Abstract
Anterior lumbar interbody fusion (ALIF) has become a widely recognized surgical technique for degenerative pathology of the lumbar spine. Spinal fusion has evolved dramatically ever since the first successful internal fixation by Hadra in 1891 who used a posterior approach to wire adjacent cervical vertebrae in the treatment of fracture‐dislocation. Advancements were made to reduce morbidity including bone grafting substitutes, metallic hardware instrumentation and improved surgical technique. The controversy regarding which surgical approach is best for treating various pathologies of the lumbar spine still exists. Despite being an established treatment modality, current indications of ALIF are yet to be clearly defined in the literature. This article discusses the current literature on indications on ALIF surgery.
Keywords: Anterior lumbar interbody fusion, Indications
Introduction
Anterior lumbar interbody fusion (ALIF) has become a widely accepted surgical technique for various degenerative pathologies of the lumbar spine. Spinal fusion has evolved significantly since the first successful internal fixation by Hadra in 1891 who used a posterior approach to wire adjacent cervical vertebrae in the treatment of fracture‐dislocation1, 2, 3, 4. The use of autologous bone grafts began with Hibbs in 1910 when he used fragments of spinous processes and lamina to perform bony ankylosis in patients with Pott's disease (extra‐pulmonary tuberculosis) of the spine1, 5, 6, 7. It was soon realised that immobilisation of the spine was the key to successful fusion and this marked the start of the hardware age featuring wiring techniques, facet and pedicle screws, and Harrington rods8. In 1932, Capener was the first to describe ALIF in the treatment of spondylolisthesis5, 9, 10. Since that time little progress was made with the ALIF technique until the 1980s. After 1980, several advancements were made to reduce morbidity including bone grafting substitutes, metallic hardware instrumentation, improved surgical technique and superior lighting and retraction2, 11, 12, 13, 14. However, the controversy regarding which surgical approach is best for treating various pathologies of the lumbar spine still exists3, 15, 16, 17, 18, 19. Despite being an established treatment option, current indications of ALIF are yet to be clearly defined in the literature20. Note should be made that the ALIF technique is usually followed by some form of internal fixation, such as integral fixation within the ALIF implant, anterior plate fixation or posterior fixation such as pedicle screws. The literature is not been specific enough in terms of distinction of the additional fixation and therefore this article relates to ALIF as the primary procedure, with or without additional bone fixation.
Rationale
The indications for ALIF surgery depend largely on the surgeon and his/her comfort with the approach and procedure, and varies with the pathology from patient to patient3, 5. Early in its history, ALIF was avoided due to difficult technical elements required in the surgery and approach based complications were considered too high a risk for the potential benefits3, 21, 22, 23. Progress in recent times with instrumentation, retraction and approach issues has led to ALIF's status as a mainstay of spinal surgery particularly in degenerative pathologies as it restores biomechanical and structural integrity3, 5, 21, 24, 25, 26, 27, 28, 29.
There are several advantages of the anterior approach to the lumbar spine. First and foremost, there is direct visualization and efficient access to the anterior column allowing for an easy and complete discectomy, better distraction increasing the neuroforaminal volume and the placement of a large interbody fusion device2, 3, 21, 23, 25, 26, 30, 31, 32, 33. All these benefits contribute to higher fusion rates and potential positive clinical outcomes5, 25, 30. Furthermore, ALIF restores lumbar lordosis, reduces anterior listhesis with distraction, and achieves coronal and sagittal balance2, 3, 31. Additionally, this alleviates pain particularly because loss of physiological lordosis and sagittal imbalance is a potential cause of pain in the lumbar spine23, 34.
Reduced blood loss, short operative times and lack of blood transfusion are other advantages with ALIF compared to other approaches2, 21, 25. Burke reported an average blood loss of 200–300 mL during a single level surgery. ALIF has a relatively short operating time compared with posterior fusion surgery, and studies show that it can take 90 minutes or less2, 8, 21. Moreover, several studies show a reduced perioperative morbidity compared to other approaches resulting in shorter hospital stay and bed rest25, 30.
In a normal lumbar spine in the upright standing position, the anterior and middle weight‐bearing columns of the spine support approximately 80% of spinal loads while the posterior column only supports 20%23, 31, 35, 36. However, with aging and the consequences of the degenerative cascade including dehydration of the nucleus and repetitive annular injuries reducing the height of the disc, the weight bearing shifts such that the posterior column supports a greater percentage of the axial load (Fig. 1). With ALIF, the interbody fusion device is utilized to redistribute the weight‐bearing to the original ratio. Furthermore, according to Woolf's Law, fusion potential increases if grafts are placed under direct compression which supports placement of the graft in the anterior column. Additionally, the anterior and middle columns provide 90% of the osseous surface area containing more vascularity than the posterolateral space and this wide cancellous bed for graft contact enhances the fusion potential13, 31. This also facilitates placing a larger interbody device that contacts the apophyseal ring and increases the segmental lordosis13. While Chow et al. found “no relation between bony fusion and symptomatic relief”, other studies show that there is generally correlation between successful fusion rate and good clinical outcomes37. However, it is possible to achieve a reasonable clinical outcome in cases of non‐union and this is attributed to indirect nerve decompression and reduction of frontal or sagittal plane deformities38, 39.
Figure 1.
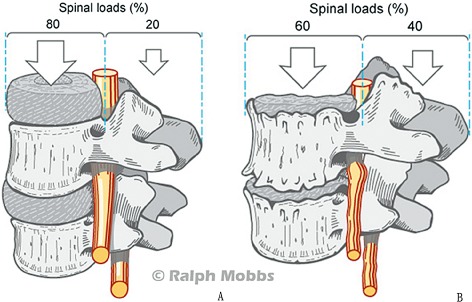
(A) Distribution of spinal loads on the anterior and posterior weight‐bearing columns in a normal lumbar spine. (B) Shifting of spinal loads to the posterior column after degenerative pathology to the lumbar spine.
Compared to posterior lumbar interbody fusion (PLIF) or the transforaminal route (TLIF), the retroperitoneal approach in ALIF spares iatrogenic trauma to the paraspinal musculature, posterior spinal nerves and posterior bony elements25, 30, 31, 40. The lateral interbody technique (LLIF) has similar advantages25, however, it is out of the scope of this article. Another advantage over the posterior approach is that nerve root retraction and entrance into the spinal canal is unnecessary and thus avoids epidural scarring and perineural fibrosis2, 5, 41, 42, 43. Furthermore, there is decreased morbidity from pulmonary complications with regard to other approaches30.
The spine is usually a versatile structure taking part in a range of movements but it has been established that the key to any bony fusion is the successful immobilization of a joint2. Stabilizing the spine by fixation and induction of osteoblastic activity to form new bony trabeculae reduces pain, corrects deformity and improves arthrodesis11. Some reports show that pseudoarthrosis and non‐union are a possible complication of stand‐alone ALIF surgery and hence supplementary posterior instrumentation achieving circumferential fusion should be utilized21, 31, 41. Instead of two staged operation involving poster lateral instrumentation, which requires additional time, expense and morbidity, evidence shows that using a minimally invasive percutaneous pedicle screw fixation maintains fusion rates but has less perioperative morbidity5, 25, 26, 31, 35, 44, 45, 46, 47, 48. Pedicle screws allow for strong fixation from the posterior elements to the anterior column allowing the spine to withstand correction forces49, 50, 51.
