Abstract
Femoral bone loss due to periprosthetic fracture, a challenging problem in total hip arthroplasty (THA), is increasingly encountered due to a rise in the number of revision THAs performed. Allograft prosthesis composite (APC) and proximal femoral replacement (PFR) are two available options for management of patients with difficult type‐B3 Vancouver periprosthetic fractures. The treatment algorithm for patients with these fractures has been extensively studied and is influenced by the age and activity level of the patient. APC is often preferred in young and active patients in an attempt to preserve bone stock while older and less active patients are considered candidates for PFR. In spite of the high rate of overall complications with these two procedures, reported survivorship is acceptable. Treating patients with these complicated fractures is fraught with complications and, even with successful treatment, the outcomes are not as promising as those associated with primary hip replacement. In this paper, we aimed to review available published reports about PFR and APC for treatment of periprosthetic fractures around THAs.
Keywords: Allograft prosthesis composite, Hip arthroplasty, Periprosthetic fracture, Proximal femoral replacement
Introduction
Femoral bone loss is a challenging problem that is increasingly encountered during total hip arthroplasty (THA)1. With the increasing number of revision THAs being performed, durable solutions for femoral bone loss are needed2. Numerous mechanisms, including infection, mechanical loosening, osteolysis secondary to particle debris, stress‐shielding with adaptive bone remodeling, and non‐union may cause loss of proximal femoral bone stock after THA1, 3, 4, 5. The integrity of the bone stock in the proximal part of femur can be compromised by insertion and removal of implants during prior reconstructive procedures as well as by periprosthetic fractures6. This paper aims to review management of periprosthetic fractures around THA with significant bone loss.
Methods
We limited the literature search for this review to PubMed and Google Scholar. We used the key phrases of “proximal femoral replacement”, “allograft prosthesis composite” and “periprosthetic fracture” to identify related articles. We expanded the literature search in PubMed using related citation options.
Epidemiology
Periprosthetic fractures are an important cause of proximal femoral bone loss. These fractures can be divided broadly into two groups: intraoperative and postoperative. Intraoperative fractures generally occur during insertion of stems7. With the significant increase in the number of THAs being performed and the longevity of patients after THA, the incidence of periprosthetic fractures is expected to increase8, 9, 10.
The reported incidence of periprosthetic fractures around THAs varies based on type of prosthesis (cemented or cementless) and type of surgery (primary or revision). Periprosthetic fractures of the femur are more frequent during cementless arthroplasties and following revision THA. Berry reported an incidence of 0.3% in primary cemented and 5.4% in primary cementless THAs11. In revision surgeries, the incidence is reportedly as low as 3.6% for cemented prostheses and as high as 20.9% for cementless implants7. Overall, the reported rate of periprosthetic fractures varies from 0.1% to 46%1, 12. Risk factors for intraoperative periprosthetic fractures include the use of minimally invasive techniques, female sex, metabolic bone disease, bone diseases leading to altered bone morphology (e.g. Paget's disease), and technical errors at the time of surgery12, 13.
Classification of Periprosthetic Fractures
Of all the suggested classification systems for periprosthetic fractures, the Vancouver Classification is the most widely utilized14. This validated classification system has been shown to have high inter‐ and intra‐observer reliability, and therefore is an accurate tool for guiding therapeutic plans15, 16. The Vancouver classification divides periprosthetic fractures into types A, B, and C and further categorizes type B fractures into three subtypes, B1, B2, and B3 fractures. In addition, it defines type‐A fractures as fractures around the trochanteric region of the femur and subdivides them into AG (involvement of greater trochanter) and AL (fracture of lesser trochanter). Vancouver type‐B1 periprosthetic fractures are fractures distal to the intertrochanteric region around prostheses in which the femoral stem remains well‐fixed. Vancouver type‐B2 fractures also occur around the femoral stem but lead to loosening of the stem or involve the cement mantle around the femoral stem. Vancouver type‐B3 fractures occur in the proximal femur with deficient bone and have associated loosening of the femoral stem. Vancouver type‐C fractures are below the tip of the component. Vancouver types B2 and B3 periprosthetic fractures are displayed in Fig. 1.
Figure 1.
