Key Points
Question
What is the 3-dimensional anatomy of polypoidal complexes in polypoidal choroidal vasculopathy?
Findings
In this cohort study of 47 patients with polypoidal choroidal vasculopathy, optical coherence tomography angiography quantitatively demonstrated polypoidal complexes in 36.2% (polypoidal structures) and 55.3% (branching vascular networks). Branching vascular networks and vessels sprouting from the choriocapillaris level were arranged in a 3-dimensional architecture, with polypoidal structures within the inner part of the level of the retinal pigment epithelium reference plane, the branching vascular networks on the outer side of the retinal pigment epithelium reference plane, and a stalk even farther out into the choroidal level.
Meaning
These findings suggest that optical coherence tomography angiography identifies quantitative 3-dimensional structures of polypoidal lesions that may help understanding of the pathogenesis of polypoidal choroidal vasculopathy.
Abstract
Importance
Investigating the quantitative 3-dimensional (3-D) anatomy of polypoidal complex is important for a better understanding of the pathogenesis of polypoidal choroidal vasculopathy (PCV).
Objective
To quantitatively evaluate the 3-D characteristics of polypoidal structures, branching vascular networks (BVNs), and origin of PCV using optical coherence tomography angiography (OCTA) and multiple image systems.
Design, Setting, and Participants
A prospective, observational study was conducted in 47 consecutive Taiwanese patients (47 eyes) from May 21, 2015, to April 30, 2017. All participants were scanned with the Optovue-RTVue-XR-Avanti OCTA system. Patients in whom PCV was identified on OCTA were examined to define characteristics and structures of the original spouting vessels (stalks) from the choroid, polypoidal structures, and BVNs on OCTA.
Main Outcomes and Measures
Quantitative analysis of 3-D structures of the polypoidal complex.
Results
Among the 47 patients, the mean (SD) patient age was 68.9 (8.0) years, and 28 (59.6%) men were included. Clear images of polypoidal structures could be detected in 17 eyes (36.2%, 22 polypoidal structures), BVNs in 26 eyes (55.3%, 26 tufts of BVNs), and stalks of origin from the choroid in 26 eyes (55.3%, 26 stalks) on the en face plane on OCTA. All polypoidal structures were found at a mean (SD) height of 45.3 (36.1) μm above the retinal pigment epithelium (RPE) reference plane that was preset by the machine, while the BVNs were found at a mean (SD) depth of 28.6 (14.2) μm below the RPE reference plane and the choroidal stalks at 80.4 (24.4) μm below RPE reference plane. The mean (SD) thickness of polypoidal structures was 38.4 (15.5) μm and of BVNs, 60.2 (25.0) μm. The polypoidal structures were all above the Bruch membrane within the dome of the RPE detachment, the choroidal stalks were all in the choroid layer. The BVNs could be either above (up to 18 μm), within, or below (up to 28 μm) the Bruch membrane and were in proximity to the double layers of flattened RPE detachment.
Conclusions and Relevance
These results demonstrate a 3-D architecture of PCV that may be helpful for a better understanding of the anatomy, pathophysiology, and pathogenesis of PCV.
This cohort study assesses the use of 3-dimensional imaging by means of optical coherence tomography angiography to show polypoidal complexes in patients with polypoidal choroidal vasculopathy.
Introduction
Polypoidal choroidal vasculopathy (PCV) was first described by Yannuzzi and associates1 as a specific type of age-related macular degeneration characterized by the appearance of subretinal orange-red and nodular lesions that were best detected as bright, hyperfluorescent, polypoidal lesions in indocyanine green angiography (ICGA) examination. The polypoidal lesions may be located at the margin of an associated, less hyperfluorescent branching vascular network (BVN) in ICGA and may cause multiple, recurrent serosanguinous detachments of the retinal pigment epithelium (RPE) and neurosensory retina as well as secondary bleeding or leakage.
The pathogenesis of the polypoidal complex in PCV is not fully understood.2 Yannuzzi et al1 described PCV as a primary abnormality of the inner choroid. Subsequent histopathologic studies have revealed that PCV lesions were located either in the inner choroidal vasculature (beneath the Bruch membrane)3,4 or within an intra-Bruch membrane fibrovascular complex.5,6,7 Further study using optical coherence tomography (OCT) has revealed that patients with PCV often have highly elevated RPE detachment with associated double-reflective layers that consist of RPE and another highly reflective layer beneath the RPE (termed the double-layer sign) in the area of the BVN.8 Studies using high-resolution spectral-domain OCT have revealed that the possible location of PCV may be between the RPE and Bruch membrane, where the polypoidal structures may be situated high beneath the roof of the RPE detachment, while the BVNs were most likely located within the double-layer structure of the RPE and Bruch membrane.8,9,10,11,12,13,14
En face OCT angiography (OCTA) is novel technology that can visualize the actual structure by using the blood flow of the chorioretinal microcirculation without intravenous dye injection.15,16,17 Furthermore, with appropriate adjustment of the segmentation slabs throughout the full thickness of chorioretinal structures, OCTA can allow us to visualize 3-dimensional (3-D) analysis of flow structures of pathologic vascular tissues, such as choroidal neovascularization in age-related macular degeneration18,19,20,21,22,23 and polypoidal structures in PCV.24,25,26,27,28 Several studies have investigated the flow structures of PCV and suggested that the polypoidal structures and BVNs are situated in the compartment space between the RPE and Bruch membrane rather than in the choroid.24,25,26,27 In the present study, by using a layer-by-layer analysis according to the RPE reference plane, we further investigated the quantitative anatomic relationship of the 3-D structures of the polypoidal complex, including the origin of the choroidal stalks, BVN, and polypoidal structures in PCV that may be helpful for a full understanding of the pathogenesis of the disease.