There are many variations on ALIF surgery including bone grafting and implant/instrument options. There have been numerous publications reviewing the graft options for anterior cervical fusion with relatively fewer papers on ALIF graft options such as autografts, allografts, bone morphogenic proteins (BMP) and other bone graft substitutes52, 53, 54, 55, 56, 57. Moreover, the ALIF interbody cages have different instrumentation namely ALIF with posterior instrumentation, ALIF with anterior plate and ALIF interbody device with internal fixation (Fig. 2).
Figure 2.
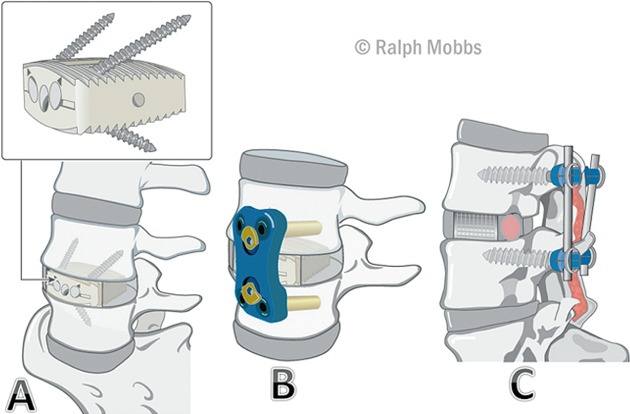
(A) ALIF interbody device with integral fixation. (B) ALIF implant with anterior plate fixation. (C) ALIF implant with posterior instrumentation.
While the long‐term success rates of ALIF have made it a viable option for many indications, there have been complications. These complications can be considered as approach based or spine specific58. With the anterior approach, mobilization of the great blood vessels and peritoneal contents, and exposure of the superior hypogastric sympathetic plexus place them at risk of iatrogenic injury13. The literature reports a host of approach based complications but the most common are retrograde ejaculation, vascular injury, superficial infection, urological injury and abdominal muscle damage59, 60. Retrograde ejaculation and sterility has been reported in many studies due to injury of the superior hypogastric sympathetic nerve plexus particularly when operating at L4/L5 level2, 3, 5, 21, 30, 31, 61, 62, 63, 64. Vascular injury is more common when operating at L4/L5 level due to the anatomy of the iliac vessels and iliolumbar vein13, 65. Spine specific complications include implant migration, graft collapse and expulsion and pseudoarthrosis2, 3, 5, 21, 23, 30, 31, 61.
Indications
The ideal candidate for ALIF has chronic, disabling back pain of discogenic origin for 1 or 2 levels with loss of height, stability and mobility of the diseased segment or neurological deficit2, 5, 25, 66. All conservative, medical approaches must be exhausted and pain is refractory to these methods2, 25, 67. Patient selection is crucial for successful outcomes and they must not have contraindicating factors such as osteoporosis or infection68. ALIF is now a common procedure but its indications are controversial and confusing3. There have been numerous uses of ALIF in the past69, 70. The following indications are the most commonly discussed in the literature.
Spondylolisthesis
Spondylolisthesis can be classified as isthmic, degenerative, dysplastic or traumatic. The literature illustrates that ALIF has been employed widely in two of these conditions, isthmic and degenerative spondylolisthesis, with good outcomes71 (Fig. 3).
Figure 3.
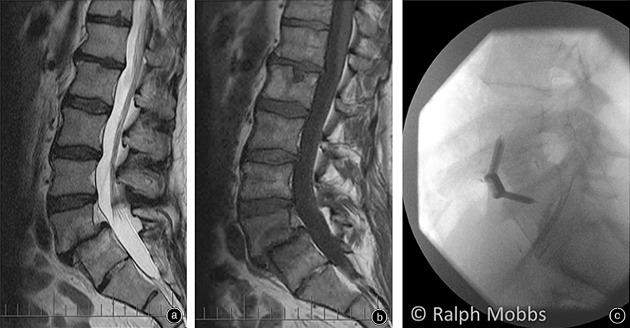
(A) T2 MRI of a L5S1 spondylolisthesis. (B) T1 MRI of a L5S1 spondylolisthesis. (C) X‐ray after ALIF surgery with internal fixation and interbody device.
Isthmic spondylolisthesis occurs when there is a fracture to the pars interarticularis resulting in a forward listhesis72. If conservative measures are ineffective to treat the symptoms including lower back and radiating pain, neurologic dysfunction and abnormal posture and gait, ALIF is an effective long term treatment option as it provides slip reduction and a biomechanical solution to the anterior translational instability39, 73. Radical discectomy and restoration of intervertebral height maintained by an interbody graft achieve indirect foraminal decompression39, 74. Non‐union has been reported, resulting in residual lower back pain. Despite this, the literature shows that ALIF is a long term solution to radicular symptoms such as leg pain, reduced walking ability, and neurological disturbances75.
Ishihara et al. recommend the use of posterior instrumentation as there are higher long term fusion rates39. Additionally, Kim and Lee expressed the importance of postoperative immobilisation76. According to Kim et al., these are the two most important factors to improve radiological and clinical outcomes77.
Degenerative spondylolisthesis (DS) was noted by Newman in 1933 as the slippage of the vertebrae with an intact neural arch resulting from arthritic degeneration of the lumbar facet joints78, 79. Most commonly seen in women at the L4–5 disc level, characteristic symptoms include lower back pain, leg pain and intermittent claudication. Pain in degenerative spondylolisthesis is produced by three different mechanisms. Listhesis causing concomitant spinal stenosis compounded by hypertrophy of the ligamentum flavum and osteophytes from facet arthritis encroaching into the spinal canal manifests as neurogenic claudication. Radicular pain occurs due to compression of the nerve root in the lateral recess or foramen. Mechanical low back pain is generated from degenerative intervertebral discs or arthritic facet joints. Apart from chronic pain, the other symptoms that warrant surgery include progressive neurological deficit and bladder or bowel dysfunction78.
ALIF is indicated as it stabilises the spinal column, restores disc height indirectly decompressing nerve roots, removes the pain‐generating intervertebral disc and posterior instrumentation corrects listhesis or kyphosis. Satomi et al. showed that ALIF had higher fusion rates, better clinical outcomes and less neurological deficits like dysesthesia and dysuria in Grade 1 and 2 DS80.
Takahashi et al. in a study of 39 patients who underwent ALIF surgery for degenerative spondylolisthesis had a 76% clinical success rate and recommend that better long‐term outcomes are experienced in patients under the age of 65 years71. The literature shows that fusion for spondylolisthesis is a better option than stand‐alone decompression and indication for instrumentation remains controversial as it has a higher complication rate compared with graft only, but also a higher long term fusion rate78, 81, 82. Several studies support ALIF for both isthmic and degenerative spondylolisthesis with clinical success rates between 72% and 94%25, 26, 39, 76, 80, 83, 84 (Table 1).
Table 1.
