Abstract
Objective
To explore the therapeutic effects of a traditional Chinese medicine (TCM) regimen on patients with ruptured lumbar disc herniation, including assessing its effects on prognosis and protrusion size.
Methods
From June 2008 to December 2011, 102 patients with ruptured lumbar disc herniation who chose conservative treatment with TCM as their first choice were followed up for 2 years to assess their final surgical rate, improvement according to Japanese Orthopaedic Association (JOA) scores, and to calculate the volume and rate of resorption of their protrusions by magnetic resonance imaging (MRI).
Results
(i) Eighty‐three of the 102 patients (81.37%) experienced partial or complete relief; the remaining 19 (18.63%) eventually needed surgery. (ii) In the 83 patents who underwent conservative treatment, rates of excellent JOA scores at 3 months, 6 months, 1 year and 2 years were 79.52%, 81.93%, 81.93% and 83.13% respectively; differences between these and pretreatment scores are all statistically significant (P < 0.01). (iii) The volume of protrusion in the patients who chose conservative treatment decreased from 1433.89 ± 525.49 mm3 (mean ± SD) to 1002.01 ± 592.95 mm3, which is statistically significant (t = 6.854, P < 0.01). The average resorption rate was 27.25% ± 32.97%; in 20 patients (24.10%) the resorption rate was >50%. The remaining 63 patients had no obvious resorption; their excellent rate was 77.77%. The difference in rate of achieving an excellent outcome differed significantly between those who did and did not have resorption of their protrusions (P = 0.018).
Conclusion
Conservative treatment with a TCM regimen is effective for ruptured lumbar disc herniation and can promote resorption of the protrusion; however, patients who develop specific indications for surgery during such treatment should undergo surgery in a timely manner.
Keywords: Intervertebral disc displacement, Lumbar vertebra, Posterior longitudinal ligament, Resorption, Traditional Chinese Medicine
Introduction
Magnetic resonance imaging (MRI) has become the most important imaging technique for diagnosing lumbar disc herniation. Lumbar disc herniation can be subdivided into the categories of ruptured or non‐ruptured according to the continuity of the posterior longitudinal ligament on MRI1. Ruptured lumbar disc herniation with large or sequestered protrusions produces serious symptoms. Many clinicians routinely offer operative treatment as the first choice when MRI shows huge ruptured protrusions, rather than assessing clinical signs and symptoms and considering trying conservative treatment. We therefore lack reports of research describing long‐term follow‐up and prognosis with conservative treatment of this disease. Many clinical guidelines and the Spine Patient Prognosis Research Trial acknowledge the effectiveness of conservative treatment for patients with lumbar disc herniation, including those with protrusions2, 3, 4. Ruptured lumbar disc herniation is included in the categories of “lumbago”, “paralysis” and so on in traditional Chinese Medicine (TCM). In China, the symptoms of many patients with ruptured protrusions are relieved, sometimes completely, by conservative treatment with TCM: as has been shown in the present clinical study. Follow‐up with MRI has shown that some patients have imaging evidence of excellent therapeutic effects on protrusion resorption.
In this study, 102 patients with ruptured lumbar disc herniation whose first choice was conservative treatment with a TCM regimen were observed, and the rate of surgery, therapeutic effects of conservative treatment and changes in protrusion size analyzed.
Materials and Methods
General Data
From June 2008 to December 2011, data on 102 patients with ruptured lumbar disc herniation whose first choice was conservative treatment with a TCM regimen and who attended the Suzhou Hospital of Traditional Chinese Medicine were studied. Inclusion criteria were as follows: (i) radicular leg pain and positive straight‐leg raising test and Lasegue sign accompanied by decreased muscle strength and paresthesiae in the corresponding parts of the legs2; (ii) ruptured lumbar disc herniation diagnosed by MRI (the “black line” on T2 weighing sagittal views being disconnected and appearing distorted or lumpy, indicating the presence of a large or free protrusion with coarse and irregular edges)5; and (iii) the locations of the protruded segment and the symptoms being consistent. Exclusion criteria were as follows: (i) pregnancy, liver and kidney disease; (ii) rheumatism and immune system diseases such as combined rheumatic arthritis and ankylosing spondylitis; (iii) previous spinal surgery, scoliosis, spinal cord injury, tuberculosis, tumor, and cauda equina syndrome accompanied by impairment of nerve function; and (iv) osteoporotic fracture of lumbar vertebra, serious spinal deterioration, lumbar spondylolisthesis, protrusion of multiple segments, all causing symptoms.
