Key Points
Question
What is the relationship between cornea preservation time and endothelial cell loss 3 years after successful Descemet stripping automated endothelial keratoplasty?
Findings
In a randomized clinical trial evaluating 945 eyes with graft success, endothelial cell loss was 37% with preservation time 0 to 7 days and 40% with preservation time 8 to 14 days, with mean endothelial cell density of 1722 and 1642 cells/mm2, respectively, at 3 years.
Meaning
Although endothelial cell loss 3 years after Descemet stripping automated endothelial keratoplasty is greater with longer preservation time, the effect of preservation time on endothelial cell loss is comparable from 4 to 13 days of preservation time.
Abstract
Importance
Demonstrating that endothelial cell loss following Descemet stripping automated endothelial keratoplasty (DSAEK) is independent of donor cornea preservation time (PT) could increase the pool of corneal tissue available for keratoplasty.
Objective
To determine whether endothelial cell loss 3 years after successful DSAEK is related to PT.
Design, Setting, and Participants
A multicenter, double-masked, randomized clinical trial included 40 clinical sites (70 surgeons) in the United States, with donor corneas provided by 23 US eye banks. A total of 945 eyes of 769 participants were included in the Cornea Preservation Time Study that had not experienced graft failure 3 years after DSAEK, performed primarily for Fuchs endothelial corneal dystrophy (96% of the cohort). The study was conducted from April 16, 2012, to June 5, 2017.
Interventions
DSAEK with random assignment of a donor cornea with PT of 0 to 7 days (0-7d PT) or 8 to 14 days (8-14d PT).
Main Outcomes and Measures
Endothelial cell density (ECD) at 3 years determined by a central image analysis reading center from clinical specular or confocal central endothelial images.
Results
Nine hundred forty-five eyes of 769 participants (median age, 70 years [range, 42-90 years], 60.8% women, 93.0% white) in the Cornea Preservation Time Study that had not experienced graft failure 3 years after DSAEK were included. At the initial eye bank tissue screening, mean (SD) central ECD was 2746 (297) cells/mm2 in the 0-7d PT group (n = 485) and 2723 (284) cells/mm2 in the 8-14d PT group (n = 460). At 3 years, the mean (SD) ECD decreased from baseline by 37% (21%) in the 0-7d PT group and 40% (22%) in the 8-14d PT group to 1722 (626) cells/mm2 and 1642 (631) cells/mm2, respectively (mean difference, 73 cells/mm2; 95% CI, 8-138 cells/mm2; P = .03). When analyzed as a continuous variable (days), longer PT was associated with lower ECD (mean difference by days, 15 cells/mm2; 95% CI, 4-26 cells/mm2; P = .006). Endothelial cell loss (ECL) was comparable from 4 to 13 days’ PT (n = 878; 36%-43% when tabulated by day). Available extension study ECD results at 4 years mirrored those at 3 years in the 203 eyes in the 0-7d PT group (mean [SD] ECD, 1620 [673] cells/mm2 and mean [SD] ECL, 41% [23%]) and 209 eyes in the 8-14d PT group (mean [SD] ECD, 1537 [683] cells/mm2 and mean [SD] ECL, 44% [23%]) (mean difference, 112 cells/mm2; 95% CI, 5-219 cells/mm2; P = .04).
Conclusions and Relevance
Although ECL 3 years after Descemet stripping automated endothelial keratoplasty is greater with longer PT, the effect of PT on ECL is comparable from 4 to 13 days’ PT.
Trial Registration
clinicaltrials.gov Identifier: NCT01537393
This randomized clinical trial evaluates the preservation time of the donor cornea after Descemet stripping automated endothelial keratoplasty.
Introduction
The corneal endothelium is critical for deturgescence of the corneal stroma with its barrier and pump functions.1,2 Although central endothelial cell density (ECD) decreases with age,3 it decreases at a higher rate with corneal conditions, such as Fuchs endothelial corneal dystrophy,4 and following cataract surgery.5,6,7 Failure of the corneal endothelium to recover from endothelial cell damage is due to its limited ability to divide.8 If central ECD falls below a critical level with an insufficient number of endothelial cells and their associated sodium-potassium adenosine triphosphatase pump sites to dehydrate the stroma,2 the cornea swells, vision decreases, and keratoplasty is then indicated. The Cornea Donor Study and its ancillary study, the Specular Microscopy Ancillary Study, which evaluated the effect of donor age on graft success and endothelial cell loss (ECL) following penetrating keratoplasty (PKP),9,10 demonstrated the importance of ECL in estimating long-term graft survival. Five years after PKP, graft success rates were similar with older and younger donor age,9 but ECL was greater with corneas from older donors compared with younger donors.11 This ECL difference at 5 years presaged a higher graft failure rate in the older donor age group by 10 years.10 In addition, ECD at 6 months, 1 year, and 5 years was associated at each time point with subsequent graft failure, irrespective of donor age.12
The Cornea Preservation Time Study (CPTS) was designed to determine whether the success of Descemet stripping automated endothelial keratoplasty (DSAEK) performed for corneal conditions associated with endothelial failure is related to donor cornea preservation time (PT). With the Cornea Donor Study and the results of studies examining ECL following DSAEK13,14,15 in mind, the determination of ECL and its association with PT following DSAEK was considered an important secondary outcome in designing the CPTS protocol,16 particularly since there have been few clinical studies assessing the effect of PT on ECL following keratoplasty with hypothermic (2°C-8°C) storage solutions.17,18,19 None of these studies examined the clinical performance of these solutions for the full 14 days approved by the US Food and Drug Administration. This report complements the CPTS article20 examining the association between PT and graft success following DSAEK by describing the effect of PT on long-term ECL following this procedure.
