Abstract
Objective
The correct management of multiple‐ligament injured knees (MLIKs) remains controversial. This study aimed to summarize the epidemiological features and short‐term results of patients treated in our department.
Methods
Sixty‐six patients diagnosed with MLIKs from 2009 to 2011 were enrolled. Relevant patient characteristics and clinical variables were analyzed to characterize the epidemiology. A surgical algorithm based on a knee dislocation classification system and postoperative rating scales, including Lysholm and Tegner rating, as well as joint mobility, stability and radiography were collected for functional evaluation at 2.5‐year follow‐up.
Results
The epidemiological profile demonstrated that 30‐ to 50‐year‐old men were at the highest risk. The primary causes were vehicle accidents and falls and most common injury type cruciate combined collateral ligament injuries. Final follow‐up analysis comparing operative versus conservative management and surgically treated mild versus severe MLIKs showed significant differences in Lysholm and Tegner scale scores, as well as knee mobility and stability.
Conclusion
The therapeutic outcome of MLIKs depends on various clinical variables and a surgical algorithm. Satisfactory restoration of function was acquired in the majority of our surgically treated MLIK cases; however, most patients had not achieved their pre‐injury activity levels by the follow‐up endpoint.
Keywords: Follow‐up, Knee, Ligament injury, Reconstruction
Introduction
Multiple‐ligament injured knees (MLIKs), defined as injuries involving at least two of the main ligaments of the knee and associated with possible capsule, meniscus, cartilage and osseous compromise1, usually occur after dislocation and severe subluxation of this joint. Although previous studies documented that MLIKs were quite rare and often high‐energy injuries with high incidences of neurological and vascular crises2, the incidence of MLIK has risen exponentially and its manifestations have become increasingly complicated. We believe that these changes are attributable to the following factors: an increase in high‐intensity athletics; the increasing mean body weight of the population; more high‐energy injuries; more complicated patterns of vehicle accidents; more sensitive diagnostic techniques; and greater insights into the recognition of knee injuries.
As a result, a significant number of MLIKs injuries occur and are diagnosed, many of which require professional treatment. However, outcomes differ between various diverse treatments. Annual official statistics showed that a total of 3.9 million vehicle accidents resulted in 254,075 people being injured in China in 2010. Approximately 8% of the patients had knee trauma among their injuries3. However, 30%–50% of multi‐ligament injuries went unrecognized during primary treatment. Serious sequelae, such as intractable pain, instability, deformity and stiffness, will predictably present over the coming decades.
Surgical reconditioning of MLIKs remains a challenge for most orthopaedic surgeons because of the complicated ligamentous, meniscal, cartilaginous, osseous and neurovascular structures surrounding the knee4. Because MLIKs primarily consist of complex ruptures of the anterior/posterior cruciate ligaments (ACL/PCL), combined with damage to the extra‐capsular ligaments (medial/lateral collateral ligaments, MCL/LCL), treatment aims to recondition these ligaments in a multidirectional and multilayered environment (Fig. 1). Additionally, different patients have varying subjective goals (depending on personal preferences, physical activity needs, cost, and so on) according to their occupations and life circumstances. Moreover, different decisions with regard to surgical timing, therapeutic algorithms, operative plans and postoperative rehabilitation have great impacts on functional recovery. Therefore, restoration of the knee complex both anatomically and functionally is both a medical and a social issue.
Figure 1.

Schematic outlines of (A, B) lateral and medial layered structures of the knee and (C) thee general principles for ligamentous repair. ①, patellar tendon; ②, iliotibial tract; ③, popliteal tendon; ④, LCL; ⑤, biceps femoris tendon; ⑥, ACL; ⑦, superficial MCL; ⑧, deep MCL; ⑨, pes anserinus.
A series of invited clinical commentaries on the topic of management of multiple‐ligament injured (dislocated) knees was published in Operative Techniques in Sports Medicine in 20105, 6, 7, 8, 9; these commentaries presented the points of view of teachers and sports medicine surgeons on the rationale and treatment of MLIKs. Although many issues, including general principles, indications, surgical techniques, rehabilitation, and complications were discussed, no consensus was reached on several key points. For example, previous reports have stated different optimal times between injury and surgery, including 7–10 days, 1–2 weeks, and 2–3 weeks4, 5, 10. A therapeutic level III study suggested that acute surgery is strongly associated with flexion deficits, whereas delayed reconstruction potentially yields equivalent outcomes in terms of stability11. In addition, Merritt et al. advocated an “all‐or‐none” approach to addressing all injuries with a single‐stage approach7, 8, whereas Vyas and Shelbourne et al. repaired/reconstructed the collateral injuries immediately and delayed cruciate reconstruction. Furthermore, reviews of published reports have found that multi‐stage treatments yield the highest percentage of excellent and good subjective outcomes4, 11, 12. The controversies surrounding these topics can confuse and even mislead surgeons participating in surgical training programs.
The aim of the present study was to review our MLIK cases over the past two years and to summarize the epidemiological profiles, peri‐operative management, short‐term results and surgical techniques of the enrolled patients. We postulated that an individualized, and staged surgical algorithm for optimum management of MLIK could be constructed based on local epidemiological characteristics. Accordingly, we designed a retrospective comparative study to evaluate this possibility.
Materials and Methods
Patients and Evaluated Variables
A retrospective review of our patient database revealed that 72 patients diagnosed with MLIKs were evaluated in our department between March 2009 and March 2011. Thirteen of these patients were excluded because of conflicting variables that affected their treatment. The reasons for exclusion included open trauma, neurovascular emergencies, compartment syndrome, any associated fractures requiring external fixators and others. Using a standardized protocol to extract the required information from the medical records, we evaluated relevant patient characteristics (sex, age, and affected half of the body) and clinical variables (mechanism, affected ligaments, classification, and time since injury).
