Abstract
Importance
Demonstrating that success of Descemet stripping automated endothelial keratoplasty is similar across donor cornea preservation times (PTs) could increase the donor pool.
Objective
To determine whether the 3-year rate of graft success using corneal donor tissue preserved 8 to 14 days is noninferior to that of donor tissue preserved 7 days or less.
Design, Setting, and Participants
A multicenter, double-masked, randomized noninferiority clinical trial was conducted from April 16, 2012, to June 5, 2017, at 40 clinical sites (70 surgeons) in the United States, with donor corneas provided by 23 US eye banks. A total of 1090 individuals (1330 study eyes) underwent Descemet stripping automated endothelial keratoplasty (1255 eyes [94.4%] for Fuchs endothelial corneal dystrophy).
Interventions
Descemet stripping automated endothelial keratoplasty with random assignment of a donor cornea with a PT of 7 days or less (0-7d PT) or 8 to 14 days (8-14d PT).
Main Outcomes and Measures
Graft success at 3 years.
Results
Of the 1090 participants (1330 study eyes; 60.2% women and 39.8% men; median age at enrollment, 70 years [range, 42-90 years]), the 3-year cumulative probability of graft success was 95.3% (95% CI, 93.6%-96.9%) in the 0-7d PT group and 92.1% (95% CI, 89.9%-94.2%) in the 8-14d PT group (difference, 3.2%). The upper limit of the 1-sided 95% CI on the difference was 5.4%, exceeding the prespecified noninferiority limit of 4%. The difference was mostly owing to more primary donor failures in the 8-14d PT group, with the conditional probability of failure after the first month being 2.4% in the 0-7d PT group and 3.1% in the 8-14d PT group. In preplanned secondary analyses, longer PT was associated with a lower rate of graft success (unadjusted hazard ratio for graft failure per additional day of PT, 1.10; 95% CI, 1.03-1.18; P = .008 [PT analyzed as days]), with success rates of 96.5% (95% CI, 92.3%-98.4%) for PT of 4 days or less, 94.9% (95% CI, 92.5%-96.6%) for PT of 5 to 7 days, 93.8% (95% CI, 91.0%-95.8%) for PT of 8 to 11 days, and 89.3% (95% CI, 84.4%-92.7%) for PT of 12 to 14 days (P = .01 [PT analyzed as categorical variable]).
Conclusions and Relevance
The 3-year success rate in eyes undergoing Descemet stripping automated endothelial keratoplasty was high irrespective of PT. However, the study was unable to conclude that the success rate with donor corneas preserved 8 to 14 days was similar to that of corneas preserved 7 days or less with respect to the prespecified noninferiority limit. Although longer PT was associated with a lower success rate, the difference in rates was small when PT was less than 12 days.
Trial Registration
clinicaltrials.gov Identifier: NCT01537393
This randomized noninferiority clinical trial examines whether the 3-year rate of graft success using corneal donor tissue preserved 8 to 14 days is noninferior to that of donor tissue preserved 7 days or less.
Key Points
Question
What is the effect of donor cornea preservation time on the success of Descemet stripping automated endothelial keratoplasty 3 years after the procedure?
Findings
In this randomized noninferiority clinical trial, the 3-year success rate of Descemet stripping automated endothelial keratoplasty using a donor cornea preserved 8 to 14 days did not meet the study’s definition of noninferiority compared with a preservation time of 7 days or less (92.1% vs 95.3%), principally owing to a lower success rate with preservation time of 12-14 days.
Meaning
Success of Descemet stripping automated endothelial keratoplasty is higher with shorter preservation time, but preservation time up to 11 days can be expected to have little influence on outcomes.
Introduction
In the United States and many other countries, hypothermic (2°C-8°C) corneal donor storage1 is preferred because it is less expensive than organ culture.2,3 In the United States, the most commonly used storage solution is Optisol-GS (Bausch and Lomb)4,5 and, less commonly, Life 4°C (Numedis)6,7 and Eusol (Alchimia).8,9 These solutions contain chondroitin sulfate, nonessential amino acids, antioxidants, and other components, varying between the storage solutions, that support endothelial viability until keratoplasty.9,10 Although these solutions are approved as devices by the US Food and Drug Administration for the storage of donor corneas up to 14 days prior to keratoplasty, storage time (called preservation time [PT] prior to the adoption of the ISBT [International Society of Blood Transfusion] 128 in 201511) for most cases has been less than 8 days. A survey of 19 eye banks in 2010-2011 revealed that 96% of donor corneas used for keratoplasty in the United States were stored less than 8 days, while 27% of donor corneas exported outside of North America were stored for 8 days or more.12 This practice in the United States is a result of many surgeons’ refusal to accept donor corneas with a PT longer than 7 days, although there is no scientific evidence to support this practice. Although available clinical13,14 and nonclinical evidence7,15,16 supports storage up to 14 days, consistent with US Food and Drug Administration–approved labeling, this limited evidence and continued practice pattern of the use of donor tissue stored less than 8 days for keratoplasty provide strong support for a prospective randomized clinical trial.
The Cornea Preservation Time Study (CPTS) was designed to definitively determine the effect of PT on graft success and endothelial cell loss.12 Eyes undergoing Descemet stripping automated endothelial keratoplasty (DSAEK) for conditions associated with endothelial dysfunction and moderate risk for graft failure (Fuchs endothelial corneal dystrophy [FECD] or pseudophakic or aphakic corneal edema) were considered optimal for addressing the study objective. Descemet stripping automated endothelial keratoplasty was chosen since it was the most common keratoplasty procedure being performed for these conditions at the time of study initiation.17 Primary graft outcome results for the 3-year randomized clinical trial are reported herein. Results on PT and long-term endothelial cell loss are reported in a companion article.18
Methods
Enrollment occurred between April 16, 2012, and February 20, 2014, and follow-up ended on June 5, 2017. Study oversight was provided by an independent data and safety monitoring committee. Study methods have been published12 and are summarized below. The protocol is available at https://clinicaltrials.gov/ct2/show/NCT01537393 and Supplement 1. The protocol was approved by institutional review boards for participating sites (eAppendix 2 in Supplement 2), and each participant provided written informed consent, with consent requested again to extend follow-up from 36 months to a common study end date.
Clinical sites, eye banks, surgeons, and ancillary staff were certified prior to study participation. Surgeons were required to have performed at least 50 DSAEK cases, with a reported primary donor failure rate of less than 3% and a dislocation rate requiring repositioning or rebubbling of less than 15% in the prior year. All eye banks were accredited by the Eye Bank Association of America.