Methods
Participants were enrolled at 40 clinical sites and donor corneas were provided by 23 eye banks across the United States.16 Enrolled CPTS participants were aged 42 to 90 years (median, 70 years) with a corneal disease associated with endothelial dysfunction, including Fuchs endothelial corneal dystrophy (94% of eyes) and pseudophakic or aphakic corneal edema (6% of eyes). Eyes were randomly assigned to receive a donor cornea with PT of 0 to 7 days (0-7d PT group) or 8 to 14 days (8-14d PT group); for participants with both eyes eligible, the first eye was assigned randomly to a PT group and the second eye was assigned to the other PT group. The 1330 eyes completing surgery with a CPTS-assigned cornea were considered the study eyes. These CPTS-assigned corneas were from donors aged 12 to 75 years (median age, 61 years) with a minimum eye bank–measured central ECD of 2300 cells/mm2 and a median PT of 6 days in the 0-7d PT group and 11 days in the 8-14d PT group. Under specific authorization from the Eye Bank Association of America, clinical investigators and participants were masked to all characteristics of the donor cornea, except for storage solution (Optisol-GS; Bausch and Lomb or Life 4°C; Numedis), residual bed thickness following lamellar dissection, and observations captured during donor tissue preparation; no Eusol (Alchimia) was used. Preoperative management, surgical technique, and postoperative care, including prescription of medications, were provided according to each investigator’s routine. Only eyes classified as a graft success at 3 years, as defined in our companion article,20 and with analyzable 3-year specular or confocal microscopic images were included in the ECD analyses reported herein. The study was conducted from April 16, 2012, to June 5, 2017.
Details of the CPTS protocol have been published16 and are available in Supplement 2. The protocol was approved by institutional review boards governing each investigational site (eAppendix 2 in Supplement 1), and individual participants gave written informed consent to participate in the study. Participants received financial compensation at each protocol visit.
Endothelial Imaging and Image Analysis
The Corneal Image Analysis Reading Center at Case Western Reserve University and University Hospitals Eye Institute (CIARC) in Cleveland, Ohio, served as the central reading center for endothelial image analysis to determine ECD and also was responsible for quality-control measures at the eye banks and clinical sites. Each specular or confocal microscope at eye banks and clinical sites used in the study underwent a prestudy external calibration determination and image quality assessment16; at sites with multiple instruments, each was calibrated. This prestudy instrument certification was designed to ascertain the specific magnification and image quality for each instrument so that ECD could accurately be measured by the CIARC. In addition, any replacement microscope installed during the study was certified before use in the trial. The eye banks used specular microscopes for endothelial imaging (HAI Laboratories Inc [n = 7]; Konan Medical Inc [n = 32]). A contact or noncontact specular microscope (Konan Medical Inc [n = 37]; Tomey Corporation USA [n = 4]) or confocal microscope (Nidek Inc [n = 16]) was used at the clinical sites. All eye bank and clinical site personnel capturing images were trained and certified to obtain good to excellent image quality according to the protocol related to endothelial imaging.
Following donor cornea procurement, eye banks obtained 1 to 3 initial specular images of the central donor cornea endothelium after their usual procedure of warming the donor tissue to room temperature and then determined ECD by their usual analysis method (referred to as screening ECD). This assessment was not standardized, since it was performed at a time when the eye bank did not yet know whether the donor cornea would be assigned to a CPTS recipient. If a donor cornea was assigned to the CPTS, eye banks also obtained 3 preoperative study images of the central endothelium either after lamellar dissection or, if the donor cornea was to be prepared by the surgeon, prior to shipment. Before preoperative images were obtained, the donor cornea was again allowed to warm to room temperature so that the best-quality images could be obtained.21 Postoperative specular or confocal microscopic images of the central corneal endothelium of the graft were obtained at 6 months and then 1, 2, and 3 years as long as a participant remained in follow-up without experiencing graft failure or undergoing regrafting. Sites were requested to retake images of poor technical quality when possible and were provided specific advice on quality improvement techniques. Images were also obtained at 4 and 5 years for participants who consented to extended observation.
ECD Determination
Preoperative donor and postoperative recipient images were evaluated for quality and ECD by the CIARC. Details of CIARC procedures have been previously described, including reader training and certification, image-quality grading, image calibration, variable frame analysis for ECD determination, and adjudication procedures for image-quality and ECD determination.11,22 Briefly, image quality was assessed as analyzable or nonanalyzable by 2 independent readers and determined by a third adjudicator (one of us, B.B.) when either reader found the quality to be nonanalyzable. The ECD of all analyzable images was independently determined by 2 readers using the variable frame analysis method.23 This method was selected for the CPTS so that the maximal number of available cells with clear centers and borders could be analyzed. If the ECDs determined by the 2 readers differed by 5.0% or more, a third independent determination of ECD was made by an adjudicator. Final ECD was the mean of all ECDs that were within 5% of each other. Readers were masked to all information about the donor corneas and study participants. Throughout the study, the CPTS Data Management and Analysis Center (Jaeb Center for Health Research, Tampa, Florida) selected eye bank and clinical images for additional masked image-quality grading and ECD determination to assess both intraobserver and interobserver variability (eTable 1 in Supplement 1).