Summary of clinical studies with spondylolisthesis as the indication for ALIF 25, 26, 29, 39, 71, 76, 80, 83, 84, 85
| Author | Study type | Indication | Surgery | Number of patients | Fusion rates (%) | Clinical success rate (%) | Serious complication rate (%) | Comments |
|---|---|---|---|---|---|---|---|---|
| Takahashi et al., 1990 | Retrospective study | Degenerative spondylolisthesis | ALIF | 39 | 90 | 76 | 3 | |
| Satomi et al., 1992 | Retrospective study | Degenerative spondylolisthesis | ALIF | 27 | 96 | 93 | 4 | |
| Muschik et al., 1997 | Retrospective study | Isthmic spondylolisthesis | ALIF | 29 | 76 | 69 | 7 | Isthmic spondylolisthesis in children and adolescents aged under 19 years |
| Muschik et al., 1997 | Retrospective study | Isthmic spondylolisthesis | ALIF + PSF | 30 | 93 | 83 | 13 | Isthmic spondylolisthesis in children and adolescents aged under 19 years |
| Kim and Lee, 1999 | Retrospective study | Isthmic spondylolisthesis | ALIF | 20 | 90 | 85 | 25 | |
| Ishihara et al., 2001 | Retrospective study | Isthmic spondylolisthesis | ALIF | 35 | 83 | — | — | 12 dropped out of this study |
| Johnson et al., 1988 | Retrospective study | Isthmic spondylolisthesis | ALIF +(−) PSF | 44 | 96 | 96 | 11 | Some cases included instrumentation with either posterior or Fibular interbody strut and H‐rods |
| Christensen et al., 1996 | Retrospective study | Isthmic spondylolisthesis | ALIF | 57 | 47 | 76 | 7 | |
| Lee et al., 2004 | Retrospective study | Isthmic spondylolisthesis | ALIF + PSF | 73 | 97 | 94 | 16 | |
| Shim et al., 2011 | Retrospective study | Isthmic spondylolisthesis | ALIF + PSF/PLF | 49 | 84 | 88 | 4 | |
| Kim et al., 2010 | Retrospective study | Isthmic spondylolisthesis | ALIF + PSF | 63 | 100 | 89 | — | Low‐grade adult spondylolisthesis |
| Suk et al., 2001 | Retrospective study | Spondylolisthesis | ALIF + PSF | 21 | 100 | — | 14 | |
| Min et al., 2007 | Retrospective study | Spondylolisthesis | ALIF | 25 | 100 | 92 | 16 |
ALIF, anterior lumbar interbody fusion; PLF, posterolateral fusion; PSF, pedicle screw fixation.
Degenerative Disc Disease
Degenerative disc disease (DDD) is a potentially painful condition that causes mechanical, chronic lower back pain and in a sizeable minority can limit function significantly13. DDD may be accompanied by foraminal stenosis due to loss of disc height, compressing the nerve root causing radiculopathy. DDD with mechanical pain is a different indication to cases with foraminal stenosis. In the literature, however, these two indications are often grouped under DDD.
In DDD with mechanical pain, the disc is considered the primary pain generator and therefore, surgical intervention is targeted at removing the intervertebral disc and restoring the structural and biomechanical integrity of the spinal column3, 86. Delamination and degeneration of the disc, and posterior annular fissuring are causes of mechanical pain due to the mechanical loading to these areas resulting in sensitization of annular receptors87. During the degenerative cascade, neovascularization, neuronal penetration with unmyelinated nerve fibres and in‐growth of Schwann cells occur and this neo‐innervation is a potential pain generator86. Therefore, removing the vertebral disc is essential in pain reduction and implanting the interbody device restores segmental stabilization and corrects abnormal loading13.
DDD with foraminal stenosis presents differently as the patient experiences an element of mechanical pain but the overriding issue is radiculopathy caused by nerve root compression35, 88, 89. Generally, segmental stenosis and radiculopathy is a result of disc herniation, posterior osteophyte formation, facets overriding and hypertrophy and infolding of the ligamentum flavum combining to reduce neuroforaminal volume90 (Fig. 4).
Figure 4.
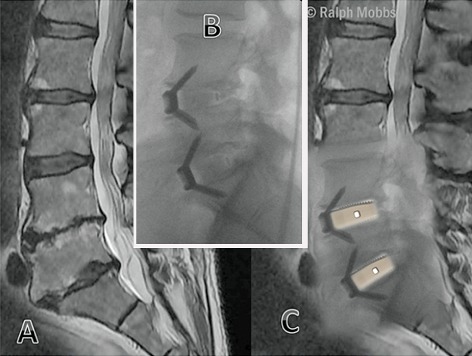
(A) MRI showing degenerative disc disease with foraminal stenosis at the L 4–5 and L5S1 levels prior to ALIF surgery. (B) Post‐operative X‐ray with anterior interbody fusion at L 4–5 and L5S1. (C) Onlay of MRI‐X‐ray showing the interbody device.
Burkus et al. have completed the largest prospective study on DDD with 279 patients investigated after ALIF treatment. Clinical outcomes were based on comparing preoperative and postoperative Oswestry disability index (ODI) scores, neurological function, back and leg pain. All these measurements indicated an overall 81% clinical success and the study had a complication rate of just 9%41. Several other studies of various sizes have produced similar results indicating high clinical success rates ranging from 71%–100%21, 23, 28, 91, 92, 93, 94, 95, 96, 97 (Table 2).
Table 2.
Summary of clinical studies with degenerative disc disease as the indication for ALIF 21, 23, 28, 41, 91, 92, 93, 94, 95, 96, 97
| Author | Study type | Surgery | Number of patients | Fusion rates (%) | Clinical success rate (%) | Serious complication rate (%) | Comments |
|---|---|---|---|---|---|---|---|
| Newmen and Grinstead, 1990 | Prospective study | ALIF | 36 | 89 | 86 | 11 | |
| Blumenthal et al., 1987 | Prospective study | ALIF | 34 | 73 | 74 | 3 | |
| Christensen et al., 1996 | Retrospective study | ALIF | 63 | 58 | 76 | 7 | |
| Boden et al., 2000 | Prospective randomized controlled trial | ALIF | 14 | 93 | 86 | NR | |
| Burkus et al., 2002 | Prospective, non‐blinded study | ALIF | 46 | 83 | 73 | 11 | |
| Burkus et al., 2002 | Prospective, randomized, non‐blinded | ALIF | 279 | 92 | 81 | 9 | |
| Kleeman et al., 2001 | Prospective, controlled, non‐randomized | ALIF | 22 | 100 | 100 | 0 | |
| Sasso et al., 2004 | Prospective, randomized, controlled | ALIF | 140 | 77 | |||
| clinical trial | |||||||
| Strube et al., 2011 | Prospective cohort study | ALIF | 40 | 71 | 91 | 6 | 6 patients dropped out |
| Moore et al., 2002 | Retrospective study | ALIF + PLF | 58 | 95 | 86 | 5 | |
| Matge & Leclercq, 2000 | Retrospective study | ALIF | 222 | 96 | 80 | 10 | |
| Pavlov et al., 2004 | Prospective study | ALIF | 58 | 99 | 98 | 10 |
ALIF, anterior lumbar interbody fusion; NR, no record; PLF, posterolateral fusion.