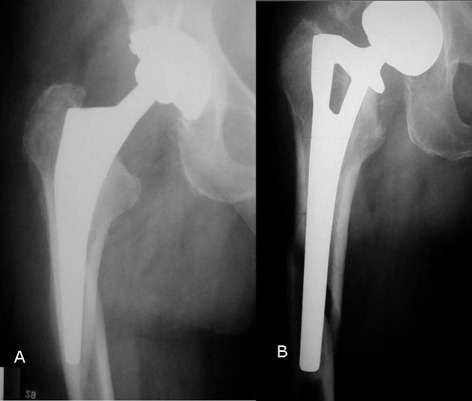
(A) Radiographs showing Vancouver type‐B 2 and (B) type‐B 3 periprosthetic fractures.
An algorithm for management of periprosthetic fractures around THAs has been described by Parvizi et al.8 In brief, type‐A fractures are commonly treated non‐operatively unless they extend into the calcar region and will therefore affect stability; they then necessitate cerclage wiring with or without bone grafting8, 17. Revision arthroplasty may be necessary in some cases of Vancouver type‐A periprosthetic fractures in which the underlying cause is wear and osteolysis17.
Cable/cerclage wiring plus or minus plate fixation is used for treatment of type‐B1 Vancouver fractures with an intact medial cortex and calcar region, whereas those with involvement of the medial cortex or short transverse fractures will benefit from both plate fixation and possibly use of a cortical strut allograft8, 17, 18. The principals of management of type‐B1 fractures include direct anatomic fracture reduction with minimal soft tissue stripping to maintain healing potential. The fracture should be stabilized with dynamic compression plates and screws as well as locking screws to maximize initial stability. The use of cables/wires and cortical allograft helps to reinforce the construct and can restore bone stock. Although there is disagreement regarding the method of fracture fixation, there is universal agreement that the stem does not need to be revised and anatomic reduction, when possible, should be the goal. In contrast, revision arthroplasty is commonly used for management of type‐B2 Vancouver fractures17. Management of type‐B3 periprosthetic fractures is more challenging and is the main focus of this review article. Treatment of these fractures depends on the patient's age and activity level. Currently, there are two main treatment options for Vancouver type‐B3 fractures with severe proximal bone loss: allograft prosthetic composite reconstruction and proximal femoral replacement17. Some studies have also described techniques involving revision arthroplasty with the use of a modular fluted stem19, 20. In this review however, we discuss the current literature on allograft prosthesis composite (APC) and proximal femoral replacement (PFR) for management of periprosthetic fractures around THAs.
Allograft Prosthetic Composite
For management of type‐B3 Vancouver periprosthetic fractures in need of reconstruction with massive femoral bone loss, long term studies have shown that APC has satisfactory results as judged by pain relief and functional outcome 21, 22. In young and active patients, APC is considered the preferred method for reconstruction of proximal femoral bone defects17. In addition to type‐B3 periprosthetic fractures, uncontained segmental femoral defects extending 8 cm into the femoral diaphysis and severe bone loss compromising distal fixation are additional indications for APC23. Elderly patients with extensive comorbidities for whom immediate mobilization and weight‐bearing is necessary and the presence of infection are contraindications to APC23.
Preoperative Planning
Presence of infection should be ruled out prior to planning for APC. The initial infection workup should include measurement of serum C‐reactive protein concentration and erythrocyte sedimentation rate. Patients with increased serum C‐reactive protein concentrations, or a concerning history such as previous infection, should undergo joint aspiration and the synovial fluid be analyzed for leukocyte count, neutrophil differential, culture, and sometimes frozen section24, 25.
Thorough preoperative planning for APC should start with similar general considerations as for revision THAs. Relevant preoperative planning issues include optimizing the medical and nutritional status of the patient, accurate physical examination, ordering of appropriate radiographs, and blood conservation strategies. Any revision THA can be associated with significant blood loss because extensive soft tissue dissection may be needed during surgery. Therefore, an appropriate blood conservation strategy should be in place for these procedures. The type of anesthesia plays an important role in perioperative blood loss; the use of hypotensive regional anesthesia techniques reportedly reduces blood loss26. Preoperative autologous blood donation27 and administration of erythropoietin preoperatively28, 29 are also reportedly effective in reducing the need for allogenic blood transfusions. Cell saver, if available, can be utilized in non‐infected cases30.
Important Specific Points about Preoperative Planning of Allograft Prosthesis Composite
Use routine radiographs to determine the approximate length of allograft needed23.
Order allografts longer than the measured femoral deficit in anticipation of the need for adjustments to the graft23.
Do not order allografts with substantial wider diameters than the host femur. The host femur should dictate the diameter of the stem because gross mismatches between host and graft can lead to difficulty when seating the stem23.