Statistical Analysis
The eye bank–determined screening ECD was considered the baseline value for all analyses evaluating the effect of PT, including calculations of ECL, since the preoperative ECD was measured after the donor corneas had already been preserved either 0 to 7 days or 8 to 14 days. The primary analysis to assess the effect of PT on 3-year ECD was conducted with a mixed linear model adjusting for baseline ECD, corneal diagnosis, and potential confounders, including storage solution, preparation by eye bank vs surgeon, and accounting for correlated data from participants with 2 study eyes or 2 corneas from the same donor. Other potential confounders were evaluated but not included in the final model (recipient and donor age, recipient and donor race, presence of glaucoma, presence of corneal vessels, history of smoking, time from lamellar dissection to surgery, and donor rim culture result). Potential confounders from univariate models with P < .10 were evaluated in a multivariate model, keeping factors with P < .01 following a backward selection process. Random effects were modeled to account for correlated data from fellow eyes of the same participant and correlated data from fellow corneas of the same donors. Preplanned secondary analyses were performed treating PT as categorical (predefined categories of 0-4 days, 5-7 days, 8-11 days, and 12-14 days) and as a continuous variable (days from initial preservation to surgery). Sensitivity analyses were performed using multiple imputation24 to impute the missing or nonanalyzable 3-year ECD of participants with a surviving graft at 3 years. Statistical models accounting for selective dropout due to graft failure, previously applied to Cornea Donor Study PKP data,25 could not be used owing to the overall low failure rate.
In another preplanned analysis, a repeated-measures least squares regression longitudinal model was fit using all available images obtained at screening, 6 months, and 1, 2, and 3 years, adjusting for corneal diagnosis and correlated data as described above. Preservation time was evaluated as both continuous and categorical. Sensitivity analyses were performed using multiple imputation24 to impute ECD at all missing time points for participants with a surviving graft at 3 years, with similar results.
Analyses of 4-year image data paralleled the primary 3-year analysis for participants with a surviving graft at 4 years. There were too few 5-year images for meaningful statistical analyses. Statistical analyses were conducted using SAS software, version 94 (SAS Institute). All P values are 2-sided and considered significant at <.05.
Results
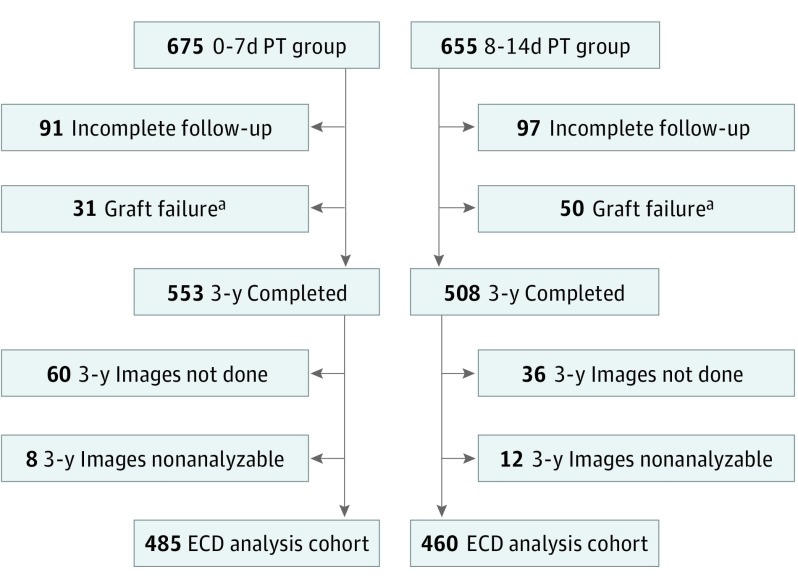
Nine hundred forty-five eyes of 769 participants (median age, 70 years [range, 42-90 years], 60.8% women, 93.0% white) were included. Of the 675 study eyes undergoing DSAEK that were assigned to the 0-7d PT group and 655 assigned to the 8-14d PT group, 493 (73.0%) and 472 (72.1%) eyes, respectively, had a clear recipient stroma, functioning graft, and ECD images at 3 years. Of these 3-year image sets (3 images per set), 8 (1.6%) and 12 (2.5%) sets, respectively, were nonanalyzable owing to poor image quality or insufficient number of cells for analysis.22 Thus, the primary ECD analysis cohort included 485 eyes in the 0-7d PT group and the 460 eyes in the 8-14d PT group (Figure 1). The recipient and donor baseline characteristics of these eyes were similar to the characteristics of the full CPTS cohort (eTable 2 and eTable 3 in Supplement 1).
Figure 1. Endothelial Cell Density (ECD) Analysis Cohort Flowchart.
0-7d PT indicates preservation time (PT) of 0 to 7 days; 8-14d PT, 8 to 14 days.
aTwo eyes (1 in each PT group) with graft failure were censored owing to severe event unrelated to PT.
In the primary ECD analysis cohort, the initial mean (SD) eye bank screening ECD was 2746 (297) cells/mm2 in the 0-7d PT group and 2723 (284) cells/mm2 in the 8-14d PT group. Mean preoperative ECD (determined by CIARC) was 2745 (354) cells/mm2 and 2764 (374) cells/mm2, respectively.