Methods
This was a prospective observational study. Consecutive patients with PCV presenting to the retinal center at Shin-Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan, from May 21, 2015, to April 30, 2017, were included. This study was conducted in accordance with the Declaration of Helsinki29 and with approval of Shin-Kong Wu Ho-Su Memorial Hospital. Informed consent was obtained verbally from all patients. The participants did not receive financial compensation.
The diagnosis of PCV was established by the finding of polyplike choroidal vessel dilatation with a BVN on ICGA, using a confocal scanning laser ophthalmoscope (Heidelberg Retina Angiograph 2; Heidelberg Engineering). Fluorescein angiography was also performed, most often simultaneously with ICGA by the same machine, for evaluation of the leaking status of the PCV lesions. Patients were excluded if they were affected by any other macular disorders, such as evidence of an angioid streak, pathologic myopia (myopia >6 diopters) or other macular degenerative diseases, and a history of other macular abnormalities related to diabetic retinopathy, retinal vein occlusion, uveitis, or other retinal diseases.
For each patient, a complete ophthalmologic examination was performed, including a best-corrected visual acuity measurement using Early Treatment Diabetic Retinopathy Study charts, intraocular pressure measurement (Non Contact Tonometer, NT-530; Nidex Co Ltd), slitlamp examination, fundus examination, scanning laser ophthalmoscopy, fluorescein angiography, and ICGA (Spectralis HRA; Heidelberg Engineering). Spectral-domain OCT was also performed in association with OCTA for a comparative examination.
Optical coherence tomography angiography was performed in all patients with a scanning area of 3 × 3 mm, centered on the fovea (when the polypoidal lesions and BVNs were near the foveal center) or centered on the polypoidal lesions and BVNs directly (RTVue XR Avanti; Optovue) to obtain amplitude-decorrelation angiography images. The characteristics of this instrument have been described in detail previously.16 Briefly, the instrument has an A-scan rate of 70 000 scans per second, using an infrared light source (centered on 840 nm with a bandwidth of 50 nm). Each OCTA volume contains 304 × 304 A-scans, using a split-spectrum amplitude decorrelation angiography algorithm to extract the OCTA information. Angiography information could be displayed perpendicularly through the thickness of vitreous-retina-choroidal tissues for layer-by-layer evaluation. The machine also allows for some manual adjustments in each segmentation method for specific evaluation purposes.
Our study used a manual adjustment for depth and width of segmentation slabs according to the machine-defined RPE reference plane (best elliptical fit of RPE) to evaluate layer-by-layer flow signals from the outermost part of the large choroidal vascular layer to the innermost part of the retina intersecting with the internal limiting membrane. We concurrently compared the images of OCTA with the pictures of fluorescein angiography and ICGA. Each PCV eye was evaluated on the detailed layer-by-layer vascular flow structures beneath the RPE detachments that correlated with the polypoidal lesions seen on ICGA. Specific features of the origin of the polypoidal complex (choroidal stalk), BVNs, and polypoidal structures were carefully delineated by comparing the en face images in OCTA with images in fluorescein angiography and ICGA. The thickness of slabs that were used for the evaluation of the polypoidal complex was set at 1 μm for initial scanning. The depths of the slabs (in micrometers) of the beginning and ending images of origin from the choroidal stalk, BVN, and polypoidal structures were recorded after scanning through the entire polypoidal complex. The thickness and mean depth of each flow structure were calculated accordingly. The thickness and depth of the slab were then adjusted to present the clearest view of the entire flow structures of the choroid stalk, BVN, and polypoidal structures for demonstration in figures.