Initial Evaluation
In general, our patients with high‐energy injuries were referred via the emergency departments, whereas the low‐energy injury patients came through the sports medicine clinic as outpatients. In emergency patients, after life‐threatening injuries (such as intracranial hemorrhage, hemopneumothorax, spine and pelvic injuries), the next most critical condition was knee dislocation/luxation. Consultations were performed to establish the mechanism of trauma and determine whether any other injuries were present. A medical history was followed by a rapid neurovascular examination, including distal pulses, skin temperature, color and a basic sensorimotor evaluation. A normal pulse does not rule out vascular injury because of establishment of a collateral circulation and the low sensitivity of pulse examinations (<80%)13. Instead, an effective index is the ankle‐brachial index (ABI). An initial ABI of less than 0.9 required an immediate arteriogram.
A thorough ligamentous examination was performed once any neurovascular emergencies had been addressed. The ACL was tested with Lachman and pivot shift tests and the PCL evaluated with the posterior drawer test and posterior sag sign. Varus and valgus stability was tested with the knee in full extension and in 30° of flexion. The posterolateral corner (PLC) was evaluated with the dial test, with the evaluator looking for asymmetric external rotation of 10° or more in 30° of flexion. The patellar tendon (PT) was examined for tendinous discontinuity and reduced active extension of the knee.
Once the knee had been reduced under epidural anesthesia, definitive radiographic assessments, such as valgus/varus stress, axial pulling‐out, and anterior/posterior translation, were obtained. Fluoroscopy included anteroposterior, lateral, and merchant views (Fig. 2). Any peri‐ and intra‐articular fractures were addressed first. Small avulsion fragments from the fibular head, internal/external condyle and tibial spine were precisely characterized by computed tomography (CT). Further magnetic resonance imaging (MRI) was recommended to detail the patterns of ligamentous injuries and to identify and evaluate other peri‐articular injuries.
Figure 2.
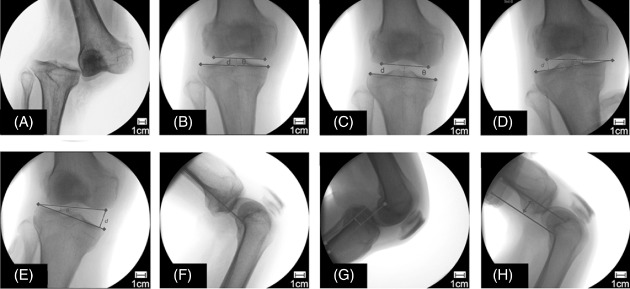
Preoperative evaluation of the ligamentous laxity under different stress conditions. (A) Radiological appearance of a dislocated knee in the emergency room. (B) The reduced knee showing a normal articular match (distance of the joint gap [d] = 7.5 mm, angle of the articular surface [θ] = 0°). (C) An axial pulling view showing equally widened medial and lateral joint spaces (d = 13.8 mm, θ = 0°). (D) A varus stress radiograph showing a Grade II injury of the LCL (d = 13.2 mm, θ = 8.5°). (E) A valgus stress radiograph showing a Grade III injury of the MCL (d = 21.3 mm, θ = 14.2°). (F) A lateral radiograph of the knee maintained in the neutral position. (G) Posterior movement of 8.8 mm of the tibia to the femur, indicating a torn PCL. (H) Anterior movement of 19.2 mm of the tibia on the femur, indicating a torn ACL.
General Surgical Management
Our recommended time frame for surgery was during the 1–2 weeks after the injury for acute cases: this allowed for the resolution of acute inflammation and soft tissue swelling and for a partial return of range of motion (ROM). For chronic cases, it was sometimes prudent to wait until the full ROM was established. Surgery was generally performed under spinal/epidural anesthesia; however, general anesthesia with an endotracheal tube was adopted for severe injuries. The patient was placed in the supine position and administered i.v. antibiotics before incision. A tourniquet (55 kPa in pressure, time of 90 min) was used to guarantee a normal ABI and arterial duplex. A full examination and fluoroscopy were repeated to confirm the injuries and to make any necessary modifications to the surgical plan.
A standard arthroscopic examination was performed using low anterolateral and high anteromedial portals. Potential injuries to the cartilage and menisci were routinely addressed and direct repair procedures for ligamentous injuries were implemented as follows (Fig. 1C). Bony avulsed ligaments, biceps femoral tendons, and the PT were anatomically reduced and fixed in situ with wire, screws or anchors. All mid‐substance lesions of the ligaments were repaired with nonabsorbable sutures (Ethicon, Cincinnati, OH, USA). If avulsed fibers comprised more than two‐thirds of the femoral or tibial insertion, the cruciate ligament was reconstructed. A specific description of the reconstruction of the ligaments is presented in the Results section below.
Details of Surgical Management and Technique
Management of Cruciate Ligament Injuries
In patients with bicruciate injuries with intact collateral ligaments, reconstruction began with drilling of transosseous tunnels: first the PCL tunnel, followed by the ACL tunnel. The guide arms were set at 50°–55° and 45°–50° for PCL and ACL reconstruction, respectively. A guidewire was started at the medial tibial cortex 4 cm distal to the joint line and advanced until it perforated the posterior cortex at the site of the PCL footprint (approximately 1 cm below the tibial plateau). For the ACL, it was drilled, exiting through the center of the ACL footprint (the posterior extent of the anterior horn of the lateral meniscus). On the femoral side, the guidewire was placed in the center of the anatomic location of the ACL footprint (i.e., 6 mm anterior to the posterior cortex of the femur at the 10 o'clock position) and the PCL footprint (7–10 mm from the articular surface). The lengths of the bone tunnels were 3–5 cm for the tibia and 5–7 cm for the femur. The graft passages (Achilles tendon for the PCL, 9–10 mm in diameter; tibialis anterior for the ACL, 7–8 mm in diameter) were fixed with an Endobutton system (Smith & Nephew, London, UK) on the femoral epicondyles and with BioRCI‐HA screws (Smith & Nephew) on the anteromedial tibia (Fig. 3).