Two to six MRI examinations were performed during the 2–24 months after commencing treatment. The initial and most recent of these were compared, the average interval between them being 6.17 ± 10.74 months. All 102 patients attended for ongoing follow‐up for over 2 years. They comprised 64 male and 38 female patients aged 16–60 years, (mean ± SD 38.66 ± 12.01 years). The locations of the protrusions were as follows: L3–4 (5 cases), L4–5 (44 cases) and L5S1 (53 cases). The duration of disease ranged from 3 days to 10 years, (mean ± SD 16.44 ± 22.87 months; the duration was less than 1 year in 47 cases and over 1 year in 55.
Treatment Schedule
Schedule of Conservative Treatment with TCM
Absolute bed rest is required for 3–4 weeks. Once permitted to ambulate, the patient is to wear a girdle for 4–8 weeks.
The patient is to take the TCM preparation Xiaosui Huahe decoction (raw Astragalus 20 g, roasted Astragalus 20 g, Radix Stephaniae Tetrandrae 10 g, Angelica sinensis 10 g, Ligusticum wallichii 10 g, Rhizoma Atractylodis Macrocephalae 10 g, Lumbricus 10 g, leech 6 g, Radix Clematidis 10 g, pawpaw 10 g and Brassica alba boiss 6 g) decocted in water and taken orally for 8–16 weeks6.
The patient is to perform the following exercises for 12–24 weeks: (i) five point support, in which the patient assumes a supine position and raises the abdomen and pelvis as high as possible while supporting the body with the head, elbows and feet for 3 seconds, then repeats this action 20–30 times on three occasions each day; and (ii) swallow style, in which the patient assumes a prone position with the hands behind the back and raises the head and chest while pushing the thighs back for 3–5 seconds, then repeats this action 10–20 times on three occasions each day.
For the first 1–2 weeks after an acute episode, if the pain is not relieved by the oral TCM prescription, the patient may take 0.1 g b.i.d. of celecoxib.
Indications for Surgery
Surgery was considered indicated when (i) conservative treatment with the TCM regimen for 3–6 months had been ineffective (Japanese Orthopaedic Association [JOA] scores <16 or improvement rate <25%); (ii) the patient experienced exacerbation or progression of radicular symptoms or cauda equina neurological signs at any stage during the treatment period; or (iii) reexamination by MRI showed that the size of the protrusion had not changed or increased.
Operative Procedures
Simple resection of the nucleus pulposus, decompression and fixation and fusion or non‐fusion and internal fixation were performed, the choice of procedure depending on the patient's age, underlying cause of the lesion and physiology of the lumbar vertebrae.
Means of Evaluation
Clinical Therapeutic Effect
This was evaluated by JOA scoring7 and calculated according to the following equation: Improvement according to JOA score (%) = (score after completion of treatment − score before treatment)/(29 − score before treatment) × 100 (the maximum possible score is 29). An improvement according to JOA score ≥75% was classified as excellent, 50%–75% as good, 25%–50% as fair and <25% as poor.
Measuring the Protrusion
Changes in protrusion size on MRI were assessed using a Siemens (Erlangen, Germany) 1.5T magnetic resonance imager with an spin‐echo sequence. Eleven sections were scanned on T1 and T2 weighted sagittal views, with an interlamellar spacing of 1.25 mm and section thickness of 5 mm. The image data was scanned and processed by Picture Archiving and Communication Systems. The volume and resorption rate of the protrusion were calculated according to the method described by Autio8. This is illustrated in Figure 1.
Figure 1.
