86 to 4.62) | 2.55 (2.11 to 2.98) | 59.0 | 1 [Reference] | |||||
| Sham | 54 | 3.85 (3.34 to 4.37) | 3.03 (2.44 to 3.62) | 0.73 (0.11 to 1.35) | .02 | 44.4 | 14.6 (−1.8 to 30.9) | .08 | 1.36 (0.98 to 1.89) | .06 |
| Waitlist | 51 | 4.01 (3.54 to 4.48) | 3.07 (2.47 to 3.67) | 0.63 (−0.03 to 1.29) | .06 | 35.3 | 23.7 (7.4 to 40.0) | .004 | 1.68 (1.12 to 2.52) | .01 |
| Week 12 | ||||||||||
| True | 101 | 4.24 (3.86 to 4.62) | 2.44 (2.00 to 2.88) | 68.3 | 1 [Reference] | |||||
| Sham | 54 | 3.85 (3.34 to 4.37) | 2.40 (1.84 to 2.96) | 0.19 (−0.40 to 0.77) | .53 | 64.8 | 3.5 (−12.1 to 19.1) | .66 | 1.06 (0.84 to 1.33) | .64 |
| Waitlist | 51 | 4.01 (3.54 to 4.48) | 3.31 (2.69 to 3.94) | 0.99 (0.36 to 1.63) | .003 | 37.3 | 31.1 (15.0 to 47.1) | <.001 | 1.85 (1.26 to 2.70) | .002 |
| Pain Severity | ||||||||||
| Week 6 | ||||||||||
| True | 100 | 4.71 (4.41 to 5.01) | 3.21 (2.85 to 3.57) | 50.0 | 1 [Reference] | |||||
| Sham | 54 | 4.75 (5.32 to 5.19) | 3.75 (3.21 to 5.28) | 0.56 (0.00 to 1.11) | .05 | 33.3 | 16.7 (0.7 to 32.6) | .04 | 1.52 (1.00 to 2.31) | .05 |
| Waitlist | 51 | 4.64 (4.25 to 5.03) | 3.82 (3.26 to 4.37) | 0.71 (0.15 to 1.28) | .01 | 33.3 | 16.7 (0.4 to 32.9) | .04 | 1.51 (0.98 to 2.34) | .06 |
| Week 12 | ||||||||||
| True | 101 | 4.71 (4.41 to 5.01) | 2.89 (2.52 to 3.26) | 57.4 | 1 [Reference] | |||||
| Sham | 54 | 4.75 (5.32 to 5.19) | 3.41 (2.86 to 3.95) | 0.53 (−0.06 to 1.11) | .08 | 44.4 | 13.0 (−3.4 to 29.4) | .12 | 1.29 (0.92 to 1.81) | .15 |
| Waitlist | 51 | 4.64 (4.25 to 5.03) | 4.25 (3.72 to 4.78) | 1.43 (0.84 to 2.02) | <.001 | 21.6 | 35.9 (21.0 to 50.7) | <.001 | 2.64 (1.53 to 4.55) | <.001 |
| Worst Stiffness | ||||||||||
| Week 6 | ||||||||||
| True | 100 | 6.65 (6.26 to 7.04) | 4.46 (3.94 to 4.98) | 52.0 | 1 [Reference] | |||||
| Sham | 54 | 6.59 (6.03 to 7.16) | 5.50 (4.86 to 6.14) | 1.00 (0.19 to 1.81) | .02 | 33.3 | 18.7 (2.7 to 34.6) | .02 | 1.56 (1.03 to 2.37) | .04 |
| Waitlist | 51 | 6.68 (6.26 to 7.10) | 5.53 (4.91 to 6.14) | 1.09 (0.26 to 1.92) | .01 | 29.4 | 22.6 (6.7 to 38.5) | .005 | 1.76 (1.11 to 2.78) | .02 |
| Week 12 | ||||||||||
| True | 100 | 6.65 (6.26 to 7.04) | 4.35 (3.82 to 4.88) | 50.0 | 1 [Reference] | |||||
| Sham | 54 | 6.59 (6.03 to 7.16) | 5.07 (4.47 to 5.68) | 0.72 (−0.08 to 1.53) | .08 | 46.3 | 3.7 (−12.8 to 20.2) | .66 | 1.09 (0.78 to 1.53) | .62 |
| Waitlist | 51 | 6.68 (6.26 to 7.10) | 6.12 (5.59 to 6.64) | 1.80 (1.03 to 2.57) | <.001 | 19.6 | 30.4 (15.7 to 45.1) | <.001 | 2.54 (1.42 to 4.54) | .002 |
Data for examination of group mean differences by study group were calculated using multivariable linear regression (adjusting for the baseline score and the stratification factor). Positive values favor true acupuncture.