Degenerative Lumbar Scoliosis
Degenerative lumbar scoliosis (DLS) is a deformity occurring after skeletal maturation causing abnormal spinal curvature and surgical correction is considered challenging98, 99. Benefits of supporting the anterior column of the spine include increased stability, better fusion rates and restoration of normal lumbar lordosis100, 101, 102. ALIF is considered a reliable option because it allows for thorough release of contracted tissue and osteophytes, complete discectomy and distraction of the intervertebral space and placement of a larger interbody fusion device103 (Fig. 5). All these factors contribute to strong anterior structural support. Moreover, an anterior approach gives increased attention to the sagittal plane enhancing segmental stability and yielding better long‐term results with low complication rates compared to posterior and transforaminal interbody fusions104. The literature shows that when supplemented with posterior instrumentation, ALIF may be a reliable surgical option because it provides anterior structural support, corrects deformity and restores lordosis103, 105.
Figure 5.
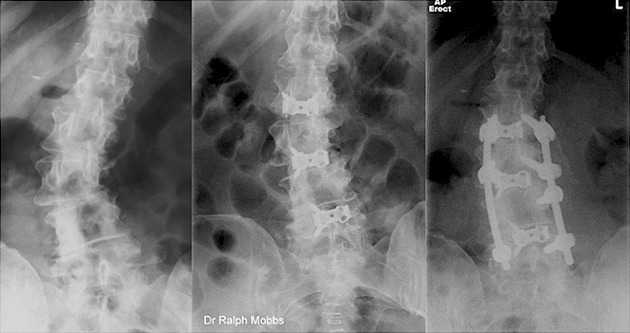
Degenerative lumbar scoliosis managed with ALIF and percutaneous pedicle screw fixation.
Pateder et al. had the largest study in the literature where 75 patients were retrospectively analyzed after receiving ALIF surgery with pedicle screw fixation for DLS. Due to the anterior thoracoabdominal approach with manipulation of major vessels and additional posterior approach, the complication rate was 24% with same day operation and 45% in anterior‐posterior staged surgery. The correction of deformity was high and clinical outcomes correlated with the fusion rate which was 88%49. Crandall and Revella retrospectively studied 20 cases of DLS patients with similar results103 (Table 3).
Table 3.
| Author | Study type | Surgery | Number of patients | Fusion rates (%) | Serious complication rate (%) | Comments |
|---|---|---|---|---|---|---|
| Crandell and Revella, 2009 | Prospective, nonrandomized consecutive single surgeon series | ALIF + Posterior instrumentation | 20 | 80 | 40 | Average ODI and VAS scores improved |
| Pateder et al., 2007 | Retrospective study. | ALIF + posterior instrumentation | 75 | 88 | 24–45 | Same day operation had lower complication to staged surgery. |
ALIF, anterior lumbar interbody fusion; ODI, Oswestry disability index; VAS, visual analog scale.
Pseudoarthrosis
Pseudoarthrosis occurs when there is a failure of union in a previous spinal fusion and in many cases, revision surgery is considered inappropriate. However, in cases of chronic pain non‐responsive to conservative management, surgical intervention is necessary106, 107, 108. There are four types of pseudoarthrosis as described by Heggeness and Esses: atrophic, transverse, shingle, and complex109. The most common form is transverse where there is remodeled bone but horizontal discontinuity108. The pain is partly attributed to the sclerotic bone adjacent to fibrous soft tissue accompanied by microfractures of cancellous bone and motion of the segment110. Diagnosis of pseudoarthrosis is difficult and requires a significant amount of time after the fusion. Bony union is defined as absence of motion between the previously mobile segments due to bridging of bony trabeculae and this is difficult to assess108.
Once conservative measures are unsuccessful and a surgical option is preferred, the aims for achieving fusion include correcting technical errors, better graft material, enhancing the biological environment for fusion and improving the biomechanical environment106, 107. ALIF is a revision surgery option indicated mostly when other approaches produced the failed fusion108, 111. If ALIF is used as the salvage procedure for a previous anterior approach, it is crucial to dissect through virgin tissue and review previous operations to see which segmental vessels were ligated112. High fusion rates can be achieved because of high bony surface area in the anterior column, excellent vascularity of well exposed end plates, cancellous bone and compression loading of the grafts108. The best outcomes are achieved with supplementary posterior instrumentation as it provides the maximum stability108 (Fig. 6).
Figure 6.
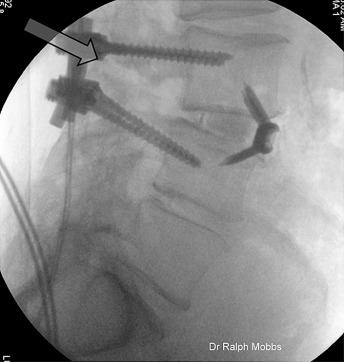
Lateral X‐ray lumbar spine demonstrating an L 3,4 ALIF, performed 18 months following posterior fusion with non‐union of the posterior elements (see arrow).
The literature shows that ALIF has been performed to correct previous failed fusions, particularly from posterior approaches. However, the overall number of cases where ALIF has been indicated for pseudoarthrosis is low. The majority of studies in the literature using other approaches show that it is costly and difficult to perform a revision procedure for pseudoarthrosis and the outcomes vary widely108. Butterman et al. had a study in the literature where 38 patients were retrospectively analyzed after receiving ALIF and posterolateral fusion surgery for pseudoarthrosis. The fusion rate was 95% and the surgical complications rate was high (64%)113.
Adjacent Segment Disease (ASD)
ASD occurs when there is degeneration to the vertebral disc directly above or below a fused spinal segment because of hypermobility and increased biomechanical stress and is considered a long‐term complication of spinal arthrodesis114, 115. Common findings include disc degeneration, disc herniation, pseudoarthrosis, faced degeneration, hypertrophic changes, lateral recess stenosis, spinal stenosis, foraminal stenosis and instability116. Surgery is uncommon but is considered when medical treatment fails to adequately manage back pain and radicular leg pain116. Biomechanical studies show that the previous lumbar fusion often increases motion and intradiscal pressure leading to ASD114. ALIF is considered a revision surgery in this context to achieve sagittal or coronal realignment, normal lordotic curvature and relieve pain117. There is a lack of clinical studies that use ALIF as the stand‐alone treatment option for ASD85.
Other Indications
The rationale of ALIF surgery demonstrates that it is a viable option in cases of instability of the lumbar spine and cases of chronic lower back pain. Hence, there are several other potential indications in the literature including Pott's disease of the spine, fracture, dislocation, trauma causing internal disc disruption, recurrent lumbar disc herniation, post‐discectomy collapse, coronal and/or sagittal plane deformity, instability after laminectomy or posterior decompression, spinal osteotomy, kyphosis and after spinal tumor resection1, 22, 23, 30, 31, 118. However, the data on these specific indications was minimal based on current literature reviews.