Figure 3.
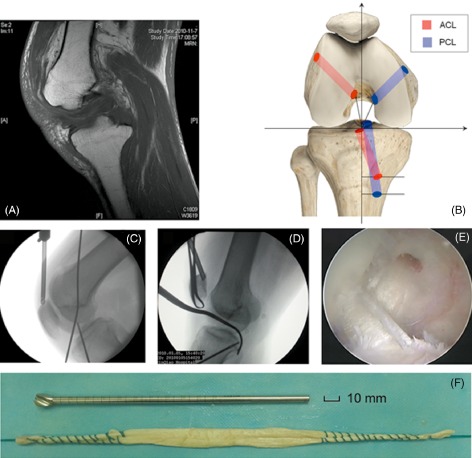
Reconstruction of bicruciate injury. (A) MRI showing discontinuity and disappearance of bicruciate ligaments. (B) Schematic representation of transosseous tunnels (anteroposterior view). (C) and (D) Intraoperative determination of transosseous tunnels for ACL and PCL by fluoroscopy (lateral views). (E) Arthroscopic view of bicruciate ligament reconstruction. (F) Preparation of a tibialis anterior allograft.
Management of Medial Structure Injuries
For cruciate combined MCL injuries, operative management was begun by reconstructing the central pivot. The repair/reconstruction approach employed a medial longitudinal incision and reconditioning of avulsion injuries of the MCL and capsule with suture anchors (Twinfix, Smith & Nephew) or repair of intra‐substance MCL tears with nonabsorbable sutures (Ethicon). However, for severe ruptures of both the superficial and deep MCL and the capsule, the anatomic structure was usually reconstructed with a “two‐tailed” technique, which consisted of one femoral and two tibial tunnels. The former was located at the attachment of the medial epicondyle, whereas the proximal and distal tibial attachments were situated at the anterior arm of the semimembranosus tendon and at the footprint at 1 cm anterior to the posteromedial crest of the tibia (an average of 6 cm distal to the joint line), respectively. A doubled‐over tibialis anterior tendon was fixed in the lateral condyle, and the two ends were embedded into the tibia under tension with BioRCI‐HA screws in an inverted “V” shape (Fig. 4).
Figure 4.
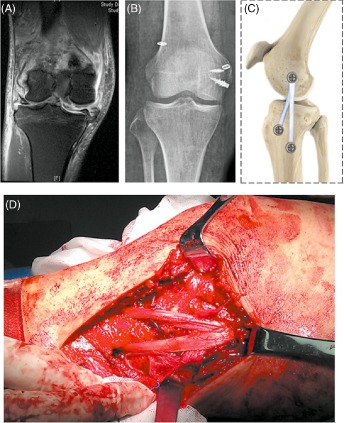
Surgical management of combined MCL injury. (A) MRI showing a proximal tear of the MCL from the medial epicondyle. (B) Postoperative fluoroscopy showing MCL repair and bicruciate reconstruction. (C) Schematic representation of a reconstructed MCL and its anatomical attachments. (D) Intraoperative reconstruction of the MCL via a medial approach.
Management of Lateral Structure Injuries
In cases of cruciate combined lateral side injuries, fibular‐based reconstruction was always performed acutely for the lateral structures (LCL, PLC, popliteus tendon, popliteofibular ligament), followed by delayed central pivot reconstruction. This approach required a lateral hockey stick incision centered between the Gerdy tubercle and the fibular head and extending proximally between the iliotibial band and the biceps femoris. The iliotibial tract, biceps femoris tendon, common peroneal nerve and popliteus tendon were identified. For LCL reconstruction, the approach consisted of a tunnel (6–7 mm in diameter) drilled through the fibular head on the sagittal plane and a transverse coronal tunnel into the lateral femur at the LCL attachment (7–8 mm in diameter). For PLC reconstruction, the “popliteus bypass” technique consisted of two transosseous tunnels at the femoral attachments of the LCL and popliteus. A tibialis anterior allograft was then passed through the fibular tunnel and embedded crosswise into the femoral tunnels with BioRCI‐HA cannulated screws (Fig. 5).
Figure 5.
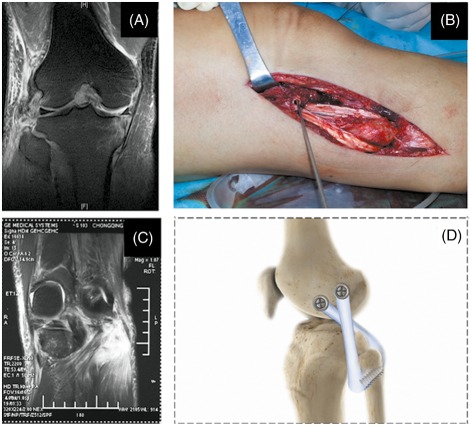
Surgical management of lateral side injury. (A) MRI showing complete avulsion of the LCL from the fibular head (Grade III). (B) LCL reconstruction via a fibular‐based approach. (C) MRI showing a Grade II popliteal tendon injury. (D) Schematic representation of PLC reconstruction technique with oblique transfibular and axial transcondylar tunnels using a “popliteal bypass” technique.