Data for examination of relative risks were calculated using Poisson regression (adjusting for the baseline score and the stratification factor). Positive values favor true acupuncture.
Data were calculated among patients with follow-up scores.
Patients randomized to the true acupuncture group were more likely than patients randomized to the sham acupuncture group to believe they were receiving true acupuncture at 6 weeks (68% vs 36%, P<.001) and at 12 weeks (70% vs 43%, P = .009). However, there was no evidence that the intervention effect with respect to the primary outcome of worst pain differed between those believing vs not believing they were receiving true acupuncture at either 6 weeks (P for interaction = .16) or 12 weeks (P for interaction = .75).
Category indicates the primary end point.
Secondary Outcomes
Patients randomized to the true acupuncture group had statistically significant improved symptom scores compared with those randomized to the sham acupuncture and waitlist control groups at 6 weeks according to BPI average pain, pain severity, and worst stiffness (Table 2). Patients randomized to true acupuncture had improved symptoms at 6 weeks compared with those in the sham acupuncture group, but not compared with the waitlist control group according to pain interference. At 12 weeks, patients randomized to the true acupuncture group compared with the sham acupuncture group had statistically significant improvements in average pain, but no significant improvement in worst pain, pain interference, pain severity, or worst stiffness (Table 2). Compared with the waitlist control group, patients randomized to the true acupuncture group had improved pain by all BPI measures at week 12 (P≤.003).
Patients randomized to the true acupuncture group had improved symptoms compared with those in the sham acupuncture group at 6 weeks according to the M-SACRAH, WOMAC, and PROMIS PI-SF measures (eTable 3 in Supplement 2). Patients randomized to the true acupuncture group had improved symptoms compared with those in the waitlist control group at 6 weeks according to the M-SACRAH and WOMAC measures. At week 12, patients randomized to the true acupuncture group had improved symptoms compared with those in the sham acupuncutre group according to the WOMAC and PROMIS PI-SF measures, and they had improved symptoms compared with those in the waitlist control group according to the M-SACRAH, WOMAC, FACT-ES, and PROMIS PI-SF measures.
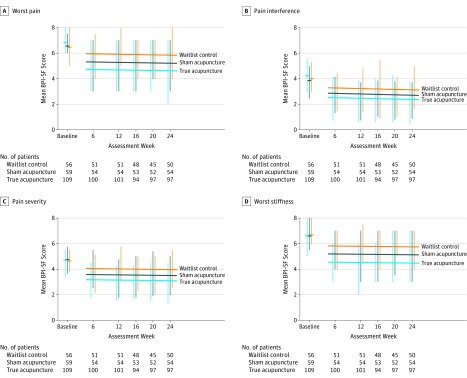
In linear mixed-model analysis, there was no significant interaction between intervention assignment and time, and there was no significant quadratic relationship of BPI-WP over time (eTable 4 in Supplement 2). Through 24 weeks, adjusted mean BPI-WP scores were 0.59 points lower (95% CI, 0.34-1.14; P = .04) in the true acupuncture group compared with the sham acupuncture group and were 1.23 points lower (95% CI, 0.66-1.80; P<.001) in the true acupuncture group compared with the waitlist control group (Figure 2). Similar findings for trends are shown for the secondary measures (eFigure 1 in Supplement 2).
Figure 2. Linear Mixed-Model Results Through 24 Weeks for Selected Brief Pain Inventory-Short Form End Points.
Horizontal bars at baseline indicate the observed baseline means. Vertical bars indicate the interquartile range (IQR) for each study group at each assessment time and are offset by a small margin to show the IQR for each group. Fitted regression lines for each group are also shown, with P values for the effect of intervention on Brief Pain Inventory-Short Form outcomes derived from multivariable linear mixed-model analyses with a random effect for patient, adjusting for continuous time, the baseline score, and indicator variables for study sites, and report values for comparisons between true acupuncture vs sham acupuncture and true acupuncture vs waitlist control for week 6, 12, 16, 20, and 24 assessment times.
A, P = .04 (vs sham acupuncture); P<.001 (vs waitlist control).
B, P = .18 (vs sham acupuncture); P = .003 (vs waitlist control).