Eligible participants were 30 to 90 years of age and undergoing DSAEK for FECD or pseudophakic or aphakic corneal edema. Eyes were excluded if they had a failed penetrating keratoplasty (PKP) or DSAEK, tube shunts, uncontrolled glaucoma, anterior chamber intraocular lenses, or peripheral anterior synechiae spanning more than one-fourth of the anterior chamber angle. Both eyes of a participant could be enrolled.
Donor corneas met Eye Bank Association of America standards for DSAEK,19 including the following criteria: donor age of 10 to 75 years, time from death to preservation at 20 hours or fewer if the donor body was refrigerated or 10 hours or fewer if not refrigerated, eye bank–determined central endothelial cell density of 2300 cells/mm2 or more, no more than mild (slight) polymorphism or polymegethism, absence of guttae, and no evidence of central endothelial cell damage, trauma, or dystrophy. Donor pairs were placed in either Optisol-GS or Life 4°C, as preferred by each eye bank.
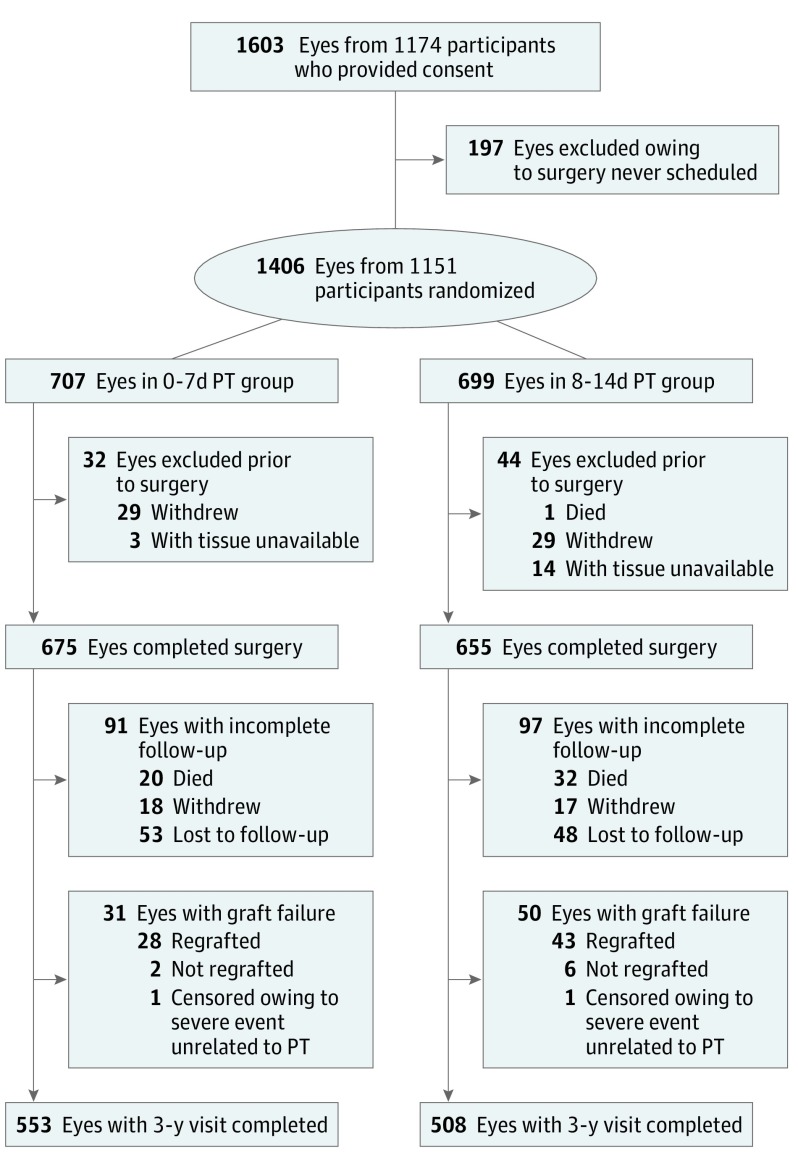
Randomization to either the group that received a donor cornea with a PT of 7 days or less (0-7d PT group) or a group that received a donor cornea with a PT of 8 to 14 days (8-14d PT group) was stratified by surgeon (G.O.R., A.J.A, S.P.D., T.M., S.P., R.D.S., M.A.T., E.Y.T., and D.D.V; eAppendix 1 in Supplement 2) using a permuted blocks design. When both eyes of a participant were included in the study, the eye undergoing surgery first was assigned randomly to a PT group and the second eye was assigned to the other PT group. A minimization algorithm was used to achieve a balance of preplanned PT subgroups (PT of 0-4 days, 5-7 days, 8-11 days, and 12-14 days). Only eyes that had surgery using the CPTS-assigned donor corneas were included in the analyses. The numbers and reasons for randomized eyes exiting the study prior to surgery and deviations from the randomization assignments for 3 cases are shown in Figure 1.
Figure 1. Enrollment and 3-Year Follow-up Flowchart.
A total of 480 study eyes completed bilateral surgery (240 participants) and 850 eyes completed unilateral surgery. Deviations from preservation time (PT) group assignment: 2 eyes had their assigned donor corneas inadvertently switched and received the opposite of their intended PT group. This switch resulted in 1 of these participants receiving a donor cornea in both study eyes from the same PT group (the group that received a donor cornea with a PT of 8 to 14 days [8-14d PT group]). These cases are counted in the PT group as treated. One participant received a donor cornea that was stored for 15 days and was included in the 8-14d PT group. The lost-to-follow-up category includes 20 eyes with no data in the 3-year window but with data in the 4-year window; these eyes were censored prior to 3 years in the primary analyses. Overall graft failure was 31 in the group with PT of 0 to 7 days [0-7d PT group] and 50 in the 8-14d PT group; 1 graft failure in each PT group was censored owing to a severe event unrelated to the PT. The 3-year-visit-completed category includes only eyes without graft failure and includes 13 eyes that did not complete a 3-year visit, but medical records were available to capture data either within the 3-year window (35-42 months) or within the acceptable late out-of-window range (42-44 months).