Management of Anterior Structure Injuries
For combined PCL‐PT cases, after PLC reconstruction the procedure was begun by drilling a cancellous bone tunnel into the patella (lower one‐third part of the patella, with a diameter of 7–8 mm) and a tibial tunnel (approximately 1 cm beneath the tibia tubercle, with a diameter of 4–5 mm). An anterior tibia graft passage was passed through the tibial tunnel without fixation and fixed to the tails with 8 mm BioRCI‐HA screws in a face‐to‐face manner within the patella (Fig. 6). The allograft was reinforced by securing it to the native tendon remnant.
Figure 6.
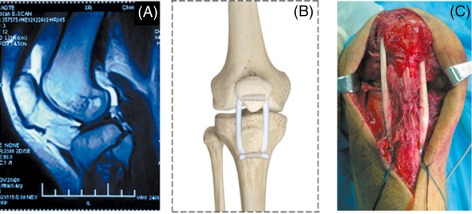
Surgical management of a cruciate combined PT injury. (A) MRI showing an associated PT rupture. (B) Schematic representation of the reconstructed PT and its attachments. (C) Intraoperative reconstruction of the PT via a medial approach.
Management of Total Injuries
For totally injured knees with ACL + PCL + MCL + LCL/PLC injuries, early surgical intervention was indicated to repair and reconstruct the MCL, LCL, and PLC, as has been described in detail above. Bicruciate reconstruction was delayed until the patient had recovered his or her ROM and strength.
Postoperative Treatment and Rehabilitation
Antibiotics were re‐administered during surgery if indicated. All patients received appropriate deep venous thrombosis (DVT) chemoprophylaxis (enoxaparin/rivaroxaban) and pain control (celecoxib/etoricoxib). A standard rehabilitation program, emphasizing early protected ROM, was launched on the first postoperative day. Briefly, the injured knee was immobilized in a hinged brace for 2–4 weeks according to the severity of the combined injuries, accompanied by standard physiotherapy and isometric quadriceps exercises. The patients were advised to remain non‐weight‐bearing for 4 weeks and partial‐weight‐bearing for an additional 2 weeks. Progression to full‐weight‐bearing out of the brace began at 6 weeks.
Follow‐up Evaluations
In all, 53 of 59 patients were followed up for a mean of 2.5 years (range, 1.75–3.75 years). A two‐part protocol was designed for short‐term evaluation. First, subjective assessment was performed by the patients using the Lysholm score system (95–100 graded as excellent, 84–94 as good, 65–83 as fair and <64 as poor) and the Tegner rating system. Second, postoperative complications, including DVTs, infections, compartment syndrome, common peroneal nerve palsy and arterial injuries, were noted in comments with regard to early results. Objective evidence in the form of ROM compared with the uninjured side, multidirectional ligament laxity and quantitative radiography were summarized to supplement the subjective assessments.
Statistical Analysis
All the data are presented as mean ± SD. The results were analyzed by Student's t‐test and the X2 test using SPSS software, version 10.0. Statistical significance was defined as P < 0.05 for 95% confidence.
Results
Epidemiological Profiles
After exclusion of the 13 patients described above, the remaining 59 cases comprised 50 men and 9 women. The mean age at the time of the surgery was 43.7 years (range, 21–63 years), and the female patients were younger (mean, 41.6 years) than the male patients (mean, 44.1 years, P < 0.05, Fig. 7A). The ligament injuries were in the right knee in 31 patients and in the left knee in 28.
Figure 7.
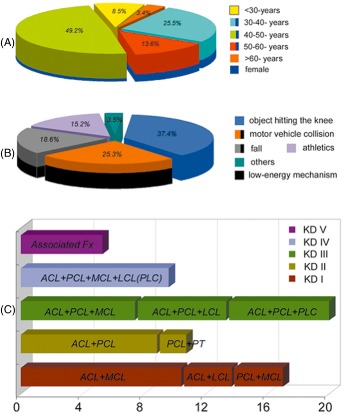
Graphs showing relevant patient characteristics and clinical variables for patients with MLIKs. (A) Patient age distribution. (B) The mechanism of injury for the MLIK population. (C) Quantitative MLIK classification according to a modified Schenk system. Knee dislocation (KD) I, single cruciate combined with either MCL or LCL injury; KD II, injury with anterior‐posterior instability; KD III, bicruciate and either MCL or LCL/PLC injury; KD IV, total bicruciate injury on both the medial and lateral sides; KD V, MLIK associated with periarticular fracture (Fx).
Regarding the traumatic energy spectrum, our database revealed that 74.6% of injuries involved high‐energy and 25.4% low‐energy mechanisms. The top three mechanisms of injury were object directly hitting the knee, motor vehicle collisions and falls from heights (Fig. 7B). Forty‐eight patients underwent acute reconstructions (<3 weeks; mean time 7.3 days, range 1–13 days) and 11 delayed reconstructions (>3 weeks; mean time 3.8 months, range 1–9 months).
We classified various combinations of MLIKs into 11 categories (Fig. 7C). Each injury type, which was based on anatomical lesion, was categorized from type I to V using a modified Schenk classification system. The most common injury types were single cruciate combined medial ligament injuries and bicruciate combined lateral side injuries. As for the most vulnerable ligament, the ACL ranked first (31%), followed by the MCL (29%), PCL (22%) and LCL (16%), in that order.
Follow‐up Evaluations
Lysholm Evaluation
The overall mean postoperative Lysholm score was 86.8 ± 11.4, compared to a mean preoperative Lysholm score of 49.3 ± 6.9 (P < 0.01). The general grading results were 77% good and 23% fair. The mean scores for patients with acute and chronic lesions were 87.6 ± 10.2 and 80.5 ± 13.3, respectively (P = 0.31). The Lysholm score in our five knee dislocation (KD)‐classified groups are listed in Table 1. Regarding injury type, the ACL + MCL, PCL + MCL, and PCL + PT groups (KD I‐II classifications) had the highest Lysholm scores (P < 0.05).