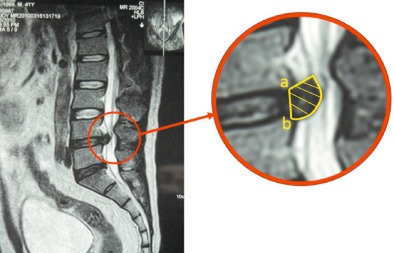
T 2 weighted sagittal view MR image showing how the resorption rate is calculated. The internal boundary of the protrusion is the connecting line between the posterior inferior margin of the upper centrum and the posterior superior margin of the lower centrum. The external boundary is the protrusion edge. A proficient MRI operator can determine the area of the protrusion, as shown in the right panel. The volume of the protrusion (mm3) = (inter‐section spacing + section thickness) (mm) × Σ area of the protrusion in each section (mm2). The resorption rate = (volume of protrusion before treatment—volume of protrusion after treatment/volume of protrusion before treatment) ×100(%).
Follow‐up
Follow‐up were performed by telephone inquiry and outpatient reexamination, which was performed 3, 6, 12 and 24 months after the first visit, and JOA scoring recorded on each occasion. MRI was performed on the first visit and the final follow‐up to calculate the volume of the protrusion. All patients completed the required follow‐up.
Statistical Analysis
Statistical analysis was performed with SPSS Statistics 20.0 software. Measurement data such as JOA scores and volume of protrusion were compared by Student's t‐test for matched data; whereas enumeration data such as improvement according to JOA score were compared by the Χ2 test or Fisher exact probability test. P < 0.05 was considered significant for all statistical tests.
Results
Symptoms improved in 83/102 patients who elected to receive conservative treatment with the TCM therapy regimen, the therapeutic effects in the remaining 19 patients 18.63% were unsatisfactory and they eventually required surgery (Figs 2, 3). Surgery was indicated in 11 of these patients because of failure to achieve improvement in symptoms after 3–6 months of conservative treatment (JOA score <16 or improvement according to JOA score <25%); five patients had initial symptomatic improvement but then had acute relapses with exacerbation and progression of symptoms; and the remaining three patients relapsed with fatigue and cauda equina syndrome. The time between commencing TCM treatment and surgery was 3–8 months (5.04 ± 1.85 months). In all patients who continued conservative treatment with the TCM regimen, JOA scores at 3 months, 6 months, 1 year and 2 years after commencing treatment were compared with those before treatment by Student's t‐test inspection for matched data; all these differences were statistically significant (Table 1).
Figure 2.
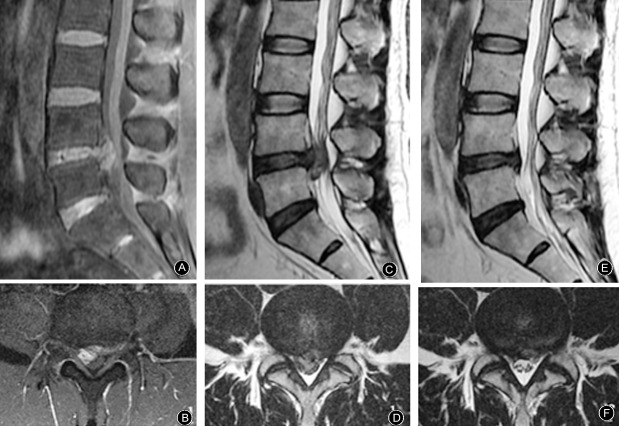
MR images from a 48‐year‐old man who presented with pain in the waist and lower right extremity for 20 days. He was unable to sleep or turn over because of the pain. He had received treatment with dexamethasone and mannitol in other hospitals; this was ineffective. Physical examination on the first visit in our hospital showed L 4,5 left paraspinal muscle tenderness radiating into the left lower extremity. Straight‐leg raising test, left 60°, right 10°; JOA score 5. (A, B) The volume of the protrusion is 2030.00 mm3, enhanced MRI indicates that the protrusion is high signal. After conservative treatment with the TCM regimen for 4 months, radiating pain in lower extremity had resolved, straight‐leg raising test, left 90°, right 90°. (C, D) MRI shows L4–5 huge ruptured protrusion. (E, F) Reexamination by MRI shows that most of the protrusion has been resorbed; the volume is now 507.50 mm3, the resorption rate is therefore 75.0%, JOA score 27. He remained free of relapse at the 2 years follow‐up.
Figure 3.