Eye banks or surgeons prepared the donor cornea according to their customary technique. Under specific authorization by the Eye Bank Association of America, surgeons were masked to all data about donor corneas except for storage solution, residual bed thickness following lamellar dissection, and observations captured during preparation of donor tissue. Preoperative care, surgical technique, and postoperative care were provided according to each investigator’s routine.
Follow-up examinations were performed at 1 day, 1 week, and 1, 6, 12, 24, and 36 months after the procedure.12 Participants consenting to the extension of the study had additional visits at 48 and 60 months, if possible, prior to study conclusion. Examination procedures were conducted according to the investigator’s standard practices. A standardized grading scale was used to categorize clarity of the recipient’s central cornea stroma by a certified examiner as clear, equivocal, or cloudy,12 as defined in eTable 1 in Supplement 2. All investigators were trained, tested, and certified on this assessment using a validated photographic guide.12
Graft Failure
Graft failure was defined as the occurrence of 1 of the following: regrafting for any reason, a cloudy or equivocally cloudy cornea on the first postoperative day that did not clear within 8 weeks, or a cornea that was initially clear postoperatively but became and remained cloudy for 90 days. The date of graft failure was the first examination during the failure event in which the cornea was cloudy or the date of regraft if the cornea was not documented as cloudy prior to regraft. The determination of graft failure was made by the certified surgeon investigator, centrally tracked, and confirmed by the coordinating center and ultimately the study chair (J.H.L.) using the above definition. Graft failure status for corneas that were cloudy for fewer than 3 months at the last available visit or the 3-year visit was determined by the study chair with advice from the executive committee. The principal causes of graft failure were classified as defined in eTable 1 in Supplement 2.
Statistical Analysis
A sample size of 1330 study eyes was computed based on a 1-sided noninferiority test under the following assumptions: 1:1 distribution of corneas between the 2 PT groups, α = .05, power = 0.90, 94% success rate in each PT group, a noninferiority limit of 4%, and 10% incomplete follow-up. Kaplan-Meier estimates of 3-year graft success with 95% CIs (Greenwood estimate of variance20) were calculated for each PT group, and a 1-sided 95% CI was constructed for the difference. A bootstrap resampling technique was used to account for potentially correlated data from 2 study eyes of the same participant; the 95% CI was calculated with bias correction. Confounding and treatment interactions were assessed in Cox proportional hazards regression models.
In preplanned secondary analyses using similar statistical methods, the effect of PT on graft success was evaluated within predefined PT categories (0-4 days, 5-7 days, 8-11 days, and 12-14 days) and as a continuous variable (days from preservation to surgery). A post hoc analysis similarly compared the subgroup with a PT of 8 to 11 days with the 0-7d PT group. Post hoc comparisons of PT groups with respect to postoperative donor positioning issues were computed using Fisher exact tests.
The 95% CI for evaluating the primary analysis of noninferiority is 1-sided; all other 95% CIs and P values are 2-sided. P < .05 was considered significant. Statistical analyses were conducted using SAS, version 9.4 (SAS Inc).
Results
Baseline Characteristics
A total of 1330 study eyes of 1090 participants (240 participants had 2 study eyes) underwent DSAEK by 70 surgeons at 40 sites, with 675 eyes receiving a donor cornea preserved for 7 days or less and 655 receiving a donor cornea preserved for 8 to 14 days. The median age of participants was 70 years (range, 42-90 years), 60.2% were women, and 90.8% were white. The most common indication for DSAEK was FECD (1255 eyes [94.4%]). Participant characteristics were balanced between the 2 PT groups (Table 1).21,22
Table 1. Baseline Participant Characteristics by Donor Cornea Preservation Time Groupa.
| Characteristic | Preservation Time Group, No. (%) | |
|---|---|---|
| 0-7 d (675 Eyes) |
8-14 d (655 Eyes) |
|
| Age at enrollment, median (IQR), yb | 70 (64-76) | 70 (64-77) |
| Female sex | 409 (60.6) | 392 (59.8) |
| Race/ethnicity | ||
| White | 613 (90.8) | 594 (90.7) |
| African American | 26 (3.9) | 21 (3.2) |
| Hispanic or Latino | 19 (2.8) | 25 (3.8) |
| Asian | 7 (1.0) | 5 (0.8) |
| American Indian or Alaskan Native | 1 (0.1) | 2 (0.3) |
| More than 1 race | 6 (0.9) | 3 (0.5) |
| Unknown or not reported | 3 (0.4) | 5 (0.8) |
| History of diabetesc | 116 (17.2) | 126 (19.2) |
| Current cigarette smokerc | 45 (6.7) | 45 (6.9) |
| Received any immunizations or vaccinations in last 3 moc | 92 (13.6) | 81 (12.4) |
| Study eye characteristics | ||
| Prior glaucoma surgery | 18 (2.7) | 13 (2.0) |
| Glaucoma medication currently being used | 45 (6.7) | 54 (8.2) |
| Diagnosis | ||
| PCE without FECD | 44 (6.5) | 29 (4.4) |
| ACE without FECD | 0 | 2 (0.3) |
| FECD12,21 | 631 (93.5) | 624 (95.3) |
| Grade 022 | 2 (0.3) | 2 (0.3) |
| Grade 1 | 0 | 3 (0.6) |
| Grade 2 | 2 (0.3) | 2 (0.3) |
| Grade 3 | 12 (1.8) | 19 (2.9) |
| Grade 4 | 95 (14.1) | 87 (13.3) |
| Grade 5 | 184 (27.3) | 180 (27.5) |
| Grade 6 | 336 (49.8) | 331 (50.5) |
| Evidence of a corneal abnormality other than FECD | ||
| No | 630 (93.3) | 623 (95.1) |
| Keratoconus | 1 (0.1) | 0 |
| EBMD | 38 (5.6) | 29 (4.4) |
| Other | 6 (0.9) | 3 (0.6) |
| Stromal corneal vessels present (but not visually significant) | 4 (0.6) | 1 (0.2) |
| Central subepithelial or stromal scarring present (but could not affect postoperative stromal clarity assessment) | 37 (5.5) | 48 (7.3) |
| Peripheral anterior synechiae present (nongonioscopic) | 4 (0.6) | 2 (0.3) |
| Preoperative lens status | ||
| Phakic | 341 (50.5) | 351 (53.6) |
| Aphakic | 0 | 2 (0.3) |
| Pseudophakic posterior chamber intraocular lens | 334 (49.5) | 302 (46.1) |
| Postoperative lens status | ||
| Phakic | 4 (0.6) | 6 (0.9) |
| Pseudophakic posterior chamber intraocular lens | 671 (99.4) | 649 (99.1) |
| IOP, median (IQR), mm Hg | 15 (12-17) | 15 (12-17) |
Abbreviations: ACE, aphakic corneal edema; EBMD, epithelial basement membrane dystrophy; FECD, Fuchs endothelial corneal dystrophy; IOP, intraocular pressure; IQR, interquartile range; PCE, pseudophakic corneal edema.