Table 1.
Subjective and objective evaluation of knee function at final follow‐up
| Groups | Lysholm score | Tegner score | δ ROM (°) | Ligament laxity | ||
|---|---|---|---|---|---|---|
| Extension | Flexion | Posterior | Lateral | |||
| KD I | 90.3 ± 9.7 | 6.0 ± 2.4 | 2.0 ± 0.3 | 3.5 ± 0.7 * | — | — |
| KD II | 86.4 ± 12.2 | 5.2 ± 2.1 | 2.8 ± 0.4 | 4.9 ± 0.5 * | — | — |
| KD III | 83.9 ± 10.5 | 4.4 ± 1.8 | 4.6 ± 1.2 | 7.2 ± 2.0 | 1 | 1 |
| KD IV | 72.7 ± 15.9 | 3.5 ± 2.5 | 15.5 ± 6.1 * | 17.1 ± 8.8 | 2 | 1 |
| KD V | 80.6 ± 12.8 | 4.0 ± 3.1 | 7.2 ± 1.8 * | 9.9 ± 3.6 | — | — |
| Average | 86.8 ± 11.4 | 5.3 ± 1.9 | 3.0 ± 0.7 | 7.6 ± 1.2 | — | — |
The mean value and SE of assessments of patients are shown.
*P < 0.05 as compared with mean findings.
Tegner Rating
Pre‐ and post‐operative Tegner scores were employed to indicate patients’ capability of returning to their pre‐injury lives/work and activity levels (Table 1). The overall average Tegner activity score was significantly less at the preoperative evaluation than before the injury (0.9 ± 2.3 vs. 6.2 ± 2.5; P < 0.01). The median Tegner score at the final follow‐up was 5.3. This change from the preoperative status reached high significance (P < 0.001).
Range of Motion
At the final post‐operative follow‐up, 42 patients (71%) were rated as normal, with extension ranges within 3° of the normal side and flexion ranges within 5° of the normal side. Fourteen (24%) patients with 3°–5° extension deficits or 6°–15° flexion deficits were rated as nearly normal. Three (5%) patients had extension deficits greater than 5° or flexion deficits greater than 15° and were rated as abnormal. The specific data on ROM differences (δ ROM, compared with the uninjured knees) in our five KD groups are also listed in Table 1. In terms of injury type, the involved injury types, according to both the KD I and KD II classifications, showed equal performances for joint ROM (2°–3° in extension and 110°–120° in flexion, P < 0.05).
Ligament Laxity
Pre‐ and post‐operative evaluations of joint stability were conducted in both the antero‐posterior and medial‐lateral directions. Positive results were recorded as significant anterior‐posterior movement (greater than 5 mm of movement) in drawer tests or a visible valgus/varus deformity (greater than 5°) in collateral stress tests. At the final follow‐up, two patients (3.4%) had visible posterior laxity, and three (5.1%) significant lateral laxity (Table 1). No anterior or medial instability was observed during the follow‐up period. The ACL + PCL + MCL + LCL (PLC) injury type was predisposed to postoperative laxity.
Radiographic Findings
Joint space radiographic findings were normal in 34 patients (58%). Sixteen patients (27%) had minimal radiological degradation characterized by slightly decreased joint space (<2 mm wide) and/or joint margin sclerosis at the final review. Nine patients (15%) had more significant joint space narrowing (2–4 mm wide) at the final follow‐up, most lesions being visualized in the medial compartment. These patients were distributed mainly among those with KD III injuries combined with lateral side injuries and KD IV.
Complications
The patients were monitored to detect early complications from immediately after surgery to discharge; these complications included DVTs, infections, compartment syndrome, peroneal nerve palsy and arterial injury. Three patients experienced wound problems (infections and fat liquefaction), which resolved following treatment with oral antibiotics and delayed removal of stitches. Five cases of tourniquet complications were indicated by skin blisters, thigh swelling and stabbing pain. No cases of DVT, compartment syndrome or iatrogenic neurovascular compromise were identified. At the endpoint of follow‐up of short‐term complications, eight patients had frequent peri‐patellar pain that was alleviated by local blocking. Two cases of knee stiffness (range of flexion within 60°) were resolved by arthrolysis. Other complications, such as loss of motion and persistent laxity, are described above. In general, there was no statistical relationship between complication incidence and injury type.
Discussion
Trauma epidemiology narrates the patterns, causes, mechanisms and effects of physical conditions in defined populations. The first objective of this analysis was to perform a systematic, retrospective study of proven surgical cases treated in our department, with the aim of clarifying their epidemiology. We found that 30‐ to 50‐year‐old men were at high risk of MLIKs. There were no significant differences between the left and right sides of the body in numbers of injured knees. As for the most vulnerable ligament, the ACL, MCL and PCL ranked as the top three sites, in that order. The most common injury types were single cruciate combined MCL injuries and bicruciate combined lateral side injuries (ACL + PCL + LCL/PLC). These general results are in agreement with most reports on knee injuries. For example, Goudie et al. reported similar patterns of sex, age, and ligament disruption types among surgically treated patients at the Royal Infirmary of Edinburgh in Scotland14.
However, this paper specifically concerns Chongqing. Chongqing municipality is a rapidly developing city of 31,442,300 located in southwest Chongqing; 61.7% of the population of this province was rural in 2009. Chongqing is also the economic and financial center of the upper Yangtze River, the demonstration area for developing a circular economy, and the national comprehensive reform pilot area for the coordination of urban and rural areas15. In this area, we identified a high proportion of high‐energy related injuries: such injuries accounted for 75% of all injuries, whereas injuries caused by athletic activities accounted for less than 16%. These findings are dramatically different from results from Western Europe and the USA. Jenkins et al. reported 45% high‐energy injuries and 40% low‐energy injuries in a population with a mean age of 21 years in the UK16. A prospective study conducted in Seattle indicated that low‐energy mechanisms accounted for almost 60% of all injuries (49% athletic activities and 10% falls), these exceeding motor vehicle collisions by up to 25%3.