C, P = .08 (vs sham acupuncture); P = .003 (vs waitlist control).
D, P = .02 (vs sham acupuncture); P<.001 (vs waitlist control).
Patients randomized to the true acupuncture group were more likely to believe they were receiving true acupuncture than those receiving sham acupuncture (68% vs 36%; P<.001). However, the intervention effect for BPI-WP did not differ between those believing vs not believing they were receiving true acupuncture when interaction tests were used (P for interaction = .16).
Post Hoc Analysis
The proportion of patients experiencing at least a 2-point reduction in BPI-WP at 6 weeks was 58.0% (n = 58) for the true acupuncture group, 33.3% (n = 18) for the sham acupuncture group (RD, 24.7% [95% CI, 8.8%-40.5%]; RR, 1.64 [95% CI, 1.10-2.44]; P = .02), and 31.4% (n = 16) for the waitlist control group (difference for true acupuncture vs waitlist control, 26.6% [95% CI, [10.6%-42.6%]; RR, 1.75 [95% CI, 1.13-2.69]; P = .01; eFigure 2 and eTable 2 in Supplement 2) Similar results by treatment group were evident for the proportion of patients experiencing an improvement of greater than 30% (Figure 2; eTable 2 and eTable 3 in Supplement 2).
Adverse Events
Bruising was the most common adverse event reported for those receiving true acupuncture or sham acupuncture. More patients in the true acupuncture group experienced grade 1 bruising (47%) than in the sham acupuncture group (25%; P = .01) (eTable 1 in Supplement 2). There was 1 episode of grade 2 presyncope in the true acupuncture group and 1 episode in the sham acupuncture group. No other differences by study group for selected adverse events were observed, and no grade 3 or higher adverse events were reported.
Discussion
In this multicenter, sham- and waitlist-controlled clinical trial of patients with early-stage breast cancer and aromatase inhibitor–related joint pain, there were statistically significant but modest improvements in pain scores with true acupuncture administered twice a week for 6 weeks compared with both sham acupuncture and waitlist control. However, the magnitude of the effect did not achieve the prespecified between-group difference of 2 points in the primary end point of BPI-WP, which had been considered to represent a clinically meaningful difference by the design.
The adjusted mean between-group difference at 6 weeks was 0.92 points for true acupuncture vs sham acupuncture and 0.96 points for true acupuncture vs waitlist control. Although there is some uncertainty about the clinical meaning of this between-group difference,27,28 the observed difference in this study is consistent with other randomized studies of pain control interventions, which have reported between-group mean differences ranging from 0.7 to 1.0 points.31,32 The pain-reporting guidelines describe the clinical meaning of an individual’s response, with studies suggesting that a reduction of 2 points on an 11-point scale (or 30%) represents a clinically important reduction (ie, much improved).21,28 A post hoc hypothesis-generating, responder analysis suggested that the proportion of patients with a 2-point improvement at 6 weeks in the true acupuncture group (58%) was greater than that in the sham acupuncture group (33%) and in the waitlist control group (31%).
In this study, the addition of maintenance true acupuncture once a week for an additional 6 weeks, compared with sham acupuncture, was associated with statistically significant improvements in average pain, but no significant improvement in worst pain, pain interference, pain severity, or worst stiffness at 12 weeks. Compared with waitlist control, additional maintenance true acupuncture was associated with significant improvements in worst pain, average pain, pain interference, pain severity and worst stiffness at 12 weeks.
Both pharmacological and nonpharmacological interventions have been studied to treat aromatase inhibitor–associated arthralgias.19,20,33,34 Although some interventions have shown a benefit from the intervention, most have been difficult to interpret due to methodological limitations. Placebo-controlled trials of pharmacological interventions, such as vitamin D, omega-3-fatty acids, and duloxetine, have consistently shown placebo effects of as much as 50%; however, only duloxetine was found to show a statistically significant between-group difference.19,33,34 Trials of unblinded, modality-based therapies, such as exercise, have shown benefit; however, no placebo effect was observed in the control group,20 raising questions about response bias.35 The lack of a placebo effect in prior acupuncture trials raised concerns about the integrity of the sham blinding, unintentional crossover in the sham treatment groups, or a possibility of a physiological effect of the sham. The current trial was designed with a waitlist control group to address prior concerns about the sham effects.