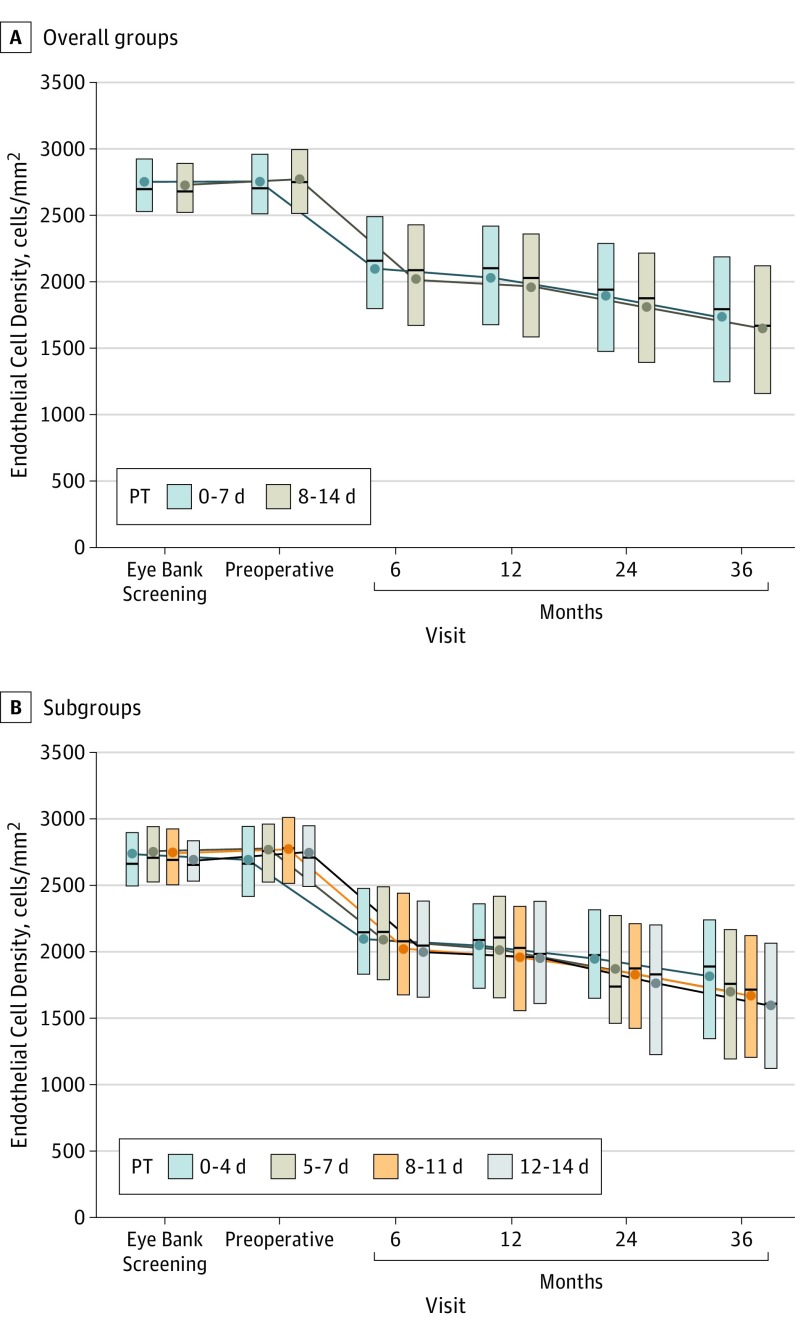
At 3 years, mean (SD) ECL decreased from baseline by 37% (21%) in the 0-7d PT group and 40% (22%) in the 8-14d PT group, resulting in a mean (SD) 3-year ECD of 1722 (626) cells/mm2 and 1642 (631) cells/mm2, respectively (mean difference, 73 cells/mm2; 95% CI, 8-138 cells/mm2; P = .03). Analysis was adjusted for screening ECD, corneal diagnosis, and other confounding factors as described above (Table). Results were similar when 3-year ECD was imputed for the additional 68 and 48 eyes with graft success at 3 years, but without analyzable 3-year images (P = .02). In both PT groups, the majority of ECL occurred in the first 6 months after DSAEK (Figure 2A), with mean ECL of 24% (19%) in the 0-7d PT group and 26% (20%) in the 8-14d PT group at that time.
Table. Three-Year ECD in Eyes With Graft Successa.
| Characteristic | No. of Eyesb | Screening ECD, Mean (SD) | Preoperative ECD, Mean (SD)c | 3-y ECD, Mean (SD) | ECL From Screening to 3 y, Mean (SD), % | P Value for 3-y ECD |
|---|---|---|---|---|---|---|
| Preservation time group | ||||||
| 0-7 d | 485 | 2746 (297) | 2745 (354) | 1722 (626) | 37 (21) | .03d |
| 8-14 d | 460 | 2723 (284) | 2764 (374) | 1642 (631) | 40 (22) | |
| Preservation time subgroup | ||||||
| 0-4 d | 122 | 2738 (302) | 2691 (390) | 1814 (619) | 34 (21) | .02e |
| 5-7 d | 363 | 2749 (296) | 2763 (340) | 1691 (626) | 39 (21) | |
| 8-11 d | 291 | 2743 (305) | 2773 (382) | 1670 (635) | 39 (22) | |
| 12-14 d | 169 | 2688 (240) | 2749 (361) | 1593 (623) | 41 (21) | |
| Preservation time, d | ||||||
| 0-3 | 51 | 2773 (335) | 2677 (403) | 1925 (568) | 30 (19) | .006f |
| 4 | 71 | 2712 (275) | 2702 (384) | 1734 (645) | 37 (22) | |
| 5 | 104 | 2753 (294) | 2706 (337) | 1679 (558) | 39 (20) | |
| 6 | 149 | 2743 (302) | 2758 (322) | 1657 (674) | 40 (22) | |
| 7 | 110 | 2754 (293) | 2825 (359) | 1750 (621) | 36 (21) | |
| 8 | 67 | 2802 (313) | 2751 (282) | 1710 (647) | 39 (21) | |
| 9 | 85 | 2726 (290) | 2765 (492) | 1671 (616) | 39 (22) | |
| 10 | 62 | 2754 (297) | 2799 (391) | 1585 (706) | 43 (24) | |
| 11 | 77 | 2703 (317) | 2778 (313) | 1703 (591) | 37 (21) | |
| 12 | 94 | 2701 (246) | 2807 (392) | 1638 (644) | 40 (21) | |
| 13 | 59 | 2695 (244) | 2679 (310) | 1596 (580) | 41 (20) | |
| 14 | 16 | 2587 (165) | 2685 (317) | 1311 (612) | 49 (24) |
Abbreviations: ECD, endothelial cell density; ECL, endothelial cell loss; PT, preservation time.