Although most injury types (10 of 11) have been described in previous studies3, our study still yielded two extraordinary findings. One important finding was that we are the first to present two cases of PCL + PT injury type; we found no published reports concerning the pathogenesis and reconstruction of this type of injury in subjects with MLIKs. One possible mechanism is a direct posterior blow to the proximal tibia injuring the PCL, followed by PT avulsion with or without a peel‐off ACL injury. Another interesting finding was that none of our patients had PCL + LCL types of injury, although this type of injury had been described by others4, 17. One possible explanation is that few of our study patients had been subjected to anterior‐lateral force on their knees as pedestrians or motor vehicle drivers.
Previous reports have indicated that MLIKs typically occur during acute dislocations, the incidence ranging from 0.001% to 0.013%18. However, because of multiple factors described previously, even under subtle stress circumstances (especially in obese individuals), knee subluxation can lead to MLIKs. Therefore, the reported incidence of MLIK was far greater than the morbidity of knee dislocation, especially in Chongqing, China. Although this observation may appear rudimentary, in the authors’ experience the primary reason for failure of surgical treatment of cruciate and collateral ligaments injuries is unrecognized posteromedial or posterolateral lesions4, 19, 20, 21, and this failure to diagnose and evaluate properly can result in a host of complications12.
We therefore advocate a complete physical examination, including X‐rays, CT and MRI studies for precise planning of surgical procedures, instrumentation needs, implants, fixators and grafts22, 23. In acute cases, knee laxity should be evaluated under anesthesia. In patients with chronic injuries, the axis of the lower limb and gait should also be evaluated9. The dogmatic flowsheet for initial evaluation proposed by Nicandri et al. could be used as a guideline24.
Despite the diverse approaches to treating MLIKs, the final goal is to achieve a well‐aligned knee with pre‐injury kinematics. The therapeutic principles when treating MLIKs are to identify and treat all pathology, for example by achieving accurate tunnel placement and anatomic graft insertion, undertaking mechanical graft tensioning and secure graft fixation and performing deliberate postoperative rehabilitation. Variations to these guidelines can occur according to the surgeon's preference, the logistics of the hospital and the availability of instruments. For example, Dong et al. presented a novel new technique for reconstruction of the MCL with a triangular shape: this technique is worth recommending because of the improved valgus and rotational stability at short‐term outcome25.
With regards to conservative versus surgical maneuvers, it is now evident that a non‐surgical approach yields consistently poor results5, 26. In our department, the vast majority of patients with MLIK are treated by surgical stabilization, exceptions being patients whose age or medical comorbidities make them poor surgical candidates. The rationale and indications for surgical treatment that we have introduced include: (i) simultaneous rupture of the bicruciate ligament; (ii) the presence of abnormal laxity under valgus/varus stress; (iii) the presence of posterolateral instability combined with rupture of the cruciate ligament; (iv) multidirectional abnormal laxity; and (v) symptomatic objective patholaxity in chronic cases.
Regarding repair versus reconstruction options, our decision depends on the ligament injured and the extent and type of injury. According to our experience, the ACL and PCL should always be reconstructed if clinically injured, but arthroscopically diagnosed PCL injuries without clinical laxity do not warrant acute reconstruction. We mostly perform single‐bundle cruciate reconstruction because there is no conclusive evidence that the results of double‐bundle reconstruction are superior to those of the single‐bundle technique27.
For the MCL, we have found that most medial injury patterns (Grades I–II) are more amenable to conservative treatment and repair than to reconstruction. Further, previous studies have also failed to show an advantage for MCL repair over reconstruction28. Whether to perform reconstruction or not depends on the location of the tear, the quality of the tissue and the time since the injury29. In our department, the MCL is reconstructed unless there is significant medical compartment widening on fluoroscopy (greater than 10 mm) or the injury is chronic. However, with lateral side injuries, reconstruction is usually a priority because it generally results in better outcomes than does repair, even in cases in which robust native tissue is available30. One study reported a 40% failure rate for repair of lateral‐sided structures without augmentation, compared with a 4% failure rate with reconstruction31.
Concerning single‐ versus multi‐stage algorithms, we prefer to address all bicruciate or combined medial side injuries with a one‐stage strategy. Our previous investigation identified no significant difference in joint function between one‐ and two‐stage treatments in these patients. However, for combined injuries of the cruciate with the lateral side or for reconstruction of the medial side or of the PT, we have had success with rational repair/reconstruction of the extra‐articular structures in the acute setting (10–14 days), followed by cruciate reconstruction after 6–8 weeks. This protocol has also been supported by many previous publications27, 32, 33.
Despite the increase in hospital time and cost, such delayed reconstruction has yielded a lower incidence of laxity and arthrofibrosis than acute reconstruction. Similarly, a level IV study reported that two‐stage surgical treatment for acute traumatic knee dislocation is effective and produces good outcomes in terms of stress radiographs, ROM, Lysholm scores, Tegner activity stages and International Knee Documentation Committee ratings34. A recent meta‐analysis showed that delayed/multi‐stage reconstruction of severe MLIKs potentially yields equivalent outcomes in terms of stability to those of acute repair11. In contrast, Miller et al. advocated single‐stage reconstruction of all injured structures during the index procedure and pursuing early surgical intervention within 2 weeks and reported reliable restoration of function and stability. Nevertheless, we still believe our multi‐stage strategy precisely balances early ROM and long‐term stability.