A total of 5 ineligible cases enrolled: 1 case of sulcus supported posterior chamber intraocular lens (protocol was subsequently amended to allow this case), 3 cases with IOP <10 mm Hg, and 1 case with IOP >25 mm Hg.
If participant had 2 study eyes, the data collected at time of enrollment of the first eye is counted in both columns.
These questions were repeated at the time of enrollment of the second eye, so the answers could differ from one eye to the next.
Donor corneas were provided by 23 eye banks. Median donor age was 61 years (range, 12-75 years), and mean (SD) eye bank–determined endothelial cell density at the time of donor screening was 2735 (298) cells/mm2 (range, 2300-4386). Donor baseline characteristics were balanced between the 2 PT groups (eTable 2 in Supplement 2). Median PT was 6 days (interquartile range, 4-6 days) in the 0-7d PT group and 11 days (interquartile range, 9-12 days) in the 8-14d PT group. Tissue was prepared by the eye bank in 987 cases (74.2%) and by the surgeon in 343 cases (25.8%).
Intraoperative and Postoperative Procedures and Complications
The numbers and types of intraoperative procedures performed in addition to DSAEK were similar between the 2 PT groups (eTable 3 in Supplement 2). For 682 of the 1330 eyes (51.3%), cataract extraction with a posterior chamber intraocular lens placement was performed concurrently with DSAEK. After DSAEK, all study eyes except 10 (4 in the 0-7d PT group and 6 in the 8-14d PT group) were pseudophakic with a posterior chamber intraocular lens. The rate of intraoperative complications was low and was similar between the 2 groups (eTable 3 in Supplement 2).
Postoperatively, 111 of 675 eyes (16.4%) in the 0-7d PT group and 143 of 655 eyes (21.8%) in the 8-14d PT group had at least 1 donor positioning issue (P = .01): dislocation (total detachment) of donor in 33 eyes (4.9%) in the 0-7d PT group and 53 eyes (8.1%) in the 8-14d PT group, graft repositioning in the absence of dislocation in 4 eyes (0.6%) in the 0-7d PT group and 12 eyes (1.8%) in the 8-14d PT group, and detachment in the absence of either dislocation or repositioning in 74 eyes (11.0%) in the 0-7d PT group and 78 eyes (11.9%) in the 8-14d PT group (eTable 4 in Supplement 2). Air injection was performed 1 or more times in 54 eyes (8.0%) in the 0-7d PT group and 85 eyes (13.0%) in the 8-14d PT group (P = .003).
One case of bacterial endophthalmitis (0.1%) occurred in the 0-7d PT group 4 days after surgery, and 2 cases of fungal keratitis (0.3%) occurred in the 8-14d PT group 13 and 43 days after surgery (eTables 5 and 6 in Supplement 2). Other ocular findings and postoperative procedures are in eTables 5-7 in Supplement 2.
Study Completion
Overall, 584 study eyes (86.5%) in the 0-7d PT group and 558 study eyes (85.2%) in the 8-14d PT group either completed a visit in the 3-year window or experienced graft failure (Figure 1). Excluding deaths prior to 3 years and accounting for participants who missed the 3-year visit but completed a subsequent visit, the study completion rate was 90.7% in the 0-7d PT group (594 of 655) and 91.2% in the 8-14d PT group (568 of 623). Data from 2 eyes with failed grafts unrelated to PT (severe blunt trauma and major surgical complication) were censored at the last examination prior to the event.
Graft Outcome
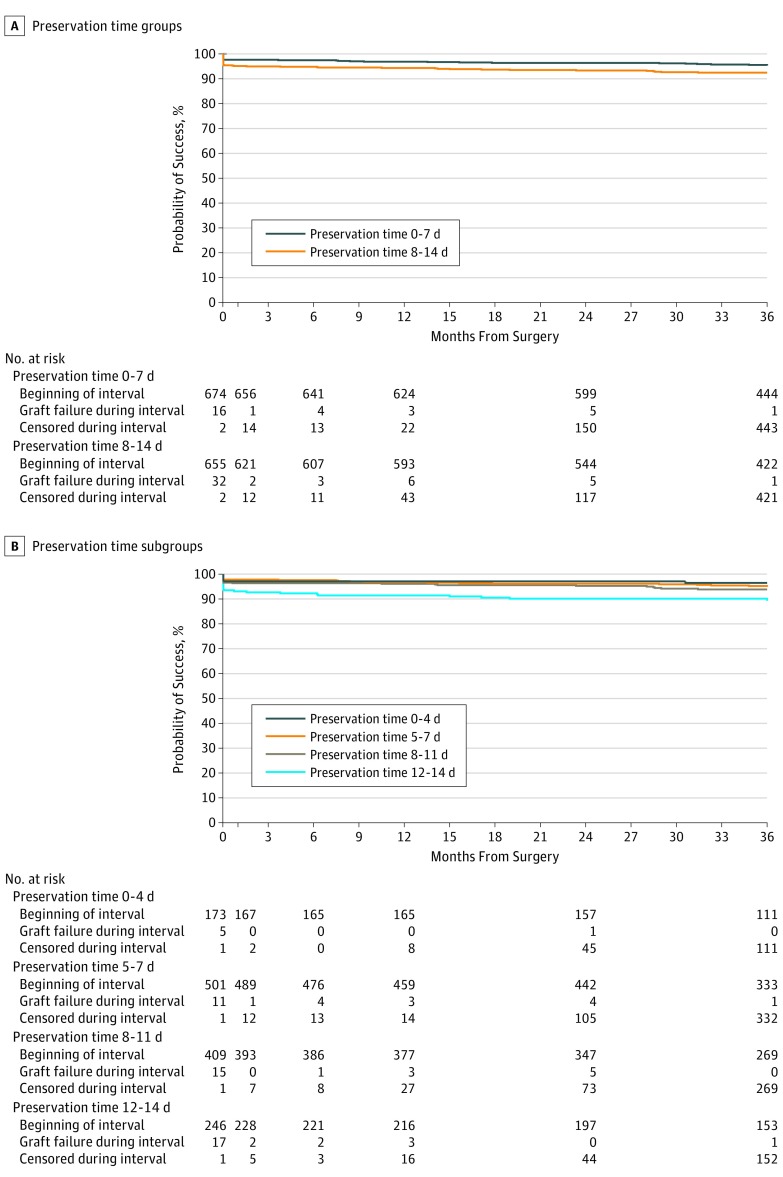
The 3-year cumulative probability of graft success was 95.3% (95% CI, 93.6%-96.9%) in the 0-7d PT group and 92.1% (95% CI, 89.9%-94.2%) in the 8-14d PT group (difference between groups, 3.2%) (Table 2). The upper limit of the 1-sided 95% CI of this difference was 5.4%, exceeding the prespecified noninferiority limit of 4%. The unadjusted hazard ratio for graft failure in the 8-14d PT group compared with the 0-7d PT group was 1.71 (95% CI, 1.09-2.71; P = .02), with no indication of confounding upon adjustment for covariates or statistical interaction (eTable 8 in Supplement 2). Most of the difference of failure rates between the groups occurred in the first postoperative month, with the probability of failure in the first month being 2.4% in the 0-7d PT group