Surgical Technique
The surgical technique for APC has been described by Kellet et al.23 In brief, APC for proximal femoral bone loss begins with preparation of the graft. The proximal femoral graft, typically smaller than the diameter of the host, should be fresh frozen allograft that has been stored at −70°C and irradiated. The allograft can be thawed in 5% povidone iodine solution and cultured. The neck is divided approximately 1 cm proximal to the lesser trochanter and a step cut osteotomy made at the distal end to enhance rotational stability. If the host trochanter with abductor attachment is available, the greater trochanter can be osteotomized and removed. The graft is reamed with straight rigid reamers and the femoral component cemented into the graft to ensure appropriate anteversion of the prosthesis23. Figures 2 and 3 show the operative technique, preoperative and postoperative radiographs of a patient with type‐B3 Vancouver periprosthetic fracture who underwent APC.
Figure 2.
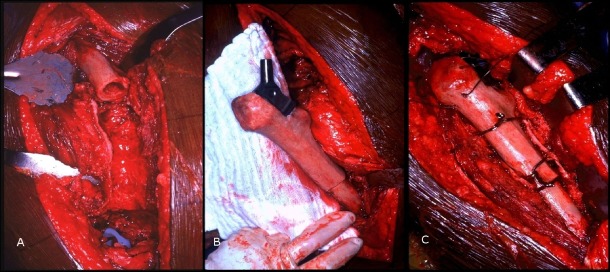
Operative stages of allograft prosthesis composite.
Figure 3.
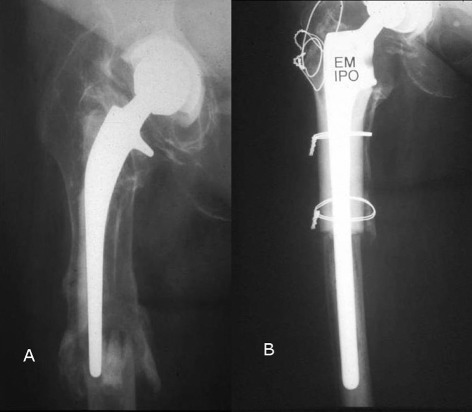
Preoperative and postoperative radiographs of a patient with a Vancouver type‐B3 periprosthetic fracture who underwent allograft prosthesis composite. (A) Preoperative radiograph (Vancouver type‐B3 periprosthetic fracture). (B) Radiograph at 2‐year follow‐up.
Once the APC has been prepared, the patient's femur is exposed by an extended trochanteric osteotomy or trochanteric slide osteotomy. The femur should be cut down to healthy stable bone and a corresponding step osteotomy made in preparation for the APC placement. The APC is telescoped 1–2 cm into the host bone and trimmed as necessary. The composite is then secured to the patient's femur with cerclage cables. The greater trochanter is then attached to the allograft by drilling holes for stainless steel cerclage wires and fastening them to the composite23.
Important Specific Points on Postoperative Care of Allograft Prosthesis Composite
Complications
In addition to the common complications of revision THA, there are reportedly specific complications in patients undergoing APC. The advantages of the use of allograft bone, such as availability and eliminating harvesting complications, need to be weighed against the disadvantages, such as a lack of osteoprogenitor cells and osteogenic factors and potential for immune reactions and disease transmission31. Junctional nonunion (13%), allograft fracture (6.7%), loosening of acetabular component (6.7%), trochanteric escape (26.7%), allograft infection (20%), and allograft resorption (20%) were reported complications in a published series of 15 patients who underwent APC32. In another report by Babis and colleagues, complications associated with revision surgery, including allograft non‐union, aseptic loosening, allograft resorption, allograft fracture, femoral stem fracture, and deep infection, were reportedly as high as 26.5%22. Additionally, 33.4% of patients in this series needed re‐operations without revision of their APCs mainly due to dislocation (11.1%) and hematoma formation (8.3%)22. The reported rate of allograft resorption (mild to severe) varies from 3%33 to 50%34 depending on the duration of follow‐up.