In this study, follow‐up of outcomes of MLIKs over a mid‐term of 2.5 years validate surgical intervention, our findings suggesting that good overall functional outcomes are achieved following surgical management in the majority of cases.
According to the Lysholm evaluation system, the outcomes in this series were 77% good and 23% fair. This finding compares favorably with those of other authors, who have reported satisfactory results in 68%–79% of patients14, 35. The different scores of different KD groups relate to varying levels of injury to knee structures; thus, anatomical lesions are possible indicators of prognosis. Physical function was very limited preoperatively, but improved significantly following surgery, most cases achieving a normal or nearly normal rating by the final follow‐up. The age and sex of the patient and the timing of surgery following the injury did not have major influences on ROM.
Although we were pleased with overall eventual joint function, we still noted a high rate (8.2%) of posterior or lateral instability and 42% of the patients developed osteoarthritis, evidenced by radiographs. However, few negative effects on daily activity were reported and no revisions were indicated. Whereas the level of activity improved greatly following surgery, most patients did not return to their pre‐injury levels of activity and some had to change their careers. This consequence was primarily due to the severity and complexity of the original injuries. In this context, a long‐term follow‐up study from Switzerland reported that, of 26 elite athletes who underwent single‐stage surgeries for MLIKs from 1983 to 2006, 73% returned to their sports with good functional outcomes and ligamentous stability; however, only 30% of these athletes reached their pre‐injury sports activity levels36. Indeed, a study on muscle strength and functional recovery showed that the quadriceps and hamstrings recovered to 85%–90% of the uninjured side's capacity at 2 years after surgery, whereas sports and quality of life factors recovered more slowly16. These results suggest that, even with complete acute restitution of anatomical structure and immediate regaining of ROM, functional restoration requires far more time; thus, more prolonged muscle rehabilitation might be worthwhile.
The main limitation of this study was the small number of cases and the heterogeneity of the groups, which made making comparisons and drawing conclusions difficult. Long‐term follow‐up data (>5 years) should be collected for conclusive results.
Standard, individualized and multi‐stage surgical strategies are beneficial for outcomes of patients with MLIKs. Although good restoration of function was achieved in the majority of cases, most patients had not reached their pre‐injury activity levels by the 2.5‐year follow‐up endpoint.
Acknowledgements
The authors thank Feng‐Xi Ma for assisting in the preparation of the illustrations and Jie He for assisting with the database and tracking of the patients.
Disclosure: The authors have no potential conflicts of interest. This study was financially supported by the Natural Science Foundation of China (No. 81171720).
References
- 1. Fanelli GC. The multiple ligament injured (dislocated) knee. J Knee Surg, 2012, 25: 261. [DOI] [PubMed] [Google Scholar]
- 2. Edwards GA, Sarasin SM, Davies AP. Dislocation of the knee: an epidemic in waiting? J Emerg Med, 2013, 44: 68–71. [DOI] [PubMed] [Google Scholar]
- 3. Wang ZG. Current situation and future of road traffic injury (Chin). Chuangshang Wai Ke Zazhi, 2011, 13: 193–196. [Google Scholar]
- 4. Fanelli GC, Stannard JP, Stuart MJ, et al Management of complex knee ligament injuries. J Bone Joint Surg Am, 2010, 92: 2235–2246. [PubMed] [Google Scholar]
- 5. Van Tongel A, MacDonald PB. How I manage the multiple‐ligament injured knee. Oper Tech Sports Med, 2010, 18: 245–249. [Google Scholar]
- 6. Vyas D, Harner CD. How I manage the multiple‐ligament injured (dislocated) knee. Oper Tech Sports Med, 2011, 19: 2–11. [DOI] [PubMed] [Google Scholar]
- 7. Gwathmey FW Jr, Shafique DA, Miller MD. Our approach to the management of the multiple‐ligament knee injury. Oper Tech Sports Med, 2010, 18: 235–244. [Google Scholar]
- 8. Merritt AL, Wahl CJ. Rationale and treatment of multiple‐ligament injured knees: the Seattle perspective. Oper Tech Sports Med, 2011, 19: 51–72. [Google Scholar]
- 9. Arciero RA. Pearls and pitfalls in the management of the chronic multiple ligament‐injured knee. Oper Tech Sports Med, 2010, 18: 250–266. [Google Scholar]
- 10. Tzurbakis M, Diamantopoulos A, Xenakis T, Georgoulis A. Surgical treatment of multiple knee ligament injuries in 44 patients: 2‐8 years follow‐up results. Knee Surg Sports Traumatol Arthrosc, 2006, 14: 739–749. [DOI] [PubMed] [Google Scholar]
- 11. Mook WR, Miller MD, Diduch DR, Hertel J, Boachie‐Adjei Y, Hart JM. Multiple‐ligament knee injuries: a systematic review of the timing of operative intervention and postoperative rehabilitation. J Bone Joint Surg Am, 2009, 91: 2946–2957. [DOI] [PubMed] [Google Scholar]
- 12. Shelbourne KD, Klootwyk TE. Low‐velocity knee dislocation with sports injuries. Treatment principles. Clin Sports Med, 2000, 19: 443–456. [DOI] [PubMed] [Google Scholar]