Results
A total of 47 consecutive Taiwanese patients (28 [59.6%] men and 19 [40.4%] women; mean [SD] age, 68.9 [8.0] years; range, 55-86 years) were included; 47 eyes (18 right eyes [38.3%] and 29 [61.7%] left eyes) with a PCV diagnosis were examined. Twenty-two polypoidal structures were detected in 17 eyes (36.2%) and 26 BVNs were detected in 26 eyes (55.3%). Twenty-six choroidal stalks were also identified in 26 eyes (55.3%). In 17 eyes (36.2%), clear images of both polypoidal structures and BVNs could be obtained on the en face plane on OCTA. Twenty-three patients had received previous intravitreal anti–vascular endothelial growth factor therapy with a mean (SD) of 8.0 (8.5) (range, 1-37) injections, and 3 patients had received verteporfin photodynamic therapy on 1 occasion. The remaining 24 patients were treatment naïve.
By the observation of layer-by-layer slabs of the polypoidal complex, the mean (SD) depth of the choroidal stalk was found to begin at 80.4 (24.4) μm below the RPE reference plane. The mean (SD) depth of the BVN was identified at a mean (SD) of 28.6 (14.2) μm (mean [SD] thickness, 60.2 [25.0] μm; range, 37-142 μm) below the RPE reference plane, and the polypoidal structures were situated within a mean (SD) of 45.3 (36.1) μm above the RPE reference plane (mean [SD] thickness, 38.4 [15.5] μm; range, 6-65 μm). The mean (SD) distance between the choroidal stalks and BVNs was 51.8 (16.8) μm, and the mean (SD) distance between BVNs and polypoidal structures was 74.4 (44.6) μm. The depth of the inner margin of the Bruch membrane was found at a mean (SD) of 30.8 (8.0) μm. The BVNs could be either above (up to 18 μm), within, or below (up to 28 μm) the Bruch membrane and were in proximity to the double layers of flattened RPE detachment. Of the 22 polypoidal structures, the locations were subfoveal in 5 (22.7%), juxtafoveal in 11 (50.0%), and extrafoveal in 6 (27.3%).
The entire images of choroidal stalk, BVN, and polypoidal structures of polypoid complexes in several eyes are depicted in Figure 1, Figure 2, and Figure 3, and eFigures 1-5 in the Supplement. Schematic drawings of the 3-D flow structures are also demonstrated in Figure 3. The flow signals of the BVN and polypoidal structures were also depicted in some of the corresponding cross-sectional B-scans, which demonstrated that the flow signal of polypoidal structures was mostly situated beneath the roof of the RPE detachment while the flow signal of the BVN was primarily detected in close association with the Bruch membrane.
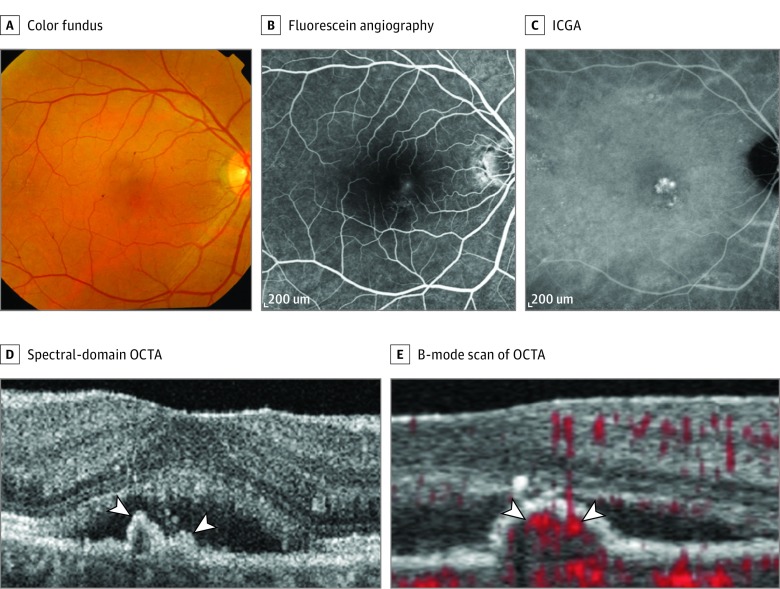
Figure 1. Multimodal Imaging of the Right Eye of a Woman in Her 50s With Subfoveal Polypoidal Choroidal Vasculopathy.
A, Color fundus photograph. B, Midphase fluorescein angiography. C, Midphase indocyanine green angiography (ICGA). D, The spectral-domain optical coherence tomography angiography (OCTA) shows the peaked retinal pigment epithelial detachment (white arrowheads). E, The B-mode scan of OCTA shows the blood flow signal (red signals [white arrowheads]) indicating polypoidal structures.