Conclusion
Overall, it is evident that spinal fusions have evolved dramatically over the past century and ALIF has experienced several advances particularly in the last decade. The rationale underlying ALIF surgery is theoretically sound and it appears to be a viable option in several degenerative pathologies of the lumbar spine. Indications vary depending on the surgeon and the patient but generally cases of instability, chronic pain and deformity of the lumbar spine are potential candidates for ALIF. While independent studies have been conducted for some specific indications, there is yet to be a clinical study relating operative outcomes against the variety of different indications for ALIF surgery.
Disclosure: No financial support was obtained for this work.
References
- 1. Howorth M. Evolution of spinal fusion. Ann Surg, 1943, 117: 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burke PJ. Anterior lumbar interbody fusion. Radiol Technol, 2001, 72: 423–430. [PubMed] [Google Scholar]
- 3. Pradhan BB, Nassar JA, Delamarter RB, Wang JC. Single‐level lumbar spine fusion: a comparison of anterior and posterior approaches. J Spinal Disord Tech, 2002, 15: 355–361. [DOI] [PubMed] [Google Scholar]
- 4. van Akkerveeken PF. Anterior lumbar interbody fusion. Acta Orthop Scand Suppl, 1993, 251: 105–107. [DOI] [PubMed] [Google Scholar]
- 5. Shen FH, Samartzis D, Khanna AJ, Anderson DG. Minimally invasive techniques for lumbar interbody fusions. Orthop Clin North Am, 2007, 38: 373–386. [DOI] [PubMed] [Google Scholar]
- 6. Albee FH. Transplantation of a portion of the tibia into the spine for Pott's disease: a preliminary report 1911. Clin Orthop Relat Res, 2007, 460: 14–16. [DOI] [PubMed] [Google Scholar]
- 7. Crock HV. Anterior lumbar interbody fusion: indications for its use and notes on surgical technique. Clin Orthop Relat Res, 1982, 165: 157–163. [PubMed] [Google Scholar]
- 8. Ray CD. Spinal interbody fusions: a review, featuring new generation techniques. Neurosurg Q, 1997, 7: 135–142. [Google Scholar]
- 9. Capener N. Spondylolisthesis. Br J Surg, 1932, 19: 374–386. [Google Scholar]
- 10. Holte D, O'Brien J, Renton P. Anterior lumbar fusion using a hybrid interbody graft. Eur Spine J, 1994, 3: 32–38. [DOI] [PubMed] [Google Scholar]
- 11. Lipson SJ. Spinal‐fusion surgery—advances and concerns. N Engl J Med, 2004, 350: 643–644. [DOI] [PubMed] [Google Scholar]
- 12. Fang H, Ong G, Hodgson A. Anterior spinal fusion. Clin Orthop Relat Res, 1964, 35: 16–25. [PubMed] [Google Scholar]
- 13. Truumees E, Majid K, Brkaric M. Anterior lumbar interbody fusion in the treatment of mechanical low back pain. Semin Spine Surg, 2008, 20: 113–125. [Google Scholar]
- 14. Werlinich M. Anterior interbody fusion and stabilization with metal fixation. Int Surg, 1974, 59: 269–273. [PubMed] [Google Scholar]
- 15. Stauffer RN, Coventry MB. Anterior interbody lumbar spine fusion analysis of Mayo Clinic series. J Bone Joint Surg Am, 1972, 54: 756–768. [PubMed] [Google Scholar]
- 16. Flynn J, Hoque M. Anterior fusion of the lumbar spine. End‐result study with long‐term follow‐up. J Bone Joint Surg (Am), 1979, 61: 1143–1150. [PubMed] [Google Scholar]
- 17. Gibson JN, Waddell G. Surgery for degenerative lumbar spondylosis. Cochrane Database Syst Rev, 2005, (4): CD001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turner JA, Herron L, Deyo RA. Meta‐analysis of the results of lumbar spine fusion. Acta Orthop Scand Suppl, 1993, 251: 120–122. [DOI] [PubMed] [Google Scholar]
- 19. Hacker RJ. Comparison of interbody fusion approaches for disabling low back pain. Spine (Phila Pa 1976), 1997, 22: 660–665. [DOI] [PubMed] [Google Scholar]
- 20. Calandruccio R, Benton B. Anterior lumbar fusion. Clin Orthop Relat Res, 1964, 35: 63–68. [PubMed] [Google Scholar]
- 21. Strube P, Hoff E, Hartwig T, Perka CF, Gross C, Putzier M. Stand‐alone anterior versus anteroposterior lumbar interbody single‐level fusion after a mean follow‐up of 41 months. J Spinal Disord Tech, 2012, 25: 362–369. [DOI] [PubMed] [Google Scholar]
- 22. Mayer HM. The ALIF concept. Eur Spine J, 2000, 9 (Suppl. 1): S35–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matgé G, Leclercq TA. Rationale for interbody fusion with threaded titanium cages at cervical and lumbar levels. Results on 357 cases. Acta Neurochir (Wien), 2000, 142: 425–433. [DOI] [PubMed] [Google Scholar]
- 24. Lee CS, Hwang CJ, Lee DH, Kim YT, Lee HS. Fusion rates of instrumented lumbar spinal arthrodesis according to surgical approach: a systematic review of randomized trials. Clin Orthop Surg, 2011, 3: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim JS, Kim DH, Lee SH, et al Comparison study of the instrumented circumferential fusion with instrumented anterior lumbar interbody fusion as a surgical procedure for adult low‐grade isthmic spondylolisthesis. World Neurosurg, 2010, 73: 565–571. [DOI] [PubMed] [Google Scholar]
- 26. Shim JH, Kim WS, Kim JH, Kim DH, Hwang JH, Park CK. Comparison of instrumented posterolateral fusion versus percutaneous pedicle screw fixation combined with anterior lumbar interbody fusion in elderly patients with L5‐S1 isthmic spondylolisthesis and foraminal stenosis. J Neurosurg Spine, 2011, 15: 311–319. [DOI] [PubMed] [Google Scholar]
- 27. Sørensen KH. Anterior interbody lumbar spine fusion for incapacitating disc degeneration and spondylolisthesis. Acta Orthop Scand, 1978, 49: 269–277. [DOI] [PubMed] [Google Scholar]
- 28. Sasso RC, Kitchel SH, Dawson EG. A prospective, randomized controlled clinical trial of anterior lumbar interbody fusion using a titanium cylindrical threaded fusion device. Spine (Phila Pa 1976), 2004, 29: 113–122. [DOI] [PubMed] [Google Scholar]
- 29. Johnson LP, Nasca RJ, Dunham WK. Surgical management of isthmic spondylolisthesis. Spine(Phila Pa 1976), 1988, 13: 93–97. [DOI] [PubMed] [Google Scholar]