All analyses (except analysis with imputation) included eyes with graft success and a gradable image at 3 years. Endothelial cell density reported as cells per square millimeter.
Total of 945 eyes.
Preoperative image ECD is missing for 15 eyes in each PT group, owing to imaging not done or not gradable.
The final model for the primary analysis was a mixed linear model, adjusting for screening ECD and corneal diagnosis (predefined to include), as well as storage solution, preparation by eye bank vs surgeon, and accounting for correlated data from participants with 2 study eyes or 2 corneas from the same donor. Other potential confounders were evaluated but not included in the final model (recipient and donor age, recipient and donor race, presence of glaucoma, presence of corneal vessels, history of smoking, time from lamellar dissection to surgery, and donor rim culture result).
The same primary analysis model was used, except PT subgroups were analyzed as categorical.
The same primary analysis model was used, except PT was analyzed as continuous.
Figure 2. Endothelial Cell Density (ECD) Over Time for Participants With Graft Success and a Gradable Image at 3 Years.
The primary ECD analysis cohort stratified by preservation time (PT) group (0-7, 8-14 days) (A) and by preservation time subgroup (0-4, 5-7, 8-11, 12-14 days) (B). The upper and lower edges of each box represent the interquartile range (25th-75th percentile). The circle in each box is the mean ECD and the line inside each box is the median.
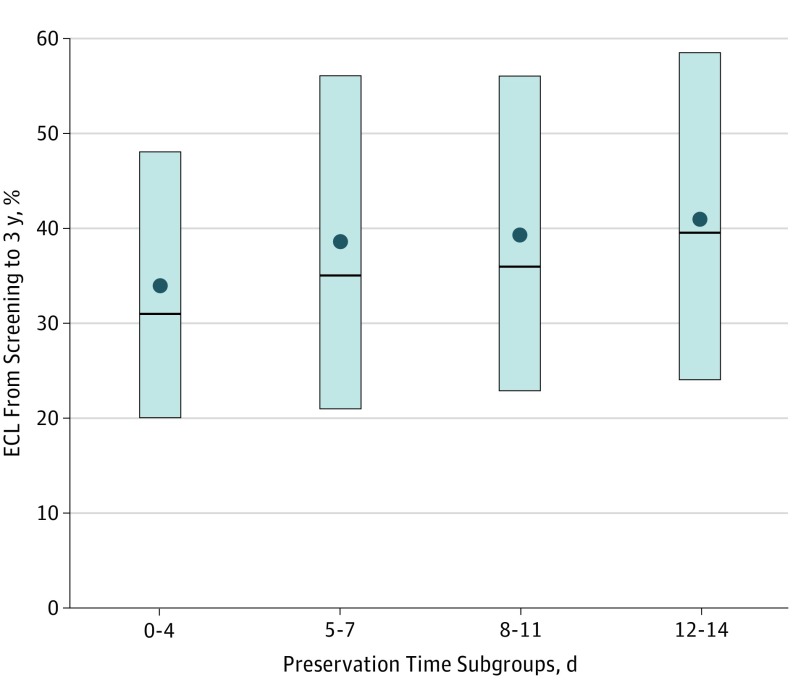
In preplanned secondary analyses, there was greater ECL with longer PT (P = .006 treating PT as continuous [days] with mean difference by days, 15 cells/mm2; 95% CI, 4-26 cells/mm2 and P = .02 treating PT as 4 predefined subgroups [0-4, 5-7, 8-11, and 12-14 days]) (Table, Figure 2B, Figure 3). Endothelial cell loss was comparable from 4 to 13 days’ PT (n = 878; 36%-43% when tabulated by day). In a longitudinal statistical model including all available images from baseline through 3 years, ECL was greater with longer PT (P = .29 comparing the 2 PT groups and P = .005 with PT as a continuous variable; P = .22 and P = .004, respectively, from multiple imputation sensitivity analysis).
Figure 3. Percentage of Endothelial Cell Loss (ECL) From Baseline Screening at 3 Years, by Preservation Time Subgroup for Participants With Graft Success and a Gradable Image at 3 Years.
The upper and lower edges of each box represent the interquartile range (25th-75th percentile). The circle in each box is the mean and the line inside each box is the median.