Outcome
Treatment of type‐B3 Vancouver periprosthetic fractures is generally associated with high rates of failure and patients with femoral periprosthetic fractures are at increased risk of death35, 36. In a study published in 2004, outcomes of 15 patients who had undergone the APC technique to reconstruct failed THAs were retrospectively reviewed32. The average length of allograft was 11 cm. At a mean follow‐up period of 7.6 years, 10 patients (67%) retained their allograft‐prosthesis constructs. The average postoperative Harris hip score was higher than preoperative values. Despite acceptable results, the authors concluded that long‐term follow‐up was needed to assess potential late complications such as infection and graft resorption32. Most recently, Babies et al. reported a 10‐year survivorship of 69% for APC and suggested that pre‐operative bone loss (Paprosky type IV), multiple previous hip revisions, and the length of the utilized allograft were predictors of survivorship22. We have summarized survivorship of APC in various studies in Table 1. Treatment of failed APC varies according to the underlying cause of failure. Where the host bone‐allograft junction has failed to unite, treatment can be observation in asymptomatic cases, internal fixation and bone grafting, or a second APC21. Loosening of the proximal allograft can be treated with another APC21. The graft is revised in cases of graft resorption. Infection of the proximal allograft can be treated either by another APC as a two‐stage revision or by long‐term antibiotic suppression therapy34.
Table 1.
Reported survivorship of allografts after allograft prosthesis composite in different studies
| Author (Year) | Number of hips | Survivorship of allograft (mean follow‐up) |
|---|---|---|
| Head et al. (1987)37 | 22 | 73% (2 years) |
| Gross et al. (1995)38 | 130 | 85% (4.8 years) |
| Haddad et al. (2000)34 | 55 | 89% (8.8 years)a |
| Blackley et al. (2001)39 | 48 | 77% (11 years) |
| Maury et al. (2006)21 | 25 | 84% (5.1 years) |
| Safir et al. (2009)40 | 93 | 82.2% (15 years) |
Five additional patients who also needed revision due to acetabular failure were not included for calculation of survivorship.
Proximal Femoral Replacement
Elderly and low demand patients with type‐B3 periprosthetic fractures are potential candidates for PFRs. Proximal femoral replacements should be thoroughly planned. However, many consider them to be less technically demanding than APC reconstruction17. The presence of superficial or deep infection around the hip is an absolute contraindication to insertion of a megaprosthesis. Relative contraindications for PFR include uncooperative patients at higher risk of dislocation, vascular insufficiency that may prevent healing, inadequate distal femoral bone stock into which to insert the femoral component, and presence of significant medical comorbidities that preclude administration of anesthesia41.
Preoperative Planning
General preoperative planning for PFR is similar to that described for APC. For preoperative radiologic evaluation, anteroposterior and lateral radiographs are ordered. Plain radiographs may underestimate the amount of bone loss, particularly if significant osteolysis is present42. If there has been a femoral fracture, or if an osteotomy or another surgical procedure has been performed on the femur, anteroposterior and lateral radiographs of the entire femur are critical6. Although several classification systems have been suggested for bone loss43, the Paprosky classification44 is the most universally accepted and utilized. The authors' preferred methods for describing the location of bone loss and the presence of lucent lines in radiographs of the femur and acetabulum are those outlined by Gruen et al.45 and DeLee and Charnley46. Osseous integration of the prosthesis is assessed based on the presence or absence of radiolucent lines and so‐called spot welding47. Radiographs are also evaluated for the presence of heterotopic ossification, which are graded according to Brooker et al.48
The length of the femoral component is determined through careful preoperative and intraoperative assessments. Correct leg length can be determined by two methods. The first is to apply traction to the limb and measure from the cup to the host bone osteotomy site. The second and preferred method is to place a Steinmann pin in the iliac crest to measure a fixed reference point on the femur before dislocation. The soft tissue tension about the hip is the main determinant of femoral prosthesis length6, 41.
Surgical Technique
Only extensile surgical approaches allowing for wide visualization should be employed for PFR placement. It is the authors' experience that either modified Hardinge or posterolateral Moore approaches should be used for dissection when performing PFRs. If the proximal femur is intact, an osteotomy to split it may be required to facilitate removal of the previous prosthesis and/or hardware. Efforts should be made to maximize the length of the native femur because the outcome of PFR is influenced directly by the length of the remaining femur41. The prosthesis can be assembled and then cemented distally, or the stem can be cemented and the body then assembled onto it. In any case, extreme care needs to be exercised to prevent rotational mal‐positioning, which can affect the final stability of the hip. Once the long‐stem trial prosthesis is in place, correct leg length can be accurately restored. Balancing tension, restoration of limb length, and avoidance of excessive tension on the sciatic nerve is of greatest importance6, 41. Figure 4 shows preoperative and postoperative radiographs of a patient with a type‐B3 Vancouver periprosthetic fracture managed by PFR.
Figure 4.