- 30. Gumbs AA, Bloom ND, Bitan FD, Hanan SH. Open anterior approaches for lumbar spine procedures. Am J Surg, 2007, 194: 98–102. [DOI] [PubMed] [Google Scholar]
- 31. Mummaneni PV, Haid RW, Rodts GE. Lumbar interbody fusion: state‐of‐the‐art technical advances. J Neurosurg Spine, 2004, 1: 24–30. [DOI] [PubMed] [Google Scholar]
- 32. Chen D, Fay LA, Lok J, Yuan P, Edwards WT, Yuan HA. Increasing neuroforaminal volume by anterior interbody distraction in degenerative lumbar spine. Spine (Phila Pa 1976), 1995, 20: 74–79. [DOI] [PubMed] [Google Scholar]
- 33. Dennis S, Watkins R, Landaker S, Dillin W, Springer D. Comparison of disc space heights after anterior lumbar interbody fusion. Spine(Phila Pa 1976), 1989, 14: 876–878. [DOI] [PubMed] [Google Scholar]
- 34. Fritzell P, Hägg O, Wessberg P, Nordwall A, Swedish Lumbar Spine Study Group . Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group. Spine (Phila Pa 1976), 2002, 27: 1131–1141. [DOI] [PubMed] [Google Scholar]
- 35. Wang JC, Mummaneni PV, Haid RW. Current treatment strategies for the painful lumbar motion segment: posterolateral fusion versus interbody fusion. Spine (Phila Pa 1976), 2005, 30 (16 Suppl.): S33–S43. [DOI] [PubMed] [Google Scholar]
- 36. Duggal N, Mendiondo I, Pares HR, et al Anterior lumbar interbody fusion for treatment of failed back surgery syndrome: an outcome analysis. Neurosurgery, 2004, 54: 636–643. [DOI] [PubMed] [Google Scholar]
- 37. Chow SP, Leong JC, Ma A, Yau AC. Anterior spinal fusion for deranged lumbar intervertebral disc: a review of 97 cases. Spine (Phila Pa 1976), 1980, 5: 452–458. [DOI] [PubMed] [Google Scholar]
- 38. Burkus JK, Schuler TC, Gornet MF, Zdeblick TA. Anterior lumbar interbody fusion for the management of chronic lower back pain: current strategies and concepts. Orthop Clin North Am, 2004, 35: 25–32. [DOI] [PubMed] [Google Scholar]
- 39. Ishihara H, Osada R, Kanamori M, et al Minimum 10‐year follow‐up study of anterior lumbar interbody fusion for isthmic spondylolisthesis. J Spinal Disord, 2001, 14: 91–99. [DOI] [PubMed] [Google Scholar]
- 40. Harmon PH. Anterior excision and vertebral body fusion operation for intervertebral disk syndromes of the lower lumbar spine: three‐to five‐year results in 244 cases. Clin Orthop Relat Res, 1963, 26: 107–127. [PubMed] [Google Scholar]
- 41. Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP‐2 with tapered interbody cages. J Spinal Disord Tech, 2002, 15: 337–349. [DOI] [PubMed] [Google Scholar]
- 42. Cheng C, Fang D, Lee P, Leong J. Anterior spinal fusion for spondylolysis and isthmic spondylolisthesis. J Bone Joint Surg Br, 1989, 71: 264–267. [DOI] [PubMed] [Google Scholar]
- 43. Chung SK, Lee SH, Lim SR, et al Comparative study of laparoscopic L5–S1 fusion versus open mini‐ALIF, with a minimum 2‐year follow‐up. Eur Spine J, 2003, 12: 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Greenough C, Taylor L, Fraser R. Anterior lumbar fusion: results, assessment techniques and prognostic factors. Eur Spine J, 1994, 3: 225–230. [DOI] [PubMed] [Google Scholar]
- 45. Schwarzenbach O. Hybrid stabilization with ALIF L5/S1 and total disc replacement L4/L5. Eur Spine J, 2009, 18: 1995–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Beutler WJ, Peppelman WC Jr. Anterior lumbar fusion with paired BAK standard and paired BAK Proximity cages: subsidence incidence, subsidence factors, and clinical outcome. Spine J, 2003, 3: 289–293. [DOI] [PubMed] [Google Scholar]
- 47. Schofferman J, Slosar P, Reynolds J, Goldthwaite N, Koestler M. A prospective randomized comparison of 270 degrees fusions to 360 degrees fusions (circumferential fusions). Spine (Phila Pa 1976), 2001, 26: E207–E212. [DOI] [PubMed] [Google Scholar]
- 48. Christensen FB, Hansen ES, Eiskjaer SP, et al Circumferential lumbar spinal fusion with Brantigan cage versus posterolateral fusion with titanium Cotrel‐Dubousset instrumentation: a prospective, randomized clinical study of 146 patients. Spine (Phila Pa 1976), 2002, 27: 2674–2683. [DOI] [PubMed] [Google Scholar]
- 49. Pateder DB, Kebaish KM, Cascio BM, Neubaeur P, Matusz DM, Kostuik JP. Posterior only versus combined anterior and posterior approaches to lumbar scoliosis in adults: a radiographic analysis. Spine (Phila Pa 1976), 2007, 32: 1551–1554. [DOI] [PubMed] [Google Scholar]
- 50. Foley KT, Gupta SK. Percutaneous pedicle screw fixation of the lumbar spine: preliminary clinical results. J Neurosurg, 2002, 97 (1 Suppl.): 7–12. [DOI] [PubMed] [Google Scholar]
- 51. Madan S, Boeree N. Comparison of instrumented anterior interbody fusion with instrumented circumferential lumbar fusion. Eur Spine J, 2003, 12: 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kozak JA, Heilman AE, O'Brien JP. Anterior lumbar fusion options. Technique and graft materials. Clin Orthop Relat Res, 1994, 300: 45–51. [PubMed] [Google Scholar]
- 53. Wimmer C, Krismer M, Gluch H, Ogon M, Stöckl B. Autogenic versus allogenic bone grafts in anterior lumbar interbody fusion. Clin Orthop Relat Res, 1999, 360: 122–126. [DOI] [PubMed] [Google Scholar]
- 54. Kuslich SD, Danielson G, Dowdle JD, et al Four‐year follow‐up results of lumbar spine arthrodesis using the Bagby and Kuslich lumbar fusion cage. Spine(Phila Pa 1976), 2000, 25: 2656–2662. [DOI] [PubMed] [Google Scholar]
- 55. Kuslich SD, Ulstrom CL, Griffith SL, Ahern JW, Dowdle JD. The Bagby and Kuslich method of lumbar interbody fusion: history, techniques, and 2‐year follow‐up results of a United States prospective, multicenter trial. Spine(Phila Pa 1976), 1998, 23: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 56. Thalgott JS, Giuffre JM, Klezl Z, Timlin M. Anterior lumbar interbody fusion with titanium mesh cages, coralline hydroxyapatite, and demineralized bone matrix as part of a circumferential fusion. Spine J, 2002, 2: 63–69. [DOI] [PubMed] [Google Scholar]