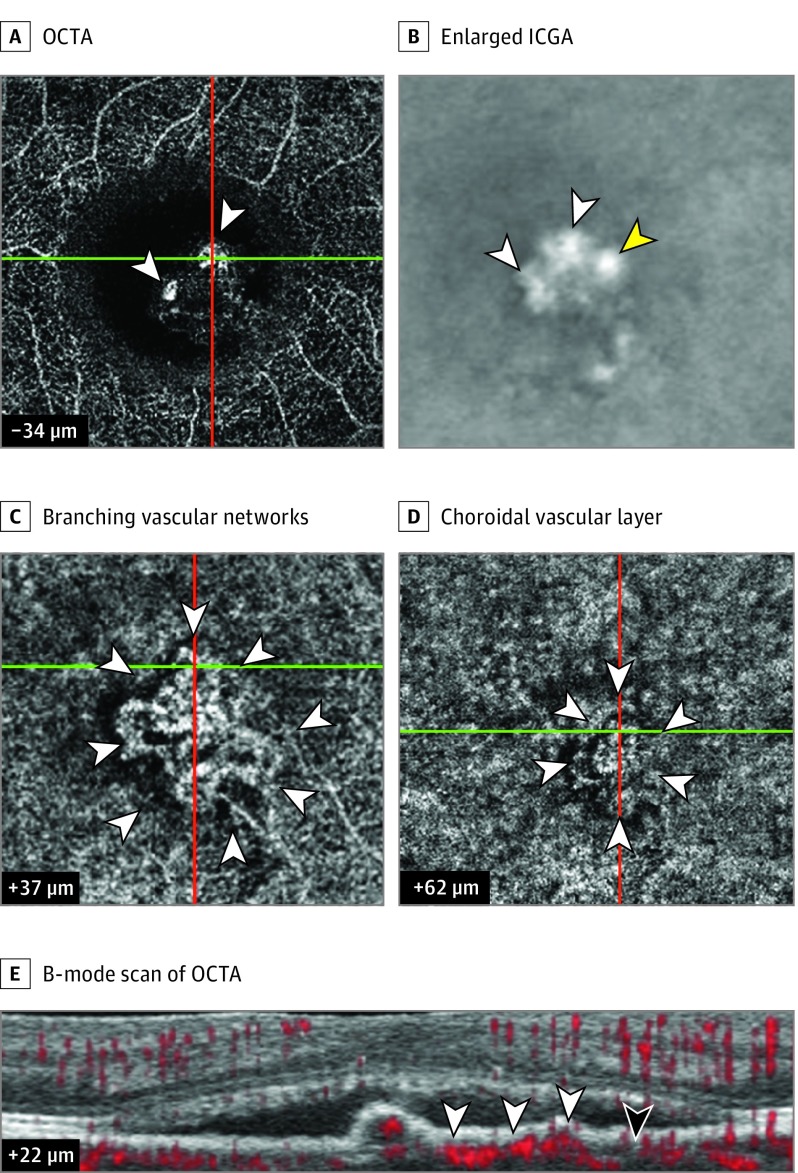
Figure 2. Further Multimodal Imaging of the Right Eye of a Woman in Her 50s With Subfoveal Polypoidal Choroidal Vasculopathy.
A, Optical coherence tomography angiography (OCTA) shows polypoidal structures (white arrowheads). B, A corresponding enlarged image part A with the use of indocyanine green angiography (ICGA) reveals polypoidal structures (white arrowheads). The yellow arrowhead reveals 1 polypoidal structure present in ICGA but absent in OCTA. C, OCTA shows full-size branching vascular networks (BVN) (white arrowheads). D, A stalklike vascular structure (white arrowheads) in OCTA is in the choroidal vascular layer. E, The B-mode scan of OCTA indicates the blood flow signal of the BVN (white arrowheads). The black arrowhead shows the Bruch membrane (+22 µm indicates the level of Bruch membrane). The plus sign indicates below retinal pigment epithelium reference and the minus sign indicates above retinal pigment epithelium reference. The green horizontal and red vertical lines represent the original markers of OCTA for indicating the localization of the lesions.