and 4.9% in the 8-14d PT group, and the conditional probability of 3-year failure after the first month being 2.4% in the 0-7d PT group and 3.1% in the 8-14d PT group (Figure 2A). Twenty-eight of 30 eyes in the 0-7d PT group and 44 of 49 eyes in the 8-14d PT group had a regraft; the other 2 failures in the 0-7d PT group and 5 failures in the 8-14d PT group met the criteria for cloudy cornea failure (of these, 1 eye in the 8-14d PT group had a cloudy cornea for less than 3 months at the last study visit). The principal causes of graft failure are summarized in Table 3. As shown in Table 2, in a preplanned secondary analysis treating PT as a continuous variable, graft success tended to decrease as PT increased (unadjusted hazard ratio for graft failure per additional day of PT, 1.10; 95% CI, 1.03-1.18; P = .008). The 3-year cumulative probability of graft success was lower (89.3%; 95% CI, 84.4%-92.7% [N = 246]) for PT of 12 to 14 days, compared with 96.5% (95% CI, 92.3%-98.4% [N = 173]) for PT of 4 days or less, 94.9% (95% CI, 92.5%-96.6% [N = 502]) for PT of 5 to 7 days, and 93.8% (95% CI, 91.0%-95.8% [N = 409]) for PT of 8 to 11 days (Table 2 and Figure 2B; P = .01). In a post hoc analysis, the difference in 3-year graft success rates comparing the 0-7d PT group (95.3%) with PT of 8 to 11 days (93.8%) was 1.5% (95% CI, –1.3% to 4.4%). Graft success results through 4 years were consistent with the 3-year primary outcome analysis (eFigure 1 in Supplement 2).
Table 2. Effect of Preservation Time on 3-Year Graft Failurea.
| Characteristic | No. | 3-y Graft Success Rate, % (95% CI) | Unadjusted Hazard Ratio (95% CI) | Unadjusted P Value | Adjusted Hazard Ratio (95% CI) | Adjusted P Value |
|---|---|---|---|---|---|---|
| Preservation time group | ||||||
| 0-7 d | 675 | 95.3 (93.6-96.9) | 1 [Reference] | .02 | 1 [Reference] | .02b |
| 8-14 d | 655 | 92.1 (89.9-94.2) | 1.71 (1.09-2.71) | 1.74 (1.10-2.75) | ||
| Preservation time subgroup | ||||||
| 0-4 d | 173 | 96.5 (92.3-98.4) | 1 [Reference] | .01 | 1 [Reference] | .01c |
| 5-7 d | 502 | 94.9 (92.5-96.6) | 1.41 (0.57-3.47) | 1.35 (0.55-3.34) | ||
| 8-11 d | 409 | 93.8 (91.0-95.8) | 1.74 (0.71-4.27) | 1.67 (0.67-4.12) | ||
| 12-14 d | 246 | 89.3 (84.4-92.7) | 3.09 (1.26-7.58) | 3.11 (1.27-7.65) | ||
| Preservation time, d | 1.10 (1.03-1.18) | .008 | 1.10 (1.03-1.19) | .007d | ||
| 1 | 1 | 100.0 | NA | NA | NA | NA |
| 2 | 18 | 94.4 (66.6-99.2) | ||||
| 3 | 58 | 98.3 (88.4-99.8) | ||||
| 4 | 96 | 95.7 (89.0-98.4) | ||||
| 5 | 145 | 97.1 (92.4-98.9) | ||||
| 6 | 206 | 92.2 (87.4-95.3) | ||||
| 7 | 151 | 96.5 (91.8-98.5) | ||||
| 8 | 98 | 90.1 (81.8-94.7) | ||||
| 9 | 109 | 95.3 (89.2-98.0) | ||||
| 10 | 94 | 93.4 (85.8-97.0) | ||||
| 11 | 108 | 96.1 (89.8-98.5) | ||||
| 12 | 134 | 90.9 (84.5-94.7) | ||||
| 13 | 88 | 89.6 (80.9-94.4) | ||||
| 14 | 23 | 81.2 (56.8-92.6) | ||||
| 15 | 1 | 100.0 |
All models accounted for correlated data from study participants with 2 study corneas. (Since no donor pairs had multiple failures, models did not adjust for random donor effect.)
Potential confounders evaluated included the following: baseline endothelial cell density, recipient and donor age, recipient and donor race/ethnicity, presence of glaucoma, presence of corneal vessels, history of smoking, storage solution, time from lamellar dissection to surgery, lamellar dissection preparation by eye bank vs surgeon, and donor rim culture result. Factors from univariate models with P < .10 were evaluated in a multivariate model, keeping factors with P < .01 following a backward selection process. The final model adjusted only for corneal diagnosis (prespecified to be forced in the analysis). Random surgeon effects were explored using a frailty model with similar results (P = .02).
The primary model comparing 2 preservation time groups was used. Preservation time subgroups were analyzed as categorical.
The primary model comparing 2 preservation time groups was used. Preservation time was analyzed as a continuous variable in which preservation time days’ calculation was fractional based on hours.
Figure 2. Graft Success During 3 Years.