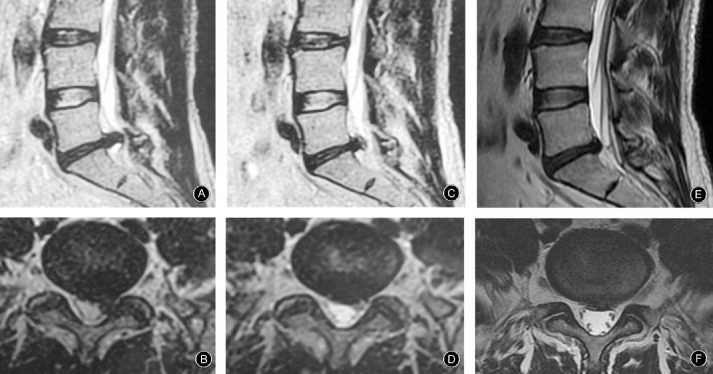
MR images from a 47 year‐old man who presented with cute pain in the left waist and lower extremity caused by sprain 3 days previously. On physical examination: he had lost lumbar curvature and had obvious L 5‐S 1 left paraspinal muscle tenderness, straight‐leg raising test, left 15°, right 70°, with normal muscle strength and sensation in both lower extremities. (A, B) Lumbar MRI shows L 5 S 1 ruptured protrusion of volume 1918.35 mm3; JOA score 5. The pain in his waist and lower extremities resolved after conservative treatment with the TCM regimen for 2 months, when physical examination showed obvious improvement in L 5 S 1 paraspinal muscle tenderness; straight‐leg raising test, left 75°, right 90°. (C, D) MRI shows the protrusion has resorbed, its volume now being 1026.67 mm3; JOA score 24. (E, F) After treatment for 19 months, his straight‐leg raising become normal, and the protrusion had resorbed further, its volume now being 653.66 mm3, the resorption rate is therefore 65.93%; JOA score 28.
Table 1.
Changes in JOA scores and improvement according to JOA scores at the indicated times during treatment
| Duration of follow‐up | n | JOA score [mean ± s.d.] | Treatment effect (n) | Excellent and good rate (%) | |||
|---|---|---|---|---|---|---|---|
| Excellent | Good | Fair | Poor | ||||
| Before treatment | 83 | 11.65 ± 4.25 | — | — | — | — | — |
| 3 months | 83 | 22.13 ± 2.79 | 18 | 48 | 13 | 4 | 79.52 |
| 6 months | 83 | 23.66 ± 2.83 | 32 | 36 | 14 | 1 | 81.93 |
| 1 year | 83 | 24.11 ± 2.96 | 36 | 32 | 15 | 0 | 81.93 |
| 2 years | 83 | 24.22 ± 3.06 | 35 | 34 | 14 | 0 | 83.13 |
In the 83 patients who continued with conservative treatment with the TCM regimen, MRI showed that protrusion resorption occurred in some patients. The volume of the protrusion decreased from 1433.89 ± 525.49 mm3 before treatment to 1002.01 ± 592.95 mm3 at the end of treatment; this difference was statistically significant (t = 6.854, P < 0.01). The rate of resorption of protrusions was 27.25% ± 32.97%. Table 2 shows the relationships between degree of protrusion resorption and improvements according to JOA scores. Obvious resorption was >50% in 20/83 patients (24.10%) who continued conservative treatment and 100% of these patients had excellent therapeutic effects during the 2‐year follow‐up. The remaining 63 patients had no obvious resorption; their excellent rate was 77.77%. The difference in rate of achieving an excellent outcome differed significantly between those who did and did not have resorption of their protrusions (P = 0.018).
Table 2.
Relationship between resorption of protrusion and treatment effect
| Resorption rate | Cases | Treatment effect* (cases) | Excellent and good rate (%) | |||
|---|---|---|---|---|---|---|
| Excellent | Good | Fair | Poor | |||
| Obvious resorption (≥50%) | 20 | 18 | 2 | 0 | 0 | 100 |
| Partial resorption (20%–50%) | 22 | 8 | 13 | 1 | 0 | 95.45 |
| Essentially unchanged (−20% to 20%) | 38 | 8 | 19 | 11 | 0 | 44.73 |
| Increase (≤−20%) | 3 | 1 | 0 | 2 | 0 | 33.33 |
*Treatment effect 2 years after initiating treatment.