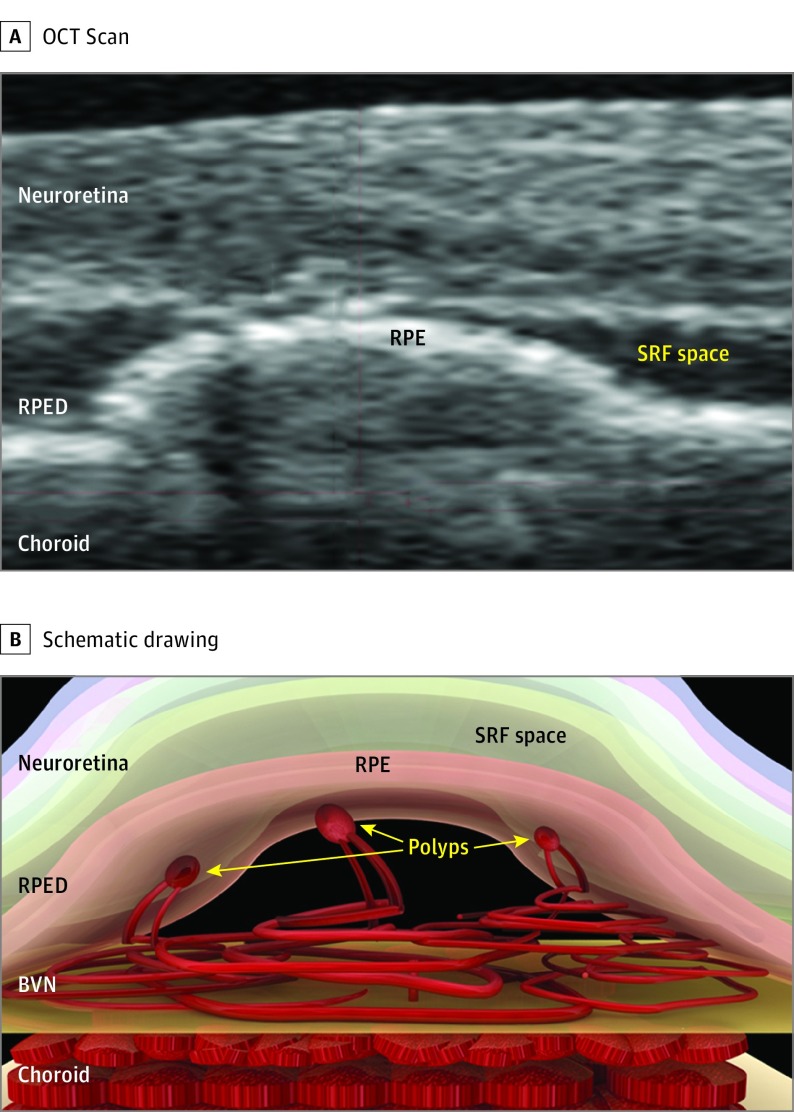
Figure 3. Scan and Schematic Drawing of the 3-Dimensional Structure of Polypoidal Choroidal Vasculopathy Represented in Figure 1 and Figure 2.
A, Optical coherence tomography (OCT) scan. B, Schematic drawing of polypoidal choroidal vasculopathy scan. Polypoidal structures sprout from the flattened branching vascular network (BVN) and are farther in than the plane of the BVN. The BVN resembles a flattened vascular tuft and seems to lie in close apposition with the Bruch membrane and is originated from the choroid. RPE indicates retinal pigment epithelium; RPED, retinal pigment epithelial detachment; and SRF, subretinal fluid.
Discussion
By applying a layer-by-layer analysis of en face OCTA using the RPE layer (segmentation layer parallel to machine-set choriocapillaris layer) as a reference plane, our study clearly demonstrated a particular characteristic in the 3-D structures in PCV: in most conditions, the polypoidal structures are seated farther inward than the BVN. In our patients, the BVN was situated within a mean of 28.6 (14.2) μm below the RPE reference plane while the polypoidal structures were situated within a mean of 45.3 (36.1) μm above the RPE reference plane, with a mean (SD) distance of 74.4 (44.6) μm. This condition is also clearly demonstrated in the recent version of the machine software (OCTA; RTVue XR Avanti; Optovue, version 2016.1.0.23) in which the flow signal can be depicted in the corresponding cross-sectional B-scans showing that the flow signals of polypoidal structures were mostly situated beneath the roof of RPE detachments while the flow signals of BVNs were mostly detected in close association with the Bruch membrane, either above (up to 18 μm), within, or below (up to 28 μm) the Bruch membrane. In addition, with layer-by-layer segmentation going from the Haller layer of the choroid to the outer retinal layer, a stalklike structure could be identified in 55.3% of the eyes (26 of 47 eyes) in our patients. These stalklike flow signals were situated at the outermost position of the entire PCV complex and were separated by a mean (SD) of 51.8 (16.8) μm from the BVN plane. These stalks may indicate the origin of the PCV that sprouted from the choroid vasculature.
These peculiar 3-D structures of PCV have long been suspected by many authors by observing the detailed structures using multimodal imaging techniques combining ICGA with spectral-domain OCT. For example, De Salvo et al30 reported that, in PCV, the spectral-domain OCT could sometimes reveal rounded, hyporeflective areas resembling the polypoidal lumen within the hyperreflective lesions adherent to the underside of the RPE. However, since the conventional spectral-domain OCT could only demonstrate the structural image of polypoidal structures without detecting blood flow, the 3-D structures could only be inferred by the morphologic similarities of sacculated images that resemble the polypoidal structures within the RPE detachment space. In addition, with the limitation of blockage of most light sources by RPE layer, a clear unequivocal image could not always be obtained in most conditions, which further makes the interpretation of the structures of PCV difficult. The unique characteristics of OCTA that could detect active blood flow in all layers of the retina-RPE-choroidal complex make this interpretation much easier. By detecting active flow in the sacculated structures lying high in the roof of RPE detachment and in the tubular structures lying near the floor of the Bruch membrane, we can accurately identify that these structures are vascular tissues. With the assistance of en face imaging and ICGA for contrast and by images superimposed on each other, these 3-D architectural characteristics of PCV could further be confirmed.