A, Graft success estimate by preservation time groups (0-7 days [n = 674], 95.3% [95% CI, 93.6%-96.9%] and 8-14 days [n = 655], 92.1% [95% CI, 89.9%-94.2%]). The 3-year graft success difference was 3.2%, and the upper limit of the 1-sided 95% CI on the difference was 5.4%. B, Graft success by preservation time subgroups (0-4 days [n = 173], 96.5% [95% CI, 92.3%-98.4%]; 5-7 days [n = 501], 94.9% [95% CI, 92.5%-96.6%]; 8-11 days [n = 409], 93.8% [95% CI, 91.0%-95.8%]; and 12-14 days [n = 246], 89.3% [95% CI, 84.4%-92.7%]). All failure or censoring events within the 3-year visit window were mapped to month 36. Available data beyond the designated 3-year visit (or the cutoff for the 3-year window when a 3-year visit was not completed) were not used in determination of failure status or censor date for the primary analyses. This protocol affected the following: 20 eyes with no data in the 3-year window but with data in the 4-year window were censored prior to 3 years in the primary analyses. One eye initiated failure before the end of the 3-year window but after the 3-year visit date that did not count as a 3-year failure in the primary analysis. Data beyond the 3-year protocol window (35-42 months) but prior to the 4-year window (42-44 months) were used to determine failure status or censor dates. This protocol affected the following: 28 eyes were permitted to complete their 3-year visit late out of window between 42 and 44 months. Four eyes did not complete a 3-year visit, but medical records were available to capture data between 42 and 44 months.
Table 3. Causes of 3-Year Graft Failure.
| Characteristic | Preservation Time Group | Preservation Time Subgroup | ||||
|---|---|---|---|---|---|---|
| 0-7 d (n = 675) |
8-14 d (n = 655) |
0-4 d (n = 173) |
5-7 d (n = 502) |
8-11 d (n = 409) |
12-14 d (n = 246) |
|
| Total 3-y graft failures, No. (%) | 30 (4.4) | 49 (7.5) | 6 (3.5) | 24 (4.8) | 24 (5.9) | 25 (10.2) |
| Primary donor failure in the absence of surgical complications, No. | 11 | 22 | 4 | 7 | 11 | 11 |
| Early failure associated with surgical complications, No. | 5 | 7 | 1 | 4 | 3 | 4 |
| Graft rejection, No. | 3 | 5 | 1 | 2 | 3 | 2 |
| Nonrejection graft failure, No. | ||||||
| Endothelial decompensation | 6 | 8 | 0 | 6 | 3 | 5 |
| Glaucoma or hypotony | 3 | 2 | 0 | 3 | 2 | 0 |
| Infection | 0 | 2 | 0 | 0 | 1 | 1 |
| Refractive or visual graft failure, No. | 2 | 2 | 0 | 2 | 1 | 1 |
| Other (write-in): suspect damage during processing, No. | 0 | 1 | 0 | 0 | 0 | 1 |
Discussion
The CPTS determined whether the success rate of DSAEK with donor corneas preserved for 8 to 14 days was noninferior to that of corneas preserved 7 days or less in eyes with endothelial dysfunction. To judge noninferiority, the end of the 1-sided 95% CI on the difference in success rates between the longer and shorter PT groups was compared with a prespecified noninferiority limit of 4% (eFigure 2 in Supplement 2). This criterion was based on judgment of the magnitude of difference that would be considered clinically insignificant. The study found that, although the observed difference between the 2 PT groups was 3.2%, the end of the 95% CI was 5.4%, which exceeded the predetermined 4% limit. Thus, the study cannot conclude that the success rate for donor corneas with PT of 8 to 14 days is similar to the success rate for donor corneas with a shorter PT.
Analyzed as a continuous variable (days), there was a significant association between longer PT and a lower graft success rate. However, up to 11 days, the effect of PT on the graft success rate was small, consistent with the endothelial cell loss data reported in a companion article.18 These findings are clinically important, as eye bank data have shown that more than 95% of donor corneas used domestically have a PT of less than 8 days.12 The CPTS results should encourage surgeons to accept corneas stored between 7 and 11 days. The success rate was still high in the group with a PT of 12 to 14 days (89.3%), which may support continued use of these stored tissues as logistics dictate. Also, additional time to evaluate and distribute the donor tissue will be most helpful to eye banks.
The combined primary and early graft failure rate in the first month was about twice as high in the 8-14d PT group as it was in the 0-7d PT group, while the difference in failure rates between groups after 1 month was small. This finding suggests that loss of endothelial cell function during longer-term storage leads to early failure in a small number of donor corneas. Although the rate of primary donor failure was twice as high in the 8-14d PT group vs the 0-7d PT group (3.4% vs 1.6%), the overall 2.5% rate (33 of 1330 cases) of primary donor failure was below the selection criterion (<3%) for our surgeons to participate in the CPTS.12 This rate was also comparable with the mean 5% rate (range, 0%-29%) cited in the American Academy of Ophthalmology report summarizing estimates of primary failure reported in the literature at the time.23
Prior studies assessing the effect of PT on graft outcomes of PKP13,14,24,25 and endothelial keratoplasty26,27,28,29,30 are limited. Several studies have not shown an association between PT and graft success, but PT was primarily 10 days or less.13,14,24,28 A retrospective PKP study showed no statistically significant association between PT and graft success; however, only 62 of the 234 reported cases (26.5%) had storage times of 11 days or more.14
The 94.1% overall success rate (1251 of 1330 eyes) for DSAEK at 3 years in this multicenter, multisurgeon prospective study is identical to what was projected in designing the study and comparable to that of single-site studies for cases of FECD managed with DSAEK.31,32,33,34 Based on prior studies, the success rate of DSAEK for pseudophakic or aphakic corneal edema and failed cases of PKP or endothelial keratoplasty is significantly lower than that for FECD.35,36,37 The CPTS protocol was designed to include only moderate-risk endothelial failure conditions (FECD and uncomplicated pseudophakic or aphakic corneal edema) to minimize the number of grafts that would be at higher risk for failure owing to other causes. The high success rate reported in this article is not necessarily reflective of the expected success rate for the entire population of patients with endothelial failure undergoing DSAEK. We report a higher graft success rate with DSAEK in the surgical management of FECD at 3 years than in the Australian38 and United Kingdom39 registries reports, possibly owing to the absence of a learning curve with surgeons meeting minimum experience requirements for study participation.