The 4 prior studies of acupuncture for aromatase inhibitor arthralgias did not evaluate maintenance or durability of effect.10,11,36,37 Most studies of acupuncture in patients with cancer have evaluated the benefits of true acupuncture among women with hot flashes, and several evaluated persistent effects following completion of the intervention, suggesting that the effects may be long lasting. For example, in a randomized unblinded trial of weekly acupuncture for 12 weeks, the intervention was associated with a significantly lower hot flash score at 3 months and also at 6 months following completion of the acupuncture intervention.38 Acupuncture is appealing to some patients because the adverse effects are generally limited compared with medications used to control pain, such as opiates or duloxetine. However, despite the benefits and limited toxicities, acupuncture is not covered by many insurance plans.
This study has several strengths. The intervention was conducted at multiple sites by acupuncturists who underwent rigorous standardized training and administered the repeat assessments throughout the time frame of the study. Acupuncturists had diverse training backgrounds, and patients were treated at both academic and community sites throughout the United States, both of which increase the generalizability of the findings. The retention and completion rates were similar across groups, reducing the concern about differential dropout.
Limitations
This study also has several limitations. First, blinding was not possible for patients randomized to the waitlist control group. Second, a significantly higher proportion of patients in the true acupuncture group compared with the sham acupuncture group believed they were receiving true acupuncture, suggesting the possibility of lack of successful masking of this group. Third, primary outcomes were based on relatively short-term measurements, and long-term follow-up beyond 12 months was not available. Fourth, the study design did not incorporate the pain literature regarding a comparison between groups of a clinically meaningful reduction for an individual, and therefore, these results are post hoc. Fifth, there was no correction for multiple comparisons for the secondary analyses, and therefore, these findings should be considered exploratory. Sixth, accrual was slower than expected due to acupuncture staff turnover and retraining, expansion of study sites, scheduling difficulties, and the widespread availability of acupuncture, limiting patients’ enthusiasm for entering a randomized trial. Seventh, uptake in the community may be limited by the expense of the intervention, logistical challenges, and uncertain experience of the acupuncturist with the study methodology.
Conclusion
Among postmenopausal women with early-stage breast cancer and aromatase inhibitor–related arthralgias, true acupuncture compared with sham acupuncture or with waitlist control, resulted in a statistically significant reduction in joint pain at 6 weeks, although the observed improvement was of uncertain clinical importance.
Trial Protocol
eFigure 1. Linear Mixed-Model Results Through 24 Weeks for the Additional Secondary Endpoints
eFigure 2. Percent With At Least a 2-Point Change on the Brief Pain Inventory Worst Pain Score
eTable 1. Number of Patients With a Given Type and Grade of Adverse Event
eTable 2. Differences in Proportions With >30% Improvement for Brief Pain Inventory Short Form Scores at Weeks 6 and 12 in Each Group
eTable 3. Observed and Fitted Group Mean Results and Differences in Proportions With >30% Improvement for M-SACRAH, WOMAC, FACT-ES, at Weeks 6 and 12 in Each Group
eTable 4. Linear Mixed-Model Results
eTable 5. Participating Study Sites
References
- 1.Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol. 2016;34(14):1689-1701. [DOI] [PubMed] [Google Scholar]
- 2.Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25(25):3877-3883. [DOI] [PubMed] [Google Scholar]
- 3.Castel LD, Hartmann KE, Mayer IA, et al. Time course of arthralgia among women initiating aromatase inhibitor therapy and a postmenopausal comparison group in a prospective cohort. Cancer. 2013;119(13):2375-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30(9):936-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goss PE, Ingle JN, Pritchard KI, et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med. 2016;375(3):209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. Treatment adherence and its impact on disease-free survival in the breast international group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol. 2016;34(21):2452-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms J. Acupuncture Energetics: A Clinical Approach for Physicians. Berkeley, CA: Medical Acupuncture Publishers; 1995. [Google Scholar]
- 9.Tedesco D, Gori D, Desai KR, et al. Drug-free interventions to reduce pain or opioid consumption after total knee arthroplasty. JAMA Surg. 2017;152(10):e172872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crew KD, Capodice JL, Greenlee H, et al. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol. 2010;28(7):1154-1160. [DOI] [PubMed] [Google Scholar]
- 11.Mao JJ, Xie SX, Farrar JT, et al. A randomised trial of electro-acupuncture for arthralgia related to aromatase inhibitor use. Eur J Cancer. 2014;50(2):267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103-115. [PubMed] [Google Scholar]
- 13.MacPherson H, Altman DG, Hammerschlag R, et al. ; STRICTA Revision Group . Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. J Evid Based Med. 2010;3(3):140-155. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health Racial and ethnic categories and definitions for NIH diversity programs and for other reporting purposes. Notice number NOT-OD-15-089. https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html. April 8, 2015. Accessed June 21, 2018.