- 13. Manske RC, Prohaska D. Physical examination and imaging of the acute multiple ligament knee injury. N Am J Sports Phys Ther, 2008, 3: 191–197. [PMC free article] [PubMed] [Google Scholar]
- 14. Goudie EB, Will EM, Keating JF. Functional outcome following PCL and complex knee ligament reconstruction. Knee, 2010, 17: 230–234. [DOI] [PubMed] [Google Scholar]
- 15. Wang H, Zhang Y, Xiang Q, et al Epidemiology of traumatic spinal fractures: experience from medical university‐affiliated hospitals in Chongqing, China, 2001–2010. J Neurosurg Spine, 2012, 17: 459–468. [DOI] [PubMed] [Google Scholar]
- 16. Jenkins PJ, Clifton R, Gillespie GN, Will EM, Keating JF. Strength and function recovery after multiple‐ligament reconstruction of the knee. Injury, 2011, 42: 1426–1429. [DOI] [PubMed] [Google Scholar]
- 17. Lill H, Glasmacher S, Korner J, Rose T, Verheyden P, Josten C. Arthroscopic‐assisted simultaneous reconstruction of the posterior cruciate ligament and the lateral collateral ligament using hamstrings and absorbable screws. Arthroscopy, 2001, 17: 892–897. [DOI] [PubMed] [Google Scholar]
- 18. Levy BA, Dajani KA, Whelan DB, et al Decision making in the multiligament‐injured knee: an evidence‐based systematic review. Arthroscopy, 2009, 25: 430–438. [DOI] [PubMed] [Google Scholar]
- 19. Ibrahim SA, Ghafar S, Salah M, et al Surgical management of traumatic knee dislocation with posterolateral corner injury. Arthroscopy, 2013, 29: 733–741. [DOI] [PubMed] [Google Scholar]
- 20. Osti M, Tschann P, Künzel KH, Benedetto KP. Posterolateral corner of the knee: microsurgical analysis of anatomy and morphometry. Orthopedics, 2013, 36: e1114–e1120. [DOI] [PubMed] [Google Scholar]
- 21. Rauh PB, Clancy WG Jr, Jasper LE, Curl LA, Belkoff S, Moorman CT 3rd. Biomechanical evaluation of two reconstruction techniques for posterolateral instability of the knee. J Bone Joint Surg Br, 2010, 92: 1460–1465. [DOI] [PubMed] [Google Scholar]
- 22. Teh J, Kambouroglou G, Newton J. Investigation of acute knee injury. BMJ, 2012, 344: e3167. [DOI] [PubMed] [Google Scholar]
- 23. Vinyard TR, Boyd J, MacDonald PB. Initial evaluation of the acute and chronic multiple ligament injured knee. J Knee Surg, 2012, 25: 275–286. [DOI] [PubMed] [Google Scholar]
- 24. Nicandri GT, Dunbar RP, Wahl CJ. Are evidence‐based protocols which identify vascular injury associated with knee dislocation underutilized? Knee Surg Sports Traumatol Arthrosc, 2010, 18: 1005–1012. [DOI] [PubMed] [Google Scholar]
- 25. Dong JT, Chen BC, Men XQ, et al Application of triangular vector to functionally reconstruct the medial collateral ligament with double‐bundle allograft technique. Arthroscopy, 2012, 28: 1445–1453. [DOI] [PubMed] [Google Scholar]
- 26. Frosch KH, Preiss A, Heider S, et al Primary ligament sutures as a treatment option of knee dislocations: a meta‐analysis. Knee Surg Sports Traumatol Arthrosc, 2013, 21: 1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fanelli GC, Edson CJ. Surgical treatment of combined PCL‐ACL medial and lateral side injuries (global laxity): surgical technique and 2‐ to 18‐year results. J Knee Surg, 2012, 25: 307–316. [DOI] [PubMed] [Google Scholar]
- 28. Dwyer T, Marx RG, Whelan D. Outcomes of treatment of multiple ligament knee injuries. J Knee Surg, 2012, 25: 317–326. [DOI] [PubMed] [Google Scholar]
- 29. Schein A, Matcuk G, Patel D, et al Structure and function, injury, pathology, and treatment of the medial collateral ligament of the knee. Emerg Radiol, 2012, 19: 489 – 498. [DOI] [PubMed] [Google Scholar]
- 30. Stannard JP, Brown SL, Robinson JT, McGwin G Jr VDA. Reconstruction of the posterolateral corner of the knee. Arthroscopy, 2005, 21: 1051–1059. [DOI] [PubMed] [Google Scholar]
- 31. Levy BA, Dajani KA, Morgan JA, Shah JP, Dahm DL, Stuart MJ. Repair versus reconstruction of the fibular collateral ligament and posterolateral corner in the multiligament‐injured knee. Am J Sports Med, 2010, 38: 804–809. [DOI] [PubMed] [Google Scholar]
- 32. Subbiah M, Pandey V, Rao SK, Rao S. Staged arthroscopic reconstructive surgery for multiple ligament injuries of the knee. J Orthop Surg (Hong Kong), 2011, 19: 297–302. [DOI] [PubMed] [Google Scholar]
- 33. Dhillon M, Akkina N, Prabhakar S, Bali K. Evaluation of outcomes in conservatively managed concomitant Type A and B posterolateral corner injuries in ACL deficient patients undergoing ACL reconstruction. Knee, 2012, 19: 769–772. [DOI] [PubMed] [Google Scholar]
- 34. Bin SI, Nam TS. Surgical outcome of 2‐stage management of multiple knee ligament injuries after knee dislocation. Arthroscopy, 2007, 23: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 35. Wang CJ, Chen HS, Huang TW, Yuan LJ. Outcome of surgical reconstruction for posterior cruciate and posterolateral instabilities of the knee. Injury, 2002, 33: 815–821. [DOI] [PubMed] [Google Scholar]
- 36. Hirschmann MT, Iranpour F, Müller W, Friederich NF. Surgical treatment of complex bicruciate knee ligament injuries in elite athletes: what long‐term outcome can we expect? Am J Sports Med, 2010, 38: 1103–1109. [DOI] [PubMed] [Google Scholar]