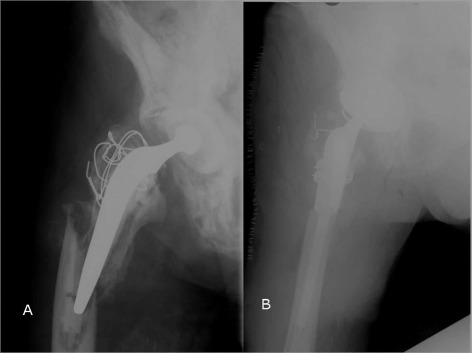
(A) Preoperative and (B) postoperative radiographs of a patient with a Vancouver type‐B3 periprosthetic fracture who underwent proximal femoral replacement.
Pearls and Pitfalls in Proximal Femoral Replacement
One of the major issues with PFR is instability because the abductor mechanism is often deficient from previous surgery, bone loss, or poor soft tissue envelope. Thus, it is very important that the abductor mechanism and proximal bone, however poor in quality, is maintained, wrapped, and attached to the prosthesis if possible. The other issue with PFR relates to poor bone stock and the difficulty in obtaining good stability of the prosthesis. Although uncemented proximal replacement components are available, most surgeons prefer to cement the prosthesis into distal bone for more predictable and secure fixation17.
Postoperative Care
Patients should commence protective weight bearing on postoperative day one. We recommend the use of abduction orthosis for all patients6. Once the fracture has healed and its components show early signs of osseo‐integration, weight bearing is increased and abductor strengthening exercises introduced. This usually occurs at approximately 6 to 8 weeks postoperatively17.
Complications
Early studies have reported various complications following PFR, including dislocation, infection, fracture, and leg length discrepancy. High rates of radiolucencies around the acetabulum and femur, aseptic loosening, and catastrophic failure of the socket in patients with poor bone stock are other reported complications of PFR6. Of these complications, dislocation is the most frequent, occcuring in 18%–50% of patients45.
Outcome
In 1981, Sim and Chao reported on the use of megaprostheses for reconstructing 21 proximal femurs without neoplastic involvement49. Of these patients, 10 had a history of failed arthroplasty with associated structural bone loss. During 25–98 months of follow‐up, all patients experienced significant pain relief. However, one instance of loosening of the acetabular component occurred in this series of patients.
Malkani et al. evaluated the outcomes of 50 PFRs for non‐neoplastic conditions50. During a mean clinical follow up period of 11.1 ± 4.0 years and mean radiographic follow‐up of 7.6 ± 3.2 years, they reported improvement in the Harris hip score. With revision surgery as the outcome measure, the authors estimated a 12‐year survivorship of 64%. Dislocation was the most frequent complication, occurring in 22% of patients. Later, Haentjens et al. reported similar results in 19 hips51. They used the Merle d'Aubigne hip‐rating scale to evaluate outcomes of patients at a mean follow‐up time of 5 years. They reported good outcomes in nine hips, fair outcomes in five and poor outcomes in two, of the remaining three patients two had died and one was lost to follow‐up. The rate of dislocation and infection were 37% and 16% respectively.
Early experience at our institution in patients with failed hip prostheses and severe bone loss mirrors the results previously discussed. Parvizi et al. reported a significant improvement in functional outcome (Harris hip score) in 48 patients from two institutions who had undergone PFR for non‐neoplastic conditions1. The functional outcome was found to be excellent or good in 22 patients, fair in 10, and poor in 11. Ten patients required a reoperation or revision because of at least one complication. Klein et al., also from our institution, reported the results of PFR following Vancouver type‐B3 fractures characterized by severe proximal bone deficiency and loose femoral stems52. Of the 21 patients enrolled in this study, all but one was able to walk and had minimal to no pain at the time of latest follow‐up (mean, 3.2 years). The rate of complications, which included persistent wound drainage requiring irrigation and debridement (two), dislocation (two), re‐fracture of the femur distal to the stem (one), and acetabular cage failure (one), was relatively high.
Most recently, Al‐Taki et al. reported on quality of life of patients who had undergone PFR53. They retrospectively reviewed 36 patients from their institution using available validated questionnaires for assessment of quality of life and functional outcome. At a mean follow‐up of 3.2 years, patients in the PFR group showed improvement in the Western Ontario and McMaster Universities Arthritis Index (WOMAC) function, WOMAC pain, Oxford score, and the mental component of the SF‐12. However, the patients in the PFR group had lower WOMAC function and Oxford scores than the control group who had undergone conventional revision THA. The authors concluded that, although the outcome scores of the PFR patients were lower than were those of the control group, the patients' quality of life was significantly improved. They also found that when dislocation remained a concern, constrained liners should be used. According to our experience and these studies, PFR is a viable option for treatment of periprosthetic fractures in low demand patients with severe bone deficiency in spite of its relatively high rate of complications. It is the authors' opinion that, when employing a PFR for revision hip surgery, the stability of the hip must be tested diligently intra‐operatively and a constrained acetabular liner utilized if instability is identified. In order to enhance the bone stock and maintain the soft tissue envelope, the proximal part of the femur, however poor in quality, should be retained and re‐approximation onto the implant encouraged52.