- 15.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655. [PubMed] [Google Scholar]
- 16.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17(2):197-210. [DOI] [PubMed] [Google Scholar]
- 17.Greenlee H, Crew KD, Capodice J, et al. Methods to standardize a multicenter acupuncture trial protocol to reduce aromatase inhibitor-related joint symptoms in breast cancer patients. J Acupunct Meridian Stud. 2015;8(3):152-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui KK, Nixon EE, Vangel MG, et al. Characterization of the “deqi” response in acupuncture. BMC Complement Altern Med. 2007;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershman DL, Unger JM, Crew KD, et al. Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. J Clin Oncol. 2015;33(17):1910-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin ML, Cartmel B, Gross CP, et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol. 2015;33(10):1104-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158. [DOI] [PubMed] [Google Scholar]
- 22.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840. [PubMed] [Google Scholar]
- 23.Sautner J, Andel I, Rintelen B, Leeb BF. Development of the M-SACRAH, a modified, shortened version of SACRAH (Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands). Rheumatology (Oxford). 2004;43(11):1409-1413. [DOI] [PubMed] [Google Scholar]
- 24.Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer. Breast Cancer Res Treat. 1999;55(2):189-199. [DOI] [PubMed] [Google Scholar]
- 25.Garcia SF, Cella D, Clauser SB, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials. J Clin Oncol. 2007;25(32):5106-5112. [DOI] [PubMed] [Google Scholar]
- 26.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963-974. [PubMed] [Google Scholar]
- 27.Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146(3):238-244. [DOI] [PubMed] [Google Scholar]
- 28.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. [DOI] [PubMed] [Google Scholar]
- 29.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. [DOI] [PubMed] [Google Scholar]
- 30.SAS Institute Inc SAS 9.4 System Options. 5th ed Cary, NC: SAS Institute Inc; 2016. [Google Scholar]
- 31.Smith EM, Pang H, Cirrincione C, et al. ; Alliance for Clinical Trials in Oncology . Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy. JAMA. 2013;309(13):1359-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroenke K, Theobald D, Wu J, et al. Effect of telecare management on pain and depression in patients with cancer. JAMA. 2010;304(2):163-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro AC, Adlis SA, Robien K, et al. Randomized, blinded trial of vitamin D3 for treating aromatase inhibitor-associated musculoskeletal symptoms (AIMSS). Breast Cancer Res Treat. 2016;155(3):501-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henry NL, Unger JM, Schott AF, et al. Randomized, multicenter, placebo-controlled clinical trial of duloxetine versus placebo for aromatase inhibitor-associated arthralgias in early-stage breast cancer: SWOG S1202. J Clin Oncol. 2018;36(4):326-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henry NL, Griggs JJ. The power of the placebo in symptom management. J Clin Oncol. 2015;33(17):1870-1872. [DOI] [PubMed] [Google Scholar]
- 36.Bao T, Cai L, Giles JT, et al. A dual-center randomized controlled double blind trial assessing the effect of acupuncture in reducing musculoskeletal symptoms in breast cancer patients taking aromatase inhibitors. Breast Cancer Res Treat. 2013;138(1):167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh B, Kimble B, Costa DS, et al. Acupuncture for treatment of arthralgia secondary to aromatase inhibitor therapy in women with early breast cancer. Acupunct Med. 2013;31(3):264-271. [DOI] [PubMed] [Google Scholar]
- 38.Lesi G, Razzini G, Musti MA, et al. Acupuncture as an integrative approach for the treatment of hot flashes in women with breast cancer. J Clin Oncol. 2016;34(15):1795-1802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Linear Mixed-Model Results Through 24 Weeks for the Additional Secondary Endpoints
eFigure 2. Percent With At Least a 2-Point Change on the Brief Pain Inventory Worst Pain Score
eTable 1. Number of Patients With a Given Type and Grade of Adverse Event
eTable 2. Differences in Proportions With >30% Improvement for Brief Pain Inventory Short Form Scores at Weeks 6 and 12 in Each Group
eTable 3. Observed and Fitted Group Mean Results and Differences in Proportions With >30% Improvement for M-SACRAH, WOMAC, FACT-ES, at Weeks 6 and 12 in Each Group
eTable 4. Linear Mixed-Model Results
eTable 5. Participating Study Sites