- 57. Chau AM, Mobbs RJ. Bone graft substitutes in anterior cervical discectomy and fusion. Eur Spine J, 2009, 18: 449–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sasso RC, Best NM, Mummaneni PV, Reilly TM, Hussain SM. Analysis of operative complications in a series of 471 anterior lumbar interbody fusion procedures. Spine(Phila Pa 1976), 2005, 30: 670–674. [DOI] [PubMed] [Google Scholar]
- 59. Johnson RM, McGuire EJ. Urogenital complications of anterior approaches to the lumbar spine. Clin Orthop Relat Res, 1981, 154: 114–118. [PubMed] [Google Scholar]
- 60. Watkins R. Anterior lumbar interbody fusion surgical complications. Clin Orthop Relat Res, 1992, 284: 47–53. [PubMed] [Google Scholar]
- 61. Madhu TS. Posterior and anterior lumbar interbody fusion. Cur Orthop, 2008, 22: 406–413. [Google Scholar]
- 62. Loguidice VA, Johnson RG, Guyer RD, et al Anterior lumbar interbody fusion. Spine (Phila Pa 1976), 1988, 13: 366–369. [DOI] [PubMed] [Google Scholar]
- 63. Flynn JC, Price CT. Sexual complications of anterior fusion of the lumbar spine. Spine (Phila Pa 1976), 1984, 9: 489–492. [DOI] [PubMed] [Google Scholar]
- 64. Tiusanen H, Seitsalo S, Österman K, Soini J. Retrograde ejaculation after anterior interbody lumbar fusion. Eur Spine J, 1995, 4: 339–342. [DOI] [PubMed] [Google Scholar]
- 65. Rajaraman V, Vingan R, Roth P, Heary RF, Conklin L, Jacobs GB. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg, 1999, 91: 60–64. [DOI] [PubMed] [Google Scholar]
- 66. Mummaneni PV, Lin FJ, Haid RW Jr, Rodts GE Jr, Subach BR, Miller JS. Current indications and techniques for anterior approaches to the lumbar spine. Contemporary Spine Surgery, 2002, 3: 57–64. [Google Scholar]
- 67. Zdeblick TA. The treatment of degenerative lumbar disorders: a critical review of the literature. Spine (Phila Pa 1976), 1995, 20 (24 Suppl.): S126–S137. [PubMed] [Google Scholar]
- 68. An H, Boden SD, Kang J, Sandhu HS, Abdu W, Weinstein J. Summary statement: emerging techniques for treatment of degenerative lumbar disc disease. Spine (Phila Pa 1976), 2003, 28 (15 Suppl.): S24–S25. [DOI] [PubMed] [Google Scholar]
- 69. Tay BBK, Berven S. Indications, Techniques, and Complications of Lumbar Interbody Fusion. New York: Thieme‐Stratton Inc., 2002; 221–230. [DOI] [PubMed] [Google Scholar]
- 70. Zdeblick TA. A prospective, randomized study of lumbar fusion. Preliminary results. Spine (Phila Pa 1976), 1993, 18: 983–991. [DOI] [PubMed] [Google Scholar]
- 71. Takahashi K, Kitahara H, Yamagata M, et al Long‐term results of anterior interbody fusion for treatment of degenerative spondylolisthesis. Spine (Phila Pa 1976), 1990, 15: 1211–1215. [DOI] [PubMed] [Google Scholar]
- 72. Suk KS, Jeon CH, Park MS, Moon SH, Kim NH, Lee HM. Comparison between posterolateral fusion with pedicle screw fixation and anterior interbody fusion with pedicle screw fixation in adult spondylolytic spondylolisthesis. Yonsei Med J, 2001, 42: 316–323. [DOI] [PubMed] [Google Scholar]
- 73. Sacks S. Anterior interbody fusion of the lumbar spine. Indications and results in 200 cases. Clin Orthop Relat Res, 1966, 44: 163–170. [PubMed] [Google Scholar]
- 74. Fujimaki A, Crock HV, Bedbrook GM. The results of 150 anterior lumbar interbody fusion operations performed by two surgeons in Australia. Clin Orthop Relat Res, 1982, 165: 164–167. [PubMed] [Google Scholar]
- 75. Molinari RW, Bridwell KH, Lenke LG, Baldus C. Anterior column support in surgery for high‐grade, isthmic spondylolisthesis. Clin Orthop Relat Res, 2002, 394: 109–120. [DOI] [PubMed] [Google Scholar]
- 76. Kim NH, Lee JW. Anterior interbody fusion versus posterolateral fusion with transpedicular fixation for isthmic spondylolisthesis in adults: a comparison of clinical results. Spine (Phila Pa 1976), 1999, 24: 812–816. [DOI] [PubMed] [Google Scholar]
- 77. Kim SS, Denis F, Lonstein JE, Winter RB. Factors affecting fusion rate in adult spondylolisthesis. Spine (Phila Pa 1976), 1990, 15: 979–984. [DOI] [PubMed] [Google Scholar]
- 78. Sengupta DK, Herkowitz HN. Degenerative spondylolisthesis: review of current trends and controversies. Spine (Phila Pa 1976), 2005, 30 (6 Suppl.): S71–S81. [DOI] [PubMed] [Google Scholar]
- 79. Mardjetko S, Connolly P, Shott S. Degenerative lumbar spondylolisthesis: a meta‐analysis of literature 1970‐1993. Spine (Phila Pa 1976), 1994, 19 (20 Suppl.): S2256–S2265. [PubMed] [Google Scholar]
- 80. Satomi K, Hirabayashi K, Toyama Y, Fujimura Y. A clinical study of degenerative spondylolisthesis. Radiographic analysis and choice of treatment. Spine (Phila Pa 1976), 1992, 17: 1329–1336. [DOI] [PubMed] [Google Scholar]
- 81. Inoue S, Watanabe T, Goto S, Takahashi K, Takata K, Sho E. Degenerative spondylolisthesis pathophysiology and results of anterior interbody fusion. Clin Orthop Relat Res, 1988, 227: 90–98. [PubMed] [Google Scholar]
- 82. Weinstein JN, Lurie JD, Tosteson TD, et al Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med, 2007, 356: 2257–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Muschik M, Zippel H, Perka C. Surgical management of severe spondylolisthesis in children and adolescents: anterior fusion in situ versus anterior spondylodesis with posterior transpedicular instrumentation and reduction. Spine (Phila Pa 1976), 1997, 22: 2036–2042. [DOI] [PubMed] [Google Scholar]
- 84. Lee SH, Choi WG, Lim SR, Kang HY, Shin SW. Minimally invasive anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation for isthmic spondylolisthesis. Spine J, 2004, 4: 644–649. [DOI] [PubMed] [Google Scholar]
- 85. Min JH, Jang JS, Lee SH. Comparison of anterior‐and posterior‐approach instrumented lumbar interbody fusion for spondylolisthesis. J Neurosurg Spine, 2007, 7: 21–26. [DOI] [PubMed] [Google Scholar]
- 86. Andersson GB, Mekhail NA, Block JE. Treatment of intractable discogenic low back pain: a systematic review of spinal fusion and intradiscal electrothermal therapy (IDET). Pain Physician, 2006, 9: 237–248. [PubMed] [Google Scholar]
- 87. Osti OL, Vernon‐Roberts B, Fraser RD. Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine (Phila Pa 1976), 1990, 1990: 762–767. [DOI] [PubMed] [Google Scholar]