Among participants with graft success and an analyzable image at 4 years (203 eyes in the 0-7d PT group and 209 eyes in the 8-14d PT group), mean (SD) ECD results at 4 years mirrored those at 3 years (ECD, 1620 [673] cells/mm2 and 41% [23%] ECL) and 209 eyes in the 8-14d PT group (mean [SD] ECD, 1537 [683] cells/mm2 and 44% [23%] ECL) (mean difference, 112 cells/mm2, 95% CI, 5-219 cells/mm2; P = .04). (eTable 4 in Supplement 1).
Discussion
Results from the CPTS in our companion article indicated that PT up to 11 days can be expected to have little influence on graft success 3 years after DSAEK.20 Although there was statistically significant greater ECL with longer PT, this companion ECL study also supports the use of corneas with a PT up to 11 days, because there was little clinical difference in ECL over the 4- to 13-day PT range. The sample size was too small for a separate analysis of the effect of 3 days or fewer or 14-day PT on ECL.
There is limited literature on the effect of PT on ECL following keratoplasty using hypothermic 2°C to 8°C, chondroitin sulfate–based storage solutions with, to our knowledge, no randomized, masked studies. Bourne17 found a positive correlation between PT and ECL in 37 corneas 2 months after PKP for corneas stored in K-Sol, with the highest individual ECLs (>30%) occurring in donor corneas stored for at least 10 days. Terry et al18 found no correlation between PT and ECL within a limited range of PT in Optisol-GS (mean, 4 days; range, 1-8 days) in 362 eyes followed up after DSAEK for up to 2 years. Price and Price26 found no significant effect of donor death to surgery time (which includes time from death to preservation plus PT), ranging from 2 to 8 days with Optisol-GS storage, on ECL in 263 eyes after DSAEK at 2 years. In the Cornea Donor Study, median death to surgery time with Optisol-GS storage was only 4 days (25th and 75th percentiles 3 and 5 days, respectively)27 and no effect on ECL was noted.28 Life 4°C has shown better endothelial viability with storage up to 14 days compared with Optisol-GS in ex vivo studies.29 In a randomized clinical trial comparing the 2 solutions, ECL was comparable at 6 months19; however, the full range of PT was not tested since PT was limited to a mean of 5 days and a maximum of 7 days. The CPTS could not examine any difference in the effect of PT on ECL with these 2 storage solutions, since only 38 of the 945 donor corneas (4.0%) were stored in Life 4°C.
There is considerable literature on long-term ECL following DSAEK, mostly single-site reports.13,14,26,30,31,32,33,34,35,36,37,38,39,40 These studies have generally found between 30% and 40% ECL at 3 years and approximately 50% to 55% at 5 years, but with great variability. Price and Price26 reported ECL of 36% at 1 year and 41% at 2 years in 263 eyes in 216 patients. They subsequently reported a median ECL of 53% with high variability (range, 7.5%-89%) at 5 years in 53 eyes,13 which was lower than observed in eyes that underwent PKP in the Specular Microscopy Ancillary Study at this same time period.11 Li et al15 reported on 144 eyes without any episodes of rejection showing a mean ECL of 31% with high variability (SD, 20%) 3 years after DSAEK. Considering these studies, it is reassuring that the mean ECL overall of 39% at 3 years in the CPTS with 70 surgeons was comparable to the percentages in these prior reports. Also, the pattern of ECL in the CPTS with a steep decline during the first 1 to 6 months and a slower rate of ECL out to 3 years was similar to the findings in these other DSAEK studies14,30,31,40 and to the mathematical modeling of ECL over 10 years.31 This finding suggests that the greatest damage to the endothelial cells associated with DSAEK occurs during the perioperative period as reflected in the ECL between 140 and 6 months14,30 with a continued slower decline thereafter,31 unlike the more gradual, progressive pattern of ECL during the first 5 years postoperatively with a slower decline thereafter with PKP.11,25,41
Strengths and Limitations
Strengths of this study included a large sample size, standardization of imaging techniques and ECD determination, highly reproducible image analyses, and masking of the surgeons and imaging analysts to PT. A limitation of all longitudinal postgraft ECL studies is that failed grafts are no longer available for ECD measurements and so the results apply only to successful grafts. Although we were unable to complete a sensitivity analysis controlling for this selective exclusion and cannot confirm its impact, we suspect that any bias is negligible owing to the small number of failures and are reassured that other studies with higher failure rates found no meaningful difference when these methods were applied.25,31 In interpreting the CPTS results, it is also important to recognize that almost all participants had Fuchs endothelial corneal dystrophy, and the results may not apply to DSAEK performed for other conditions, including failed grafts or pseudophakic or aphakic corneal edema associated with tube shunts and anterior chamber intraocular lenses. The findings also may not apply to PKP, which has a different pattern of ECL than DSAEK.25,31
Conclusions
Although there was a statistically significant association between greater ECL and longer PT in corneas that are clear 3 years after DSAEK, ECL was clinically similar among corneas stored for 4 to 13 days. With a higher frequency of graft failure in the 12- to 14-day PT group as reported in our companion article,20 the findings suggest that donor tissue with PT up to 11 days is suitable in terms of both graft success and ECL. Donor tissue stored for 12 to 14 days also may be considered suitable for logistical reasons, because the DSAEK success rate is high, irrespective of PT, although higher ECL in corneas that are clear at 3 years could portend a higher long-term failure rate.