Strengths and Limitations
Strengths of the study included a large sample size, masking of surgeon and participant to PT, experienced DSAEK surgeons in both university and community settings, good retention, and a standardized definition of graft failure. Although the CPTS cohort consisted principally of FECD cases (94.4%), there is no biologically plausible reason to expect that the effect of PT on DSAEK success would be different for other cornea conditions. We also hypothesize that these results may apply to the increasingly popular Descemet membrane endothelial keratoplasty40,41 and to PKP,13,14 although this hypothesis was not studied.
Conclusions
The 3-year success rate in eyes undergoing DSAEK predominantly for FECD was high irrespective of PT. Although the study was unable to conclude that the success rate using donor corneas preserved for 8 to 14 days was similar to that of corneas preserved 7 days or less based on the study’s predetermined definition of noninferiority, the difference in rates was small when PT was less than 12 days. Therefore, these results suggest that using donor corneas with PT up to 11 days is likely to have comparable success rates and that donor corneas with PTs beyond 11 days could be used when logistically necessary, as the success rate using these corneas was also high.
Study Protocol
eFigure 1. Graft Success Rate Over Time by 2 Preservation Time Groups Including Follow-up Beyond 3 Years
eFigure 2. Visual Representation of the Primary Noninferiority Analysis
eAppendix 1. Cornea Preservation Time Study Group: Clinical Sites
eAppendix 2. Institutional Review Boards
eTable 1. CPTS Graft Failure Definitions
eTable 2. CPTS Donor Characteristics
eTable 3. Surgical Data
eTable 4. Postoperative Donor Positioning Complications and Procedures
eTable 5. Serious Abnormalities
eTable 6. Abnormalities on Cornea Exam / AC Exam
eTable 7. Study Eye Procedures
eTable 8. Potential Interactions with Preservation Time Groups
eReferences
References
- 1.Jeng BH. Preserving the cornea: corneal storage media. Curr Opin Ophthalmol. 2006;17(4):332-337. [DOI] [PubMed] [Google Scholar]
- 2.Pels E, Beele H, Claerhout I. Eye bank issues, II: preservation techniques: warm versus cold storage. Int Ophthalmol. 2008;28(3):155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pels E, Rijneveld WJ. Organ culture preservation for corneal tissue: technical and quality aspects. Dev Ophthalmol. 2009;43:31-46. [DOI] [PubMed] [Google Scholar]
- 4.Lass JH, Gordon JF, Sugar A, et al. Optisol containing streptomycin. Am J Ophthalmol. 1993;116(4):503-504. [DOI] [PubMed] [Google Scholar]
- 5.Lass JH, Bourne WM, Musch DC, et al. A randomized, prospective, double-masked clinical trial of Optisol vs DexSol corneal storage media. Arch Ophthalmol. 1992;110(10):1404-1408. [DOI] [PubMed] [Google Scholar]
- 6.Price MO, Knight OJ, Benetz BA, et al. Randomized, prospective, single-masked clinical trial of endothelial keratoplasty performance with 2 donor cornea 4°C storage solutions and associated chambers. Cornea. 2015;34(3):253-256. [DOI] [PubMed] [Google Scholar]
- 7.Skelnik DL, Wilson RR, Wilson JR, Welch DP. Life 4°C: a new corneal preservation system. Invest Ophthalmol Vis Sci. 2006;47(13):2365. [Google Scholar]
- 8.Kanavi MR, Javadi MA, Chamani T, Fahim P, Javadi F. Comparing quantitative and qualitative indices of the donated corneas maintained in Optisol-GS with those kept in Eusol-C. Cell Tissue Bank. 2015;16(2):243-247. [DOI] [PubMed] [Google Scholar]
- 9.Yüksel B, Uzunel UD, Küsbeci T. Endothelial cell viability of donor corneas preserved in Eusol-C corneal storage medium. Exp Clin Transplant. 2016;14(4):441-444. [DOI] [PubMed] [Google Scholar]
- 10.Pham C, Hellier E, Vo M, Szczotka-Flynn L, Benetz BA, Lass JH. Donor endothelial specular image quality in Optisol GS and Life 4°C. Int J Eye Banking. 2013;1(2):1-8. doi: 10.7706/ijeb.v1i2.52 [DOI] [Google Scholar]
- 11.Eye Bank Association of America Implementation guide: use of ISBT 128 in North American eye banks. http://restoresight.org/wp-content/uploads/2016/01/ISBT-Webpage-Implementation-Guide-Use-of-ISBT-128-in-North-American-Eye-Banks-v130.pdf. Published December 2015. Accessed August 2, 2017.
- 12.Lass JH, Szczotka-Flynn LB, Ayala AR, et al. ; Writing Committee for the Cornea Preservation Time Study Group . Cornea preservation time study: methods and potential impact on the cornea donor pool in the United States. Cornea. 2015;34(6):601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doganay S, Hepsen IF, Yologlu S, Demirtas H. Effect of the preservation-to-surgery interval on corneal allograft survival in low-risk patients. Ophthalmic Surg Lasers Imaging. 2007;38(6):457-461. [DOI] [PubMed] [Google Scholar]
- 14.Wagoner MD, Gonnah S. Corneal graft survival after prolonged storage in Optisol-GS. Cornea. 2005;24(8):976-979. [DOI] [PubMed] [Google Scholar]
- 15.Means TL, Geroski DH, Hadley A, Lynn MJ, Edelhauser HF. Viability of human corneal endothelium following Optisol-GS storage. Arch Ophthalmol. 1995;113(6):805-809. [DOI] [PubMed] [Google Scholar]
- 16.Lindstrom RL. Advances in corneal preservation. Trans Am Ophthalmol Soc. 1990;88:555-648. [PMC free article] [PubMed] [Google Scholar]
- 17.Eye Bank Association of America 2012 Eye banking statistical report. http://restoresight.org/wp-content/uploads/2013/04/2012_Statistical_Report_FINAL-reduced-size-4-10.pdf. Accessed August 2, 2017.
- 18.Lass JH, Benetz BA, Verdier DD, et al. Corneal endothelial cell loss 3 years after successful Descemet stripping automated endothelial keratoplasty in the Cornea Preservation Time Study [published online November 10, 2017]. JAMA Ophthalmol. doi: 10.1001/jamaophthalmol.2017.4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eye Bank Association of America Medical standards. http://www.corneas.org/repository/docs/SurgeonDocs/EBAA-Medical-Standards-with-Appendices-June-2015.pdf. Published June 2015. Accessed August 2, 2017.