- 88. Jenis LG, An HS. Spine update: lumbar foraminal stenosis. Spine (Phila Pa 1976), 2000, 25: 389–394. [DOI] [PubMed] [Google Scholar]
- 89. Hallett A, Huntley JS, Gibson JN. Foraminal stenosis and single‐level degenerative disc disease: a randomized controlled trial comparing decompression with decompression and instrumented fusion. Spine (Phila Pa 1976), 2007, 32: 1375–1380. [DOI] [PubMed] [Google Scholar]
- 90. Kuslich S, Ulstrom C, Michael C. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am, 1991, 22: 181–187. [PubMed] [Google Scholar]
- 91. Newman MH, Grinstead GL. Anterior lumbar interbody fusion for internal disc disruption. Spine (Phila Pa 1976), 1992, 17: 831–833. [DOI] [PubMed] [Google Scholar]
- 92. Blumenthal SL, Baker J, Dossett A, Selby DK. The role of anterior lumbar fusion for internal disc disruption. Spine (Phila Pa 1976), 1988, 13: 566–569. [DOI] [PubMed] [Google Scholar]
- 93. Christensen F, Karlsmose B, Hansen E, Bünger C. Radiological and functional outcome after anterior lumbar interbody spinal fusion. Eur Spine J, 1996, 5: 293–298. [DOI] [PubMed] [Google Scholar]
- 94. Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP‐2 in interbody fusion cages: definitive evidence of osteoinduction in humans: a preliminary report. Spine (Phila Pa 1976), 2000, 25: 376–381. [DOI] [PubMed] [Google Scholar]
- 95. Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein‐2. Spine (Phila Pa 1976), 2002, 27: 2396–2408. [DOI] [PubMed] [Google Scholar]
- 96. Kleeman TJ, Michael Ahn U, Talbot‐Kleeman A. Laparoscopic anterior lumbar interbody fusion at L4‐L5: an anatomic evaluation and approach classification. Spine (Phila Pa 1976), 2002, 27: 1390–1395. [DOI] [PubMed] [Google Scholar]
- 97. Moore KR, Pinto MR, Butler LM. Degenerative disc disease treated with combined anterior and posterior arthrodesis and posterior instrumentation. Spine (Phila Pa 1976), 2002, 27: 1680–1686. [DOI] [PubMed] [Google Scholar]
- 98. Tribus CB. Degenerative lumbar scoliosis: evaluation and management. J Am Acad Orthop Surg, 2003, 11: 174–183. [DOI] [PubMed] [Google Scholar]
- 99. Ploumis A, Transfledt EE, Denis F. Degenerative lumbar scoliosis associated with spinal stenosis. Spine J, 2007, 7: 428–436. [DOI] [PubMed] [Google Scholar]
- 100. Kelly DM, McCarthy RE, McCullough FL, Kelly HR. Long‐term outcomes of anterior spinal fusion with instrumentation for thoracolumbar and lumbar curves in adolescent idiopathic scoliosis. Spine (Phila Pa 1976), 2010, 35: 194–198. [DOI] [PubMed] [Google Scholar]
- 101. Verma K, Auerbach JD, Kean KE, Chamas F, Vorsanger M, Lonner BS. Anterior spinal fusion for thoracolumbar scoliosis: comprehensive assessment of radiographic, clinical, and pulmonary outcomes on 2‐years follow‐up. J Pediatr Orthop, 2010, 30: 664–669. [DOI] [PubMed] [Google Scholar]
- 102. Majd ME, Castro FP Jr, Holt RT. Anterior fusion for idiopathic scoliosis. Spine(Phila Pa 1976), 2000, 25: 696–702. [DOI] [PubMed] [Google Scholar]
- 103. Crandall DG, Revella J. Transforaminal lumbar interbody fusion versus anterior lumbar interbody fusion as an adjunct to posterior instrumented correction of degenerative lumbar scoliosis: three year clinical and radiographic outcomes. Spine (Phila Pa 1976), 2009, 34: 2126–2133. [DOI] [PubMed] [Google Scholar]
- 104. Schwab FJ, Smith VA, Biserni M, Gamez L, Farcy JP, Pagala M. Adult scoliosis: a quantitative radiographic and clinical analysis. Spine (Phila Pa 1976), 2002, 27: 387–392. [DOI] [PubMed] [Google Scholar]
- 105. Gupta MC. Degenerative scoliosis: options for surgical management. Orthop Clin North Am, 2003, 34: 269–280. [DOI] [PubMed] [Google Scholar]
- 106. Ondra SL, Marzouk S. Revision strategies for lumbar pseudarthrosis. Neurosurg Focus, 2003, 15: E9. [DOI] [PubMed] [Google Scholar]
- 107. Gertzbein SD, Hollopeter MR, Hall S. Pseudarthrosis of the lumbar spine. Outcome after circumferential fusion. Spine (Phila Pa 1976), 1998, 23: 2352–2356. [DOI] [PubMed] [Google Scholar]
- 108. Etminan M, Girardi FP, Khan SN, Cammisa FP Jr. Revision strategies for lumbar pseudarthrosis. Orthop Clin North Am, 2002, 33: 381–392. [DOI] [PubMed] [Google Scholar]
- 109. Heggeness MH, Esses SI. Classification of pseudarthroses of the lumbar spine. Spine (Phila Pa 1976), 1991, 16 (8 Suppl.): S449–S454. [PubMed] [Google Scholar]
- 110. Heggeness MH, Esses SI, Mody DR. A histologic study of lumbar pseudarthrosis. Spine (Phila Pa 1976), 1993, 18: 1016–1020. [DOI] [PubMed] [Google Scholar]
- 111. Barrick WT, Schofferman JA, Reynolds JB, et al Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine (Phila Pa 1976), 2000, 25: 853–857. [DOI] [PubMed] [Google Scholar]
- 112. Gumbs AA, Hanan S, Yue JJ, Shah RV, Sumpio B. Revision open anterior approaches for spine procedures. Spine J, 2007, 7: 280–285. [DOI] [PubMed] [Google Scholar]
- 113. Buttermann GR, Glazer PA, Hu SS, Bradford DS. Revision of failed lumbar fusions: a comparison of anterior autograft and allograft. Spine (Phila Pa 1976), 1997, 22: 2748–2755. [DOI] [PubMed] [Google Scholar]
- 114. Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976), 2004, 29: 1938–1944. [DOI] [PubMed] [Google Scholar]
- 115. Aiki H, Ohwada O, Kobayashi H, et al Adjacent segment stenosis after lumbar fusion requiring second operation. J Orthop Sci, 2005, 10: 490–495. [DOI] [PubMed] [Google Scholar]
- 116. Whitecloud TS 3rd, Davis JM, Olive PM. Operative treatment of the degenerated segment adjacent to a lumbar fusion. Spine (Phila Pa 1976), 1994, 19: 531–536. [DOI] [PubMed] [Google Scholar]
- 117. Fisher CG, Vaccaro AR, Mulpuri K, et al Evidence‐based recommendations for spine surgery. Spine (Phila Pa 1976), 2013, 38: E30–E37. [DOI] [PubMed] [Google Scholar]
- 118. Vishteh AG, Dickman CA. Anterior lumbar microdiscectomy and interbody fusion for the treatment of recurrent disc herniation. Neurosurgery, 2001, 48: 334–337. [PubMed] [Google Scholar]