eAppendix 1. Cornea Preservation Time Study Group: Clinical Sites
eAppendix 2. Institutional Review Boards
eTable 1. Quality Control Repeat Grading Results
eTable 2. Baseline Recipient Characteristics in Eyes with Graft Success
eTable 3. CPTS Donor Characteristics in Eyes with Graft Success
eTable 4. Four Year Endothelial Cell Density (cells/mm2) in Eyes with Graft Success
Trial Protocol and CPTS Statistical Analysis Plan
References
- 1.Waring GO III, Bourne WM, Edelhauser HF, Kenyon KR. The corneal endothelium: normal and pathologic structure and function. Ophthalmology. 1982;89(6):531-590. [PubMed] [Google Scholar]
- 2.Edelhauser HF. The resiliency of the corneal endothelium to refractive and intraocular surgery. Cornea. 2000;19(3):263-273. [DOI] [PubMed] [Google Scholar]
- 3.Laule A, Cable MK, Hoffman CE, Hanna C. Endothelial cell population changes of human cornea during life. Arch Ophthalmol. 1978;96(11):2031-2035. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Patel DV. The pathophysiology of Fuchs’ endothelial dystrophy—a review of molecular and cellular insights. Exp Eye Res. 2015;130(1):97-105. [DOI] [PubMed] [Google Scholar]
- 5.Park J, Yum HR, Kim MS, Harrison AR, Kim EC. Comparison of phaco-chop, divide-and-conquer, and stop-and-chop phaco techniques in microincision coaxial cataract surgery. J Cataract Refract Surg. 2013;39(10):1463-1469. [DOI] [PubMed] [Google Scholar]
- 6.Storr-Paulsen A, Norregaard JC, Ahmed S, Storr-Paulsen T, Pedersen TH. Endothelial cell damage after cataract surgery: divide-and-conquer versus phaco-chop technique. J Cataract Refract Surg. 2008;34(6):996-1000. [DOI] [PubMed] [Google Scholar]
- 7.Bourne WM, Nelson LR, Hodge DO. Continued endothelial cell loss ten years after lens implantation. Ophthalmology. 1994;101(6):1014-1022. [DOI] [PubMed] [Google Scholar]
- 8.Joyce NC. Proliferative capacity of corneal endothelial cells. Exp Eye Res. 2012;95(1):16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gal RL, Dontchev M, Beck RW, et al. ; Cornea Donor Study Investigator Group . The effect of donor age on corneal transplantation outcome results of the cornea donor study. Ophthalmology. 2008;115(4):620-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mannis MJ, Holland EJ, Gal RL, et al. ; Writing Committee for the Cornea Donor Study Research Group . The effect of donor age on penetrating keratoplasty for endothelial disease: graft survival after 10 years in the Cornea Donor Study. Ophthalmology. 2013;120(12):2419-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lass JH, Gal RL, Dontchev M, et al. ; Cornea Donor Study Investigator Group . Donor age and corneal endothelial cell loss 5 years after successful corneal transplantation; Specular Microscopy Ancillary Study results. Ophthalmology. 2008;115(4):627-632.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugar A, Gal RL, Kollman C, et al. ; Writing Committee for the Cornea Donor Study Research Group . Factors associated with corneal graft survival in the cornea donor study. JAMA Ophthalmol. 2015;133(3):246-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price MO, Fairchild KM, Price DA, Price FW Jr. Descemet’s stripping endothelial keratoplasty five-year graft survival and endothelial cell loss. Ophthalmology. 2011;118(4):725-729. [DOI] [PubMed] [Google Scholar]
- 14.Price MO, Gorovoy M, Price FW Jr, Benetz BA, Menegay HJ, Lass JH. Descemet’s stripping automated endothelial keratoplasty: three-year graft and endothelial cell survival compared with penetrating keratoplasty. Ophthalmology. 2013;120(2):246-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li JY, Terry MA, Goshe J, Shamie N, Davis-Boozer D. Graft rejection after Descemet’s stripping automated endothelial keratoplasty: graft survival and endothelial cell loss. Ophthalmology. 2012;119(1):90-94. [DOI] [PubMed] [Google Scholar]
- 16.Lass JH, Szczotka-Flynn LB, Ayala AR, et al. ; Writing Committee for the Cornea Preservation Time Study Group . Cornea preservation time study: methods and potential impact on the cornea donor pool in the United States. Cornea. 2015;34(6):601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourne WM. Endothelial cell survival on transplanted human corneas preserved at 4 C in 2.5% chondroitin sulfate for one to 13 days. Am J Ophthalmol. 1986;102(3):382-386. [DOI] [PubMed] [Google Scholar]
- 18.Terry MA, Shamie N, Straiko MD, Friend DJ, Davis-Boozer D. Endothelial keratoplasty: the relationship between donor tissue storage time and donor endothelial survival. Ophthalmology. 2011;118(1):36-40. [DOI] [PubMed] [Google Scholar]
- 19.Price MO, Knight OJ, Benetz BA, et al. Randomized, prospective, single-masked clinical trial of endothelial keratoplasty performance with 2 donor cornea 4°C storage solutions and associated chambers. Cornea. 2015;34(3):253-256. [DOI] [PubMed] [Google Scholar]
- 20.Rosenwasser GO, Szczotka-Flynn LB, Ayala AR, et al. Effect of cornea preservation time on Descemet stripping automated endothelial keratoplasty success: results of a randomized noninferiority trial [published online November 10, 2017]. JAMA Ophthalmol. doi: 10.1001/jamaophthalmol.2017.4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham C, Hellier E, Vo M, Szczotka-Flynn L, Benetz BA, Lass JH. Donor endothelial image quality in Optisol GS and Life4°C. Int J Eye Banking. 2013;1(2):1-8. [Google Scholar]