Summary
Treatment of severe femoral bone loss in the face of periprosthetic fracture can be an extremely challenging event that should be planned as thoroughly as possible prior to entering the operating room. Limited bone stock, poor soft tissue envelope and patient co‐morbidities negatively affect treatment outcomes. Failure rates for type‐B3 periprosthetic fractures are high despite current treatments35. APC and PFR are two available options for management of type‐B3 periprosthetic fractures with significant bone loss23, 31. However, it is recommended that APC should be performed in young and active patients and PFR reserved for older and less active patients17. Finally, it should be noted that, even when these fractures are successfully treated, patients with periprosthetic femur fracture should be counseled about the multiple complications and increased risk of mortality36.
Disclosure: One author (Javad Parvizi, MD, FRCS) certifies that he has or may receive payments or benefits from Zimmer (Warsaw, IN, USA), Smith & Nephew (Memphis, IN, USA), and Cermatec (Laurens, SC, USA). Another author (William J Hozack, MD) receives royalties from Stryker Orthopaedics (Mahwah, NJ, USA) and is a paid consultant for the same company.
References
- 1. Parvizi J, Tarity TD, Slenker N, et al Proximal femoral replacement in patients with non‐neoplastic conditions. J Bone Joint Surg Am, 2007, 89: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 2. Kurtz S, Mowat F, Ong K, et al Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am, 2005, 87: 1487–1497. [DOI] [PubMed] [Google Scholar]
- 3. Roberson JR. Proximal femoral bone loss after total hip arthroplasty. Orthop Clin North Am, 1992, 23: 291–302. [PubMed] [Google Scholar]
- 4. Rubash HE, Sinha RK, Shanbhag AS, et al Pathogenesis of bone loss after total hip arthroplasty. Orthop Clin North Am, 1998, 29: 173–186. [DOI] [PubMed] [Google Scholar]
- 5. Murphy SB, Rodriguez J. Revision total hip arthroplasty with proximal bone loss. J Arthroplasty, 2004, 19 (4 Suppl. 1): S115–S119. [DOI] [PubMed] [Google Scholar]
- 6. Parvizi J, Porat M. Revision THA with femoral bone loss—proximal femoral replacement. Orthopaedia Main. In: Orthopaedia—Collaborative Orthopaedic Knowledgebase 2011‐05‐04, 2010. Available at: http://www.orthopaedia.net/display/Main/Revision+THA+with+Femoral+Bone+Loss+‐+Proximal+Femoral+Replacement 2011‐07‐26.
- 7. Lindahl H. Epidemiology of periprosthetic femur fracture around a total hip arthroplasty. Injury, 2007, 38: 651–654. [DOI] [PubMed] [Google Scholar]
- 8. Parvizi J, Rapuri VR, Purtill JJ, et al Treatment protocol for proximal femoral periprosthetic fractures. J Bone Joint Surg Am, 2004, 86 (Suppl. 2): S8–S16. [DOI] [PubMed] [Google Scholar]
- 9. Lindahl H, Garellick G, Regner H, et al Three hundred and twenty‐one periprosthetic femoral fractures. J Bone Joint Surg Am, 2006, 88: 1215–1222. [DOI] [PubMed] [Google Scholar]
- 10. Lindahl H, Malchau H, Herberts P, et al Periprosthetic femoral fractures classification and demographics of 1049 periprosthetic femoral fractures from the Swedish National Hip Arthroplasty Register. J Arthroplasty, 2005, 20: 857–865. [DOI] [PubMed] [Google Scholar]
- 11. Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am, 1999, 30: 183–190. [DOI] [PubMed] [Google Scholar]
- 12. Pike J, Davidson D, Garbuz D, et al Principles of treatment for periprosthetic femoral shaft fractures around well‐fixed total hip arthroplasty. J Am Acad Orthop Surg, 2009, 17: 677–688. [DOI] [PubMed] [Google Scholar]
- 13. Davidson D, Pike J, Garbuz D, et al Intraoperative periprosthetic fractures during total hip arthroplasty. Evaluation and management. J Bone Joint Surg Am, 2008, 90: 2000–2012. [DOI] [PubMed] [Google Scholar]