Several groups of researchers have investigated the anatomic structures of PCV by using OCTA and revealed that the polypoidal structures and the BVN are most likely situated in the compartment space between RPE and the Bruch membrane.24,25,26,27 By overlaying flow signals into different retinal layers in cross-sectional OCTA, Tomiyasu et al26 and Wang et al27 demonstrated results similar to ours that most of the flow signals of polypoidal structures (usually at the top of the underside of the RPE detachment roof) were situated more inward than the BVNs (usually close to the RPE reference plane). Our study provides further quantitative analysis of the relationship between the inner seated polypoidal structures and the outer seated BVNs, with a mean (SD) separation of 74.4 (44.6) μm. In addition, we identified a stalklike structure with flow signals indicating the origin of the sprouting of the PCV complex, which was located a mean of 51.8 (16.8) μm from the BVN plane. This finding also confirmed previous reports that PCV may have feeding vessels originating from the choroid.2,12,28,31,32 Combining these findings, a 3-D architecture of the entire PCV complex could be reconstructed, including a stalk sprouting from the choroid that was growing into a tangle or sea fan–like BVN situated in close contact with the Bruch membrane, and then terminating into polypoidal structures situated in the innermost part at the underside of RPE detachment.
Although our study revealed a relative spatial relationship between the polypoidal structures and BVNs, the absolute locations of BVNs were not confirmed in OCTA images of our cases. As indicated by the offset levels in the machine-set scale box in the monitor for indication of the depth relative to the RPE reference plane, the polypoidal structures were all located farther inward than the RPE reference plane (mean [SD], 45.3 [36.1] μm) as predicted; the locations of the BVN in our cases were mostly under (outward from) the RPE reference plane by a mean (SD) of 28.6 (14.2) μm, and the locations of the inner margin of the Bruch membrane were all under (outward from) the RPE reference plane (mean [SD], 30.8 [8.0] μm). If we assume that the machine-set RPE reference plane is correctly marked at the scale (0 as indicated in the scale box), then our results may indicate that the BVNs could be situated either above (up to 18 μm), within, or below (up to 28 μm) the Bruch membrane and in the inner choroidal layer, depending on the height of the RPE detachment and subsequent elevation of the machine-set RPE reference from the Bruch membrane. The exact location of the BVN relative to the Bruch membrane has been controversial.2 Initially, the PCV complex was thought to be in the inner choroidal level,1 while additional studies combining ICGA and spectral-domain OCT images have shown PCV lesions located between the RPE and Bruch membrane.8,9,10,11,12,13,14 This controversy was also present in the histopathologic studies showing that, in some specimens, PCV lesions were located either just beneath the Bruch membrane4 or in the inner choroidal vasculature.3 However, in other studies, the lesions were found beneath the RPE or within an intra–Bruch membrane fibrovascular complex.5,6,7 Discrepancy also exists regarding the exact location of BVNs detected by OCTA. While most OCTA studies concluded that BVNs of PCV are situated in the compartment space between the RPE and Bruch membrane,24,25,26,27 Tanaka et al28 found that BVNs were located in the choroidal level in 14 of 32 patients with PCV. Even though the article by Inoue et al24 concluded that the BVNs were situated between the RPE and Bruch membrane, the investigators found that the best images of type 1 choroidal neovascularization and the BVN could be acquired by setting the plane of segmentation on the outer aspect of the Bruch membrane when performing the OCTA imaging of PCV or polypoid choroidal neovascularization. Srour et al25 noted that, when attempting to identify the location of BVNs, their visualization in OCTA at that precise level was impaired mainly by the projection artifact mirrored from the choriocapillaris segmentation plane. This projection artifact is a well-documented pitfall in interpreting the images of OCTA that is frequently misleading as to the localization of the depth of a certain flow signal.33
In our study, the flow signals of the BVN could be detected in only 55.3% of patients with PCV (26 of 47) and the flow signals with configuration of polypoidal structures could be detected in an even lower proportion of only 36.2% of the patients (17 of 47). The low detection rate of OCTA for polypoidal structures and the BVN in PCV were also found in several previous studies that reported a detection rate of ICGA-identifiable polypoidal structures between 25% and 50%.24,25,26,34 The major conditions in our cases that impeded the detection rate included subretinal or sub-RPE hemorrhage; large, serous RPE detachment; and unstable fixation. Other factors that impeded the detection of polypoidal structures may be due to the slower flow velocity resulting from an abrupt dilatation or partial obstruction of the lumen of the polypoidal structures24 or a turbulent flow in the polypoidal structures.25 Since the OCTA could detect a flow velocity only within a limited range, it is possible that blood flow in some polypoidal structures was slower than the detection limit of OCTA and undetected by OCTA (false-negative artifact).33
Limitations
Our study had several limitations. First, it frequently was not able to visualize the structure of the PCV using OCTA owing to the presence of extensive exudation, hemorrhage, edema, and large RPE detachment in PCV cases. Second, the segmentation algorithm was preset by the OCTA system, which did not allow us to customize the segmentation slab to adapt to the contour of the lesions. This limitation could make the interpretation of the OCTA image difficult, especially for defining the exact location of the BVN. Third, although OCTA provides both 3 × 3-mm and 6 × 6-mm fields of view, a detailed image could only be obtained by the 3 × 3-mm field, which may not be enough to cover all of the extended areas of the entire BVN and polypoidal structures. In addition, the small numbers in our case series may preclude a generalized application of the results to all PCV cases.