- 22.Benetz BA, Gal RL, Ruedy KJ, et al. ; Cornea Donor Study Group . Specular microscopy ancillary study methods for donor endothelial cell density determination of Cornea Donor Study images. Curr Eye Res. 2006;31(4):319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayegh RR, Benetz BA, Lass JH. Specular microscopy In: Mannis MJ, Holland EJ, eds. Cornea: Fundamentals, Diagnosis, Management. Vol 1 New York: Elsevier; 2016:160-179. [Google Scholar]
- 24.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 25.Riddlesworth TD, Kollman C, Lass JH, et al. A mathematical model to predict endothelial cell density following penetrating keratoplasty with selective dropout from graft failure. Invest Ophthalmol Vis Sci. 2014;55(12):8409-8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price MO, Price FW Jr. Endothelial cell loss after Descemet stripping with endothelial keratoplasty influencing factors and 2-year trend. Ophthalmology. 2008;115(5):857-865. [DOI] [PubMed] [Google Scholar]
- 27.Sugar A, Gal RL, Beck W, et al. ; Cornea Donor Study Group . Baseline donor characteristics in the Cornea Donor Study. Cornea. 2005;24(4):389-396. [DOI] [PubMed] [Google Scholar]
- 28.Lass JH, Beck RW, Benetz BA, et al. ; Cornea Donor Study Investigator Group . Baseline factors related to endothelial cell loss following penetrating keratoplasty. Arch Ophthalmol. 2011;129(9):1149-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skelnik DL, Wilson RR, Wilson JR, Welch DP. Life 4°C: a new corneal preservation system. Inv Ophthalmol Vis Sci. 2006;47:2365. [Google Scholar]
- 30.Terry MA, Chen ES, Shamie N, Hoar KL, Friend DJ. Endothelial cell loss after Descemet’s stripping endothelial keratoplasty in a large prospective series. Ophthalmology. 2008;115(3):488-496.e3. [DOI] [PubMed] [Google Scholar]
- 31.Price MO, Calhoun P, Kollman C, Price FW Jr, Lass JH. Descemet stripping endothelial keratoplasty: ten-year endothelial cell loss compared with penetrating keratoplasty. Ophthalmology. 2016;123(7):1421-1427. [DOI] [PubMed] [Google Scholar]
- 32.Fajgenbaum MA, Hollick EJ. Modeling endothelial cell loss after Descemet stripping endothelial keratoplasty: data from 5 years of follow-up. Cornea. 2017;36(5):553-560. [DOI] [PubMed] [Google Scholar]
- 33.Dooren BT, Saelens IE, Bleyen I, Mulder PG, Bartels MC, Rij GV. Endothelial cell decay after descemet’s stripping automated endothelial keratoplasty and top hat penetrating keratoplasty. Invest Ophthalmol Vis Sci. 2011;52(12):9226-9231. [DOI] [PubMed] [Google Scholar]
- 34.Ishiyama S, Mori Y, Nejima R, et al. Comparison of long-term outcomes of visual function and endothelial cell survival after Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty using mixed-effects models. Cornea. 2016;35(12):1526-1532. [DOI] [PubMed] [Google Scholar]
- 35.Terry MA, Li J, Goshe J, Davis-Boozer D. Endothelial keratoplasty: the relationship between donor tissue size and donor endothelial survival. Ophthalmology. 2011;118(10):1944-1949. [DOI] [PubMed] [Google Scholar]
- 36.Terry MA, Shamie N, Chen ES, Phillips PM, Hoar KL, Friend DJ. Precut tissue for Descemet’s stripping automated endothelial keratoplasty: vision, astigmatism, and endothelial survival. Ophthalmology. 2009;116(2):248-256. [DOI] [PubMed] [Google Scholar]
- 37.Price MO, Bidros M, Gorovoy M, et al. Effect of incision width on graft survival and endothelial cell loss after Descemet stripping automated endothelial keratoplasty. Cornea. 2010;29(5):523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price MO, Baig KM, Brubaker JW, Price FW Jr. Randomized, prospective comparison of precut vs surgeon-dissected grafts for Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2008;146(1):36-41. [DOI] [PubMed] [Google Scholar]
- 39.Lee WB, Jacobs DS, Musch DC, Kaufman SC, Reinhart WJ, Shtein RM. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116(9):1818-1830. [DOI] [PubMed] [Google Scholar]
- 40.Wacker K, Baratz KH, Maguire LJ, McLaren JW, Patel SV. Descemet stripping endothelial keratoplasty for Fuchs’ endothelial corneal dystrophy: five-year results of a prospective study. Ophthalmology. 2016;123(1):154-160. [DOI] [PubMed] [Google Scholar]
- 41.Lass JH, Benetz BA, Gal RL, et al. ; Writing Committee for the Cornea Donor Study Research Group . Donor age and factors related to endothelial cell loss 10 years after penetrating keratoplasty: Specular Microscopy Ancillary Study. Ophthalmology. 2013;120(12):2428-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Cornea Preservation Time Study Group: Clinical Sites
eAppendix 2. Institutional Review Boards
eTable 1. Quality Control Repeat Grading Results
eTable 2. Baseline Recipient Characteristics in Eyes with Graft Success
eTable 3. CPTS Donor Characteristics in Eyes with Graft Success
eTable 4. Four Year Endothelial Cell Density (cells/mm2) in Eyes with Graft Success
Trial Protocol and CPTS Statistical Analysis Plan