- 14. Duncan CP, Masri BA. Fractures of the femur after hip replacement. Instr Course Lect, 1995, 44: 293–304. [PubMed] [Google Scholar]
- 15. Brady OH, Garbuz DS, Masri BA, et al The reliability and validity of the Vancouver classification of femoral fractures after hip replacement. J Arthroplasty, 2000, 15: 59–62. [DOI] [PubMed] [Google Scholar]
- 16. Rayan F, Dodd M, Haddad FS. European validation of the Vancouver classification of periprosthetic proximal femoral fractures. J Bone Joint Surg Br, 2008, 90: 1576–1579. [DOI] [PubMed] [Google Scholar]
- 17. Parvizi J, Vegari DN. Periprosthetic proximal femur fractures: current concepts. J Orthop Trauma, 2011, 25 (Suppl. 2): S77–S81. [DOI] [PubMed] [Google Scholar]
- 18. Corten K, Vanrykel F, Bellemans J, et al An algorithm for the surgical treatment of periprosthetic fractures of the femur around a well‐fixed femoral component. J Bone Joint Surg Br, 2009, 91: 1424–1430. [DOI] [PubMed] [Google Scholar]
- 19. Berry DJ. Treatment of Vancouver B3 periprosthetic femur fractures with a fluted tapered stem. Clin Orthop Relat Res, 2003, 417: 224–231. [DOI] [PubMed] [Google Scholar]
- 20. Mulay S, Hassan T, Birtwistle S, et al Management of types B2 and B3 femoral periprosthetic fractures by a tapered, fluted, and distally fixed stem. J Arthroplasty, 2005, 20: 751–756. [DOI] [PubMed] [Google Scholar]
- 21. Maury AC, Pressman A, Cayen B, et al Proximal femoral allograft treatment of Vancouver type‐B3 periprosthetic femoral fractures after total hip arthroplasty. J Bone Joint Surg Am, 2006, 88: 953–958. [DOI] [PubMed] [Google Scholar]
- 22. Babis GC, Sakellariou VI, O'Connor MI, et al Proximal femoral allograft‐prosthesis composites in revision hip replacement: a 12‐year follow‐up study. J Bone Joint Surg Br, 2010, 92: 349–355. [DOI] [PubMed] [Google Scholar]
- 23. Kellett CF, Boscainos PJ, Maury AC, et al Proximal femoral allograft treatment of Vancouver type‐B3 periprosthetic femoral fractures after total hip arthroplasty. Surgical technique. J Bone Joint Surg Am, 2007, 89 (Suppl. 2 Pt.1): S68–S79. [DOI] [PubMed] [Google Scholar]
- 24. Parvizi J, Ghanem E, Menashe S, et al Periprosthetic infection: what are the diagnostic challenges? J Bone Joint Surg Am, 2006, 88 (Suppl. 4): S138–S147. [DOI] [PubMed] [Google Scholar]
- 25. Della Valle C, Parvizi J, Bauer TW, et al Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg, 2010, 18: 760–770. [DOI] [PubMed] [Google Scholar]
- 26. Dennis DA. Blood conservation in revision total hip arthroplasty. Semin Arthroplasty, 1992, 3: 246–256. [PubMed] [Google Scholar]
- 27. Parvizi J, Chaudhry S, Rasouli MR, et al Who needs autologous blood donation in joint replacement? J Knee Surg, 2011, 24: 25–31. [DOI] [PubMed] [Google Scholar]
- 28. Bezwada HP, Nazarian DG, Henry DH, et al Preoperative use of recombinant human erythropoietin before total joint arthroplasty. J Bone Joint Surg Am, 2003, 85: 1795–1800. [DOI] [PubMed] [Google Scholar]
- 29. Keating EM, Ritter MA. Transfusion options in total joint arthroplasty. J Arthroplasty, 2002, 17 (4 Suppl. 1): S125–S128. [DOI] [PubMed] [Google Scholar]
- 30. Esper SA, Waters JH. Intra‐operative cell salvage: a fresh look at the indications and contraindications. Blood Transfus, 2011, 9: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsiridis E, Spence G, Gamie Z, et al Grafting for periprosthetic femoral fractures: strut, impaction or femoral replacement. Injury, 2007, 38: 688–697. [DOI] [PubMed] [Google Scholar]
- 32. Wang JW, Wang CJ. Proximal femoral allografts for bone deficiencies in revision hip arthroplasty: a medium‐term follow‐up study. J Arthroplasty, 2004, 19: 845–852. [DOI] [PubMed] [Google Scholar]
- 33. Gross AE, Hutchison CR. Proximal femoral allografts for reconstruction of bone stock in revision hip arthroplasty. Orthopedics, 1998, 21: 999–1001. [DOI] [PubMed] [Google Scholar]