Discussion
Resorption after Ruptured Lumbar Disc Herniation
Decrease in size or disappearance of nucleus pulposus protrusions associated with lumbar disc herniation without excision or other interventions to dissolve the nucleus pulposus is referred to as resorption. In 1984, Guinto et al. used CT to follow‐up patients with lumbar disc herniation undergoing conservative treatment; they were the first to report the phenomenon of resorption of protruded lumbar disc tissue9. In 1998, Jiang et al. were the first in China to report on the mechanism and clinical significance of this phenomenon10. Subsequent research has shown that possible mechanisms for resorption of lumbar disc herniation include the following: (i) immune and phagocytosis of inflammatory cells11, 12, 13, 14; (ii) growth of new vessels12, 15, 16; (iii) dehydration of the tissue or absorption of hematoma13, 17; (iv) degradation of tissue and apoptosis of cells18; and (v) spontaneous regression13. Phagocytosis of inflammatory cells and growth of new vessels are the key factors in resorption. Rupture of the posterior longitudinal ligament offers an opportunity for the protrusion to come into contact with the epidural blood supply, which provides favorable conditions for resorption of protrusion. This phenomenon also provides a theoretical basis for initially choosing conservative treatment for ruptured lumbar disc herniation.
TCM Therapy as the First Choice for Ruptured Lumbar Disc Herniation
In the last ten years, a number of scholars from China and other countries have reported that ruptured lumbar disc herniation is prone to undergoing resorption and that conservative treatment can achieve good therapeutic effects. These studies have provided more evidence and a basis for conservative treatment of this disease8, 19, 20, 21. In their book Lumbar Disc Herniation, Gunzburg and Szpalski explain the mechanism(s) for the natural favorable prognosis and resorption of lumbar disc herniation, using many illustrative cases in which treatment was conservative22. They point out that complete resorption of protruded nucleus pulposus most often occurs in patients with huge protrusions. High resorption rates (>70%) occur mainly in patients with huge and medium‐sized protrusions because the cells and blood vessels growing into the protruded lumbar disc can “eliminate the protrusion in a targeted manner”22. In our study, 68.29% of patients in whom resorption did not occur nevertheless achieved outcomes classified as excellent according to improvements in JOA scores. The mechanisms may have involved resolution of nerve root edema as well as changes in the deformation or displacement of the protrusion. Although large protrusions were still visible on MRI, they were no longer causing symptoms; however, these patients are at huge risk of relapse and exacerbation and therefore require long‐term follow‐up. Lebow et al. have shown that, 2 years after resection of the nucleus pulposus, about a quarter of patients have MRI evidence of (asymptomatic) lumbar disc herniation to the same extent as that before surgery23. This further indicates that the extent of protrusion seen on MRI is not always in direct proportion to the patients' symptoms or prognosis. In conclusion, whether or not resorption occurs, conservative treatment is a rational first choice for ruptured lumbar disc herniation and most patients will benefit. In the case of obvious resorption, the therapeutic effects will be excellent.
Mechanism for TCM Xiaosui Huahe Decoction's Beneficial Effects in Ruptured Lumbar Disc Herniation
The prescription of “Xiaosui Huahe decoction” used in our group's research is in line with ancient prescriptions of Radix stephaniae Tetrandrae and Astragalus decoction for invigorating yang to assist recuperation. Originating from the Synopsis of Golden Chamber, Tetrandra and Astragalus decoction is mainly used for symptoms of lung and spleen deficiency, failure of qi to transform fluid and internal stagnation of fluid‐damp type; it is a representative of classical prescriptions for tonifying qi and promoting diuresis. In terms of modern medicine, its path is consistent with that of promoting nucleus pulposus resorption and relieving nerve root edema. Decoctions that invigorate yang to assist recuperation were first recorded in Correction of the Errors of Medical Works by Wang Qingren in the Qing Dynasty. This book presents Wang's creative specialized perceptions for treating hemiplegia and a flaccidity syndrome caused by qi deficiency and blood stasis. In this prescription, qi‐tonifying drugs and preparations for promoting blood circulation to remove meridian obstruction are compatible; these inspire vigor, promote and invigorate and smooth blood circulation and are aimed at eliminating such symptoms as numbness, pain and muscle weakness.