Conclusions
By using layer-by-layer analysis of OCTA, our study demonstrated 3-D architecture of the PCV complex with actual flow signals. We provided flow-based support for the previous hypothesis that the PCV complex originates from the choroidal layer by a vascular stalk that ascends into sub-RPE space, becomes a flat tuft or tangle of BVN lying in close apposition with the Bruch membrane, and terminates even further toward the roof of the underside of the RPE detachment to form polypoidal structures. We also provide quantitative data for the extent of these 3 components of PCV that may be helpful for a better understanding of the anatomic and pathophysiologic aspects of PCV.
eFigure 1. Multimodal Imaging of the Right Eye of a 57-Year-Old Woman With Subfoveal PCV
eFigure 2. Multimodal Imaging of the Right Eye of a 57-Year-Old Woman With Subfoveal PCV
eFigure 3. Multimodal Imaging and OCTA of the Right Eye of a 67-Year-Old Man With Juxtafoveal PCV
eFigure 4. Multimodal Imaging and OCTA of the Right Eye of a 76-Year-Old Man With Subfoveal PCV
eFigure 5. Multimodal Imaging and OCTA of the Right Eye of a 79-Year-Old Man With Subfoveal PCV
References
- 1.Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10(1):1-8. [PubMed] [Google Scholar]
- 2.Ferrara D, Waheed NK, Duker JS. Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog Retin Eye Res. 2016;52:130-155. [DOI] [PubMed] [Google Scholar]
- 3.Okubo A, Sameshima M, Uemura A, Kanda S, Ohba N. Clinicopathological correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Ophthalmol. 2002;86(10):1093-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuroiwa S, Tateiwa H, Hisatomi T, Ishibashi T, Yoshimura N. Pathological features of surgically excised polypoidal choroidal vasculopathy membranes. Clin Exp Ophthalmol. 2004;32(3):297-302. [DOI] [PubMed] [Google Scholar]
- 5.Lafaut BA, Aisenbrey S, Van den Broecke C, Bartz-Schmidt KU, Heimann K. Polypoidal choroidal vasculopathy pattern in age-related macular degeneration: a clinicopathologic correlation. Retina. 2000;20(6):650-654. [DOI] [PubMed] [Google Scholar]
- 6.Rosa RH Jr, Davis JL, Eifrig CW. Clinicopathologic reports, case reports, and small case series: clinicopathologic correlation of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 2002;120(4):502-508. [DOI] [PubMed] [Google Scholar]
- 7.Terasaki H, Miyake Y, Suzuki T, Nakamura M, Nagasaka T. Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation. Br J Ophthalmol. 2002;86(3):321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato T, Kishi S, Watanabe G, Matsumoto H, Mukai R. Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina. 2007;27(5):589-594. [DOI] [PubMed] [Google Scholar]
- 9.Kokame GT. Prospective evaluation of subretinal vessel location in polypoidal choroidal vasculopathy (PCV) and response of hemorrhagic and exudative PCV to high-dose antiangiogenic therapy (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2014;112:74-93. [PMC free article] [PubMed] [Google Scholar]
- 10.Ojima Y, Hangai M, Sakamoto A, et al. Improved visualization of polypoidal choroidal vasculopathy lesions using spectral-domain optical coherence tomography. Retina. 2009;29(1):52-59. [DOI] [PubMed] [Google Scholar]
- 11.Mrejen S, Spaide RF. Optical coherence tomography: imaging of the choroid and beyond. Surv Ophthalmol. 2013;58(5):387-429. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Kang SW, Kim TH, Kim SJ, Ahn J. Structure of polypoidal choroidal vasculopathy studied by colocalization between tomographic and angiographic lesions. Am J Ophthalmol. 2013;156(5):974-980.e2. [DOI] [PubMed] [Google Scholar]
- 13.Alshahrani ST, Al Shamsi HN, Kahtani ES, Ghazi NG. Spectral-domain optical coherence tomography findings in polypoidal choroidal vasculopathy suggest a type 1 neovascular growth pattern. Clin Ophthalmol. 2014;8:1689-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan S, Engelbert M, Imamura Y, Freund KB. Polypoidal choroidal vasculopathy: simultaneous indocyanine green angiography and eye-tracked spectral domain optical coherence tomography findings. Retina. 2012;32(6):1057-1068. [DOI] [PubMed] [Google Scholar]
- 15.Coscas GJ, Lupidi M, Coscas F, Cagini C, Souied EH. Optical coherence tomography angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration: a new diagnostic challenge. Retina. 2015;35(11):2219-2228. [DOI] [PubMed] [Google Scholar]