- 34. Haddad FS, Spangehl MJ, Masri BA, et al Circumferential allograft replacement of the proximal femur. A critical analysis. Clin Orthop Relat Res, 2000, 371: 98–107. [DOI] [PubMed] [Google Scholar]
- 35. Mukundan C, Rayan F, Kheir E, et al Management of late periprosthetic femur fractures: a retrospective cohort of 72 patients. Int Orthop, 2010, 34: 485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lindahl H, Oden A, Garellick G, et al The excess mortality due to periprosthetic femur fracture. A study from the Swedish national hip arthroplasty register. Bone, 2007, 40: 1294–1298. [DOI] [PubMed] [Google Scholar]
- 37. Head WC, Berklacich FM, Malinin TI, et al Proximal femoral allografts in revision total hip arthroplasty. Clin Orthop Relat Res, 1987, 225: 22–36. [PubMed] [Google Scholar]
- 38. Gross AE, Hutchison CR, Alexeeff M, et al Proximal femoral allografts for reconstruction of bone stock in revision arthroplasty of the hip. Clin Orthop Relat Res, 1995, 319: 151–158. [PubMed] [Google Scholar]
- 39. Blackley HR, Davis AM, Hutchison CR, et al Proximal femoral allografts for reconstruction of bone stock in revision arthroplasty of the hip. A nine to fifteen‐year follow‐up. J Bone Joint Surg Am, 2001, 83: 346–354. [DOI] [PubMed] [Google Scholar]
- 40. Safir O, Kellett CF, Flint M, et al Revision of the deficient proximal femur with a proximal femoral allograft. Clin Orthop Relat Res, 2009, 467: 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parvizi J, Sim FH. Proximal femoral replacements with megaprostheses. Clin Orthop Relat Res, 2004, 420: 169–175. [DOI] [PubMed] [Google Scholar]
- 42. Puri L, Wixson RL, Stern SH, et al Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am, 2002, 84: 609–614. [DOI] [PubMed] [Google Scholar]
- 43. Della Valle CJ, Paprosky WG. The femur in revision total hip arthroplasty evaluation and classification. Clin Orthop Relat Res, 2004, 420: 55–62. [DOI] [PubMed] [Google Scholar]
- 44. Paprosky WG, Aribindi R. Hip replacement: treatment of femoral bone loss using distal bypass fixation. Instr Course Lect, 2000, 49: 119–130. [PubMed] [Google Scholar]
- 45. Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem‐type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res, 1979, 141: 17–27. [PubMed] [Google Scholar]
- 46. DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res, 1976, 121: 20–32. [PubMed] [Google Scholar]
- 47. Engh CA, Massin P, Suthers KE. Roentgenographic assessment of the biologic fixation of porous‐surfaced femoral components. Clin Orthop Relat Res, 1990, 257: 107–128. [PubMed] [Google Scholar]
- 48. Brooker AF, Bowerman JW, Robinson RA, et al Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am, 1973, 55: 1629–1632. [PubMed] [Google Scholar]
- 49. Sim FH, Chao EY. Hip salvage by proximal femoral replacement. J Bone Joint Surg Am, 1981, 63: 1228–1239. [PubMed] [Google Scholar]
- 50. Malkani AL, Settecerri JJ, Sim FH, et al Long‐term results of proximal femoral replacement for non‐neoplastic disorders. J Bone Joint Surg Br, 1995, 77: 351–356. [PubMed] [Google Scholar]
- 51. Haentjens P, De Boeck H, Opdecam P. Proximal femoral replacement prosthesis for salvage of failed hip arthroplasty: complications in a 2–11 year follow‐up study in 19 elderly patients. Acta Orthop Scand, 1996, 67: 37–42. [DOI] [PubMed] [Google Scholar]
- 52. Klein GR, Parvizi J, Rapuri V, et al Proximal femoral replacement for the treatment of periprosthetic fractures. J Bone Joint Surg Am, 2005, 87: 1777–1781. [DOI] [PubMed] [Google Scholar]
- 53. Al‐Taki MM, Masri BA, Duncan CP, et al Quality of life following proximal femoral replacement using a modular system in revision THA. Clin Orthop Relat Res, 2011, 469: 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]