In this prescription, roasted Astragalus roots tonify middle‐Jiao and Qi, which nourishes Qi to promote blood circulation and eliminate stagnation without impairing healthy energy. Because they are the sovereign medicine in this prescription, Astragalus roots should be put in a prominent position. Radix Stephaniae Tetrandrae dispels wind, eliminates dampness and induces diuresis to alleviate edema. Angelica sinensis promotes blood circulation to remove blood stasis and smooth blood circulation. Brassica alba boiss is good at warming cold phlegm, promoting qi circulation and eliminating stagnation; it is particularly good at eliminating phlegm between the skin and membranes. The aforesaid medicines were commonly used as ministerial drugs. As “a medicine for notifying qi and blood”, Ligusticum wallichii can assist Angelica sinensis to invigorate the blood circulation and remove stasis, and also promotes qi circulation to relieve pain. Atractylodis Macrocephalae tonifies the spleen, eliminates dampness and induces diuresis to alleviate edema. Pawpaw dispels dampness and dredges collaterals, nourishes the liver and is spasmolytic. It assists Radix Stephaniae Tetrandrae to induce diuresis. Radix clematidis has two functions here. First, this medicine softens hardness to dissipate stagnation and can dissolve bone (for example, a fish bone in the throat). Because it is similar to its image, it can also “dissolve” protruded nucleus pulposus. Second, with its nature of pungent taste with dispersing effect as well as moving and fleeing effects, it can guide various drugs to collaterals. It reduces irritation. Leech and Lumbricus both assist Brassica alba boiss to reduce phlegm, dissipate stagnation and dredge collaterals; they are both adjuvants. Using a combination of these various drugs can eliminate exogenous pathogenic factors, remove dampness, eliminate phlegm stagnation and smooth qi‐blood circulation. This results in relief of pain and the resolution of symptoms. The protrusion may also be resorbed.
In our study, there was MRI evidence that resorption of obvious protrusion occurred in 20 (24.1%) patients who chose conservative treatment; all these patients had excellent resolution of symptoms.
Acknowledgements
The authors thank all of the orthopedic and traumatology specialists in our department who have been providing information to our registry since 2008.
Disclosure: The authors have no conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of this manuscript. This research was founded by the Jiangsu Bureau of Traditional Chinese Medicine (NO.LZ13418).
References
- 1. Wong DA, Transfeldt E. Macnab's Backache, 4th edn. Philadelphia, PA: Lippincott Williams & Wilkins, 2007; 79. [Google Scholar]
- 2. Atlas SJ, Tosteson TD, Blood EA, Skinner JS, Pransky GS, Weinstein JN. The impact of workers' compensation on outcomes of surgical and nonoperative therapy for patients with a lumbar disc herniation: SPORT. Spine (Phila Pa 1976), 2010, 35: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rihn JA, Hilibrand AS, Radcliff K, et al Duration of symptoms resulting from lumbar disc herniation: effect on treatment outcomes: analysis of the Spine Patient Outcomes Research Trial (SPORT). J Bone Joint Surg Am, 2011, 93: 1906–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kreiner DS, Hwang SW, Easa JE, et al An evidence‐based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J, 2014, 14: 180–191. [DOI] [PubMed] [Google Scholar]
- 5. Matsubara Y, Kato F, Mimatsu K, Kajino G, Nakamura S, Nitta H. Serial changes on MRI in lumbar disc herniations treated conservatively. Neuroradiology, 1995, 37: 378–383. [DOI] [PubMed] [Google Scholar]
- 6. Jiang H. Lumbar Disc Herniation: Resorption and Treatment, 2nd edn. Nanjing: Jiangsu Science and Technology Publishing House, 2012: 153. [Google Scholar]