- 20.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons; 1980. [Google Scholar]
- 21.Louttit MD, Kopplin LJ, Igo RP Jr, et al. ; FECD Genetics Multi-Center Study Group . A multicenter study to map genes for Fuchs endothelial corneal dystrophy: baseline characteristics and heritability. Cornea. 2012;31(1):26-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbott RL, Fine BS, Webster RG Jr, Paglen PG, Spencer WH. Specular microscopic and histologic observations in nonguttate corneal endothelial degeneration. Ophthalmology. 1981;88(8):788-800. [DOI] [PubMed] [Google Scholar]
- 23.Lee WB, Jacobs DS, Musch DC, Kaufman SC, Reinhart WJ, Shtein RM. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116(9):1818-1830. [DOI] [PubMed] [Google Scholar]
- 24.Chang SD, Pecego JG, Zadnik K, Danneffel MB, Mutti DO, Mannis MJ. Factors influencing graft clarity. Cornea. 1996;15(6):577-581. [PubMed] [Google Scholar]
- 25.Abbott RL, Forster RK. Determinants of graft clarity in penetrating kerotoplasty. Arch Ophthalmol. 1979;97(6):1071-1075. [DOI] [PubMed] [Google Scholar]
- 26.Guttman C. Donor death-surgery interval may affect DSAEK outcomes. Ophthamology Times April 15, 2009. http://ophthalmologytimes.modernmedicine.com/ophthalmologytimes/news/modernmedicine/modern-medicine-feature-articles/donor-death-surgery-interval. Accessed August 2, 2017.
- 27.Price MO, Price FW Jr. Endothelial cell loss after Descemet stripping with endothelial keratoplasty influencing factors and 2-year trend. Ophthalmology. 2008;115(5):857-865. [DOI] [PubMed] [Google Scholar]
- 28.Chen ES, Terry MA, Shamie N, Hoar KL, Friend DJ. Precut tissue in Descemet’s stripping automated endothelial keratoplasty donor characteristics and early postoperative complications. Ophthalmology. 2008;115(3):497-502. [DOI] [PubMed] [Google Scholar]
- 29.Terry MA, Shamie N, Chen ES, Phillips PM, Hoar KL, Friend DJ. Precut tissue for Descemet’s stripping automated endothelial keratoplasty: vision, astigmatism, and endothelial survival. Ophthalmology. 2009;116(2):248-256. [DOI] [PubMed] [Google Scholar]
- 30.Terry MA, Shamie N, Straiko MD, Friend DJ, Davis-Boozer D. Endothelial keratoplasty: the relationship between donor tissue storage time and donor endothelial survival. Ophthalmology. 2011;118(1):36-40. [DOI] [PubMed] [Google Scholar]
- 31.Ang M, Soh Y, Htoon HM, Mehta JS, Tan D. Five-year graft survival comparing Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Ophthalmology. 2016;123(8):1646-1652. [DOI] [PubMed] [Google Scholar]
- 32.Price MO, Gorovoy M, Benetz BA, et al. Descemet’s stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the Cornea Donor Study. Ophthalmology. 2010;117(3):438-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price MO, Fairchild KM, Price DA, Price FW Jr. Descemet’s stripping endothelial keratoplasty five-year graft survival and endothelial cell loss. Ophthalmology. 2011;118(4):725-729. [DOI] [PubMed] [Google Scholar]
- 34.Fajgenbaum MA, Hollick EJ. Modeling endothelial cell loss after Descemet stripping endothelial keratoplasty: data from 5 years of follow-up. Cornea. 2017;36(5):553-560. [DOI] [PubMed] [Google Scholar]
- 35.Wiaux C, Baghdasaryan E, Lee OL, et al. Outcomes after Descemet stripping endothelial keratoplasty in glaucoma patients with previous trabeculectomy and tube shunt implantation. Cornea. 2011;30(12):1304-1311. [DOI] [PubMed] [Google Scholar]
- 36.Aldave AJ, Chen JL, Zaman AS, Deng SX, Yu F. Outcomes after DSEK in 101 eyes with previous trabeculectomy and tube shunt implantation. Cornea. 2014;33(3):223-229. [DOI] [PubMed] [Google Scholar]
- 37.Mitry D, Bhogal M, Patel AK, et al. Descemet stripping automated endothelial keratoplasty after failed penetrating keratoplasty: survival, rejection risk, and visual outcome. JAMA Ophthalmol. 2014;132(6):742-749. [DOI] [PubMed] [Google Scholar]
- 38.Coster DJ, Lowe MT, Keane MC, Williams KA; Australian Corneal Graft Registry Contributors . A comparison of lamellar and penetrating keratoplasty outcomes: a registry study. Ophthalmology. 2014;121(5):979-987. [DOI] [PubMed] [Google Scholar]
- 39.Greenrod EB, Jones MN, Kaye S, Larkin DF; National Health Service Blood and Transplant Ocular Tissue Advisory Group and Contributing Ophthalmologists (Ocular Tissue Advisory Group Audit Study 16) . Center and surgeon effect on outcomes of endothelial keratoplasty versus penetrating keratoplasty in the United Kingdom. Am J Ophthalmol. 2014;158(5):957-966. [DOI] [PubMed] [Google Scholar]
- 40.Monnereau C, Quilendrino R, Dapena I, et al. Multicenter study of Descemet membrane endothelial keratoplasty: first case series of 18 surgeons. JAMA Ophthalmol. 2014;132(10):1192-1198. [DOI] [PubMed] [Google Scholar]
- 41.Eye Bank Association of America 2015 Eye banking statistical report. http://restoresight.org/wp-content/uploads/2016/03/2015-Statistical-Report.pdf. Accessed August 2, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
eFigure 1. Graft Success Rate Over Time by 2 Preservation Time Groups Including Follow-up Beyond 3 Years
eFigure 2. Visual Representation of the Primary Noninferiority Analysis
eAppendix 1. Cornea Preservation Time Study Group: Clinical Sites
eAppendix 2. Institutional Review Boards
eTable 1. CPTS Graft Failure Definitions
eTable 2. CPTS Donor Characteristics
eTable 3. Surgical Data
eTable 4. Postoperative Donor Positioning Complications and Procedures
eTable 5. Serious Abnormalities
eTable 6. Abnormalities on Cornea Exam / AC Exam
eTable 7. Study Eye Procedures
eTable 8. Potential Interactions with Preservation Time Groups
eReferences