- 16.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puliafito CA. OCT angiography: the next era of OCT technology emerges. Ophthalmic Surg Lasers Imaging Retina. 2014;45(5):360. [DOI] [PubMed] [Google Scholar]
- 18.Kuehlewein L, Sadda SR, Sarraf D. OCT angiography and sequential quantitative analysis of type 2 neovascularization after ranibizumab therapy. Eye (Lond). 2015;29(7):932-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phasukkijwatana N, Tan AC, Chen X, Freund KB, Sarraf D. Optical coherence tomography angiography of type 3 neovascularisation in age-related macular degeneration after antiangiogenic therapy. Br J Ophthalmol. 2017;101(5):597-602 . [DOI] [PubMed] [Google Scholar]
- 20.El Ameen A, Cohen SY, Semoun O, et al. Type 2 neovascularization secondary to age-related macular degeneration imaged by optical coherence tomography angiography. Retina. 2015;35(11):2212-2218. [DOI] [PubMed] [Google Scholar]
- 21.Dansingani KK, Naysan J, Freund KB. En face OCT angiography demonstrates flow in early type 3 neovascularization (retinal angiomatous proliferation). Eye (Lond). 2015;29(5):703-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miere A, Querques G, Semoun O, El Ameen A, Capuano V, Souied EH. Optical coherence tomography angiography in early type 3 neovascularization. Retina. 2015;35(11):2236-2241. [DOI] [PubMed] [Google Scholar]
- 23.Kuehlewein L, Dansingani KK, de Carlo TE, et al. Optical coherence tomography angiography of type 3 neovascularization secondary to age-related macular degeneration. Retina. 2015;35(11):2229-2235. [DOI] [PubMed] [Google Scholar]
- 24.Inoue M, Balaratnasingam C, Freund KB. Optical coherence tomography angiography of polypoidal choroidal vasculopathy and polypoidal choroidal neovascularization. Retina. 2015;35(11):2265-2274. [DOI] [PubMed] [Google Scholar]
- 25.Srour M, Querques G, Semoun O, et al. Optical coherence tomography angiography characteristics of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2016;100(11):1489-1493. [DOI] [PubMed] [Google Scholar]
- 26.Tomiyasu T, Nozaki M, Yoshida M, Ogura Y. Characteristics of polypoidal choroidal vasculopathy evaluated by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT324-OCT330. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Zhou Y, Gao SS, et al. Evaluating polypoidal choroidal vasculopathy with optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT526-OCT532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka K, Mori R, Kawamura A, Nakashizuka H, Wakatsuki Y, Yuzawa M. Comparison of OCT angiography and indocyanine green angiographic findings with subtypes of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2017;101(1):51-55. [DOI] [PubMed] [Google Scholar]
- 29.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 30.De Salvo G, Vaz-Pereira S, Keane PA, Tufail A, Liew G. Sensitivity and specificity of spectral-domain optical coherence tomography in detecting idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol. 2014;158(6):1228-1238.e1. [DOI] [PubMed] [Google Scholar]
- 31.Alasil T, Ferrara D, Adhi M, et al. En face imaging of the choroid in polypoidal choroidal vasculopathy using swept-source optical coherence tomography. Am J Ophthalmol. 2015;159(4):634-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuzawa M. [Polypoidal choroidal vasculopathy] [in Japanese]. Nippon Ganka Gakkai Zasshi. 2012;116(3):200-231. [DOI] [PubMed] [Google Scholar]
- 33.Spaide RF, Fujimoto JG, Waheed NK. Image Artifacts in Optical Coherence Tomography Angiography. Retina. 2015;35(11):2163-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JY, Kwon OW, Oh HS, Kim SH, You YS. Optical coherence tomography angiography in patients with polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(8):1505-1510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Multimodal Imaging of the Right Eye of a 57-Year-Old Woman With Subfoveal PCV
eFigure 2. Multimodal Imaging of the Right Eye of a 57-Year-Old Woman With Subfoveal PCV
eFigure 3. Multimodal Imaging and OCTA of the Right Eye of a 67-Year-Old Man With Juxtafoveal PCV
eFigure 4. Multimodal Imaging and OCTA of the Right Eye of a 76-Year-Old Man With Subfoveal PCV
eFigure 5. Multimodal Imaging and OCTA of the Right Eye of a 79-Year-Old Man With Subfoveal PCV