- 7. Toyone T, Takahashi K, Kitahara H, Yamagata M, Murakami M, Moriya H. Visualisation of symptomatic nerve roots. Prospective study of contrast‐enhanced MRI in patients with lumbar disc herniation. J Bone Joint Surg Br, 1993, 75: 529–533. [DOI] [PubMed] [Google Scholar]
- 8. Autio RA, Karppinen J, Niinimäki J, et al Determinants of spontaneous resorption of intervertebral disc herniations. Spine (Phila Pa 1976), 2006, 31: 1247–1252. [DOI] [PubMed] [Google Scholar]
- 9. Guinto FC Jr, Hashim H, Stumer M. CT demonstration of disk regression after conservative therapy. AJNR Am J Neuroradiol, 1984, 5: 632–633. [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang H, Shi Q, Zheng QB. The spontaneous resorption and clinical value of lumbar disc herniation. Zhonghua Gu Ke Za Zhi, 1998, 18: 755–757 (in Chinese). [Google Scholar]
- 11. Yoshida M, Nakamura T, Sei A, Kikuchi T, Takagi K, Matsukawa A. Intervertebral disc cells produce tumor necrosis factor alpha, interleukin‐1beta, and monocyte chemoattractant protein‐1 immediately after herniation: an experimental study using a new hernia model. Spine (Phila Pa 1976), 2005, 30: 55–61. [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi S, Meir A, Kokubo Y, et al Ultrastructural analysis on lumbar disc herniation using surgical specimens: role of neovascularization and macrophages in hernias. Spine (Phila Pa 1976), 2009, 34: 655–662. [DOI] [PubMed] [Google Scholar]
- 13. Slavin KV, Raja A, Thornton J, Wagner FC Jr. Spontaneous regression of a large lumbar disc herniation: report of an illustrative case. Surg Neurol, 2001, 56: 333–337. [DOI] [PubMed] [Google Scholar]
- 14. Zhu Y, Ohba T, Ando T, et al Endogenous TGF‐β activity limits TSLP expression in the intervertebral disc tissue by suppressing NF‐κB activation. J Orthop Res, 2013, 31: 1144–1149. [DOI] [PubMed] [Google Scholar]
- 15. Komori H, Shinomiya K, Nakai O, Yamaura I, Takeda S, Furuya K. The natural history of herniated nucleus pulposus with radiculopathy. Spine (Phila Pa 1976), 1996, 21: 225–229. [DOI] [PubMed] [Google Scholar]
- 16. Ratsep T, Minajeva A, Asser T. Relationship between neovascularization and degenerative changes in herniated lumbar intervertebral discs. Eur Spine J, 2013, 22: 2474–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orief T, Orz Y, Attia W, Almusrea K. Spontaneous resorption of sequestrated intervertebral disc herniation. World Neurosurg, 2012, 77: 146–152. [DOI] [PubMed] [Google Scholar]
- 18. Ha KY, Koh IJ, Kirpalani PA, et al The expression of hypoxia inducible factor‐1alpha and apoptosis in herniated discs. Spine (Phila Pa 1976), 2006, 31: 1309–1313. [DOI] [PubMed] [Google Scholar]
- 19. Splendiani A, Puglielli E, De Amicis R, Barile A, Masciocchi C, Gallucci M. Spontaneous resolution of lumbar disk herniation: predictive signs for prognostic evaluation. Neuroradiology, 2004, 46: 916–922. [DOI] [PubMed] [Google Scholar]
- 20. Cribb GL, Jaffray DC, Cassar‐Pullicino VN. Observations on the natural history of massive lumbar disc herniation. J Bone Joint Surg Br, 2007, 89: 782–784. [DOI] [PubMed] [Google Scholar]
- 21. Yu PF, Jiang FD, Liu JT, Jiang H. Outcomes of conservative treatment for ruptured lumbar disc herniation. Acta Orthop Belg, 2013, 79: 726–730. [PubMed] [Google Scholar]
- 22. Gunzburg R, Szpalski M. Lumbar Disc Herniation. Philadelphia, PA: Lippincott Williams & Wilkins, 2002; 67–69. [Google Scholar]
- 23. Lebow RL, Adogwa O, Parker SL, Sharma A, Cheng J, McGirt MJ. Asymptomatic same‐site recurrent disc herniation after lumbar discectomy: results of a prospective longitudinal study with 2‐year serial imaging. Spine (Phila Pa 1976), 2011, 36: 2147–2151. [DOI] [PubMed] [Google Scholar]


