Key Points
Question
Is use of chlorhexidine 2% mouthwash, selective oropharyngeal decontamination (SOD), or selective digestive tract decontamination (SDD) associated with reduced risk of bloodstream infections due to multidrug-resistant gram-negative bacteria among ventilated patients in intensive care units (ICUs) with moderate to high prevalence of antibiotic resistance?
Findings
In this randomized trial of 8665 patients, the use of chlorhexidine 1% mouthwash, SOD, or SDD was not associated with significant differences in ICU-acquired bloodstream infections with multidrug-resistant gram-negative bacteria (adjusted hazard ratios, 1.13, 0.89, and 0.70, respectively), compared with a baseline period of chlorhexidine body washing and a hand hygiene improvement program.
Meaning
Among ventilated patients in ICUs with moderate to high prevalence of antibiotic resistance, use of chlorhexidine 1% mouthwash, SOD, or SDD was not associated with a significant difference in bloodstream infections with multidrug-resistant gram-negative bacteria compared with standard care.
Abstract
Importance
The effects of chlorhexidine (CHX) mouthwash, selective oropharyngeal decontamination (SOD), and selective digestive tract decontamination (SDD) on patient outcomes in ICUs with moderate to high levels of antibiotic resistance are unknown.
Objective
To determine associations between CHX 2%, SOD, and SDD and the occurrence of ICU-acquired bloodstream infections with multidrug-resistant gram-negative bacteria (MDRGNB) and 28-day mortality in ICUs with moderate to high levels of antibiotic resistance.
Design, Setting, and Participants
Randomized trial conducted from December 1, 2013, to May 31, 2017, in 13 European ICUs where at least 5% of bloodstream infections are caused by extended-spectrum β-lactamase–producing Enterobacteriaceae. Patients with anticipated mechanical ventilation of more than 24 hours were eligible. The final date of follow-up was September 20, 2017.
Interventions
Standard care was daily CHX 2% body washings and a hand hygiene improvement program. Following a baseline period from 6 to 14 months, each ICU was assigned in random order to 3 separate 6-month intervention periods with either CHX 2% mouthwash, SOD (mouthpaste with colistin, tobramycin, and nystatin), or SDD (the same mouthpaste and gastrointestinal suspension with the same antibiotics), all applied 4 times daily.
Main Outcomes and Measures
The occurrence of ICU-acquired bloodstream infection with MDRGNB (primary outcome) and 28-day mortality (secondary outcome) during each intervention period compared with the baseline period.
Results
A total of 8665 patients (median age, 64.1 years; 5561 men [64.2%]) were included in the study (2251, 2108, 2224, and 2082 in the baseline, CHX, SOD, and SDD periods, respectively). ICU-acquired bloodstream infection with MDRGNB occurred among 144 patients (154 episodes) in 2.1%, 1.8%, 1.5%, and 1.2% of included patients during the baseline, CHX, SOD, and SDD periods, respectively. Absolute risk reductions were 0.3% (95% CI, −0.6% to 1.1%), 0.6% (95% CI, −0.2% to 1.4%), and 0.8% (95% CI, 0.1% to 1.6%) for CHX, SOD, and SDD, respectively, compared with baseline. Adjusted hazard ratios were 1.13 (95% CI, 0.68-1.88), 0.89 (95% CI, 0.55-1.45), and 0.70 (95% CI, 0.43-1.14) during the CHX, SOD, and SDD periods, respectively, vs baseline. Crude mortality risks on day 28 were 31.9%, 32.9%, 32.4%, and 34.1% during the baseline, CHX, SOD, and SDD periods, respectively. Adjusted odds ratios for 28-day mortality were 1.07 (95% CI, 0.86-1.32), 1.05 (95% CI, 0.85-1.29), and 1.03 (95% CI, 0.80-1.32) for CHX, SOD, and SDD, respectively, vs baseline.
Conclusions and Relevance
Among patients receiving mechanical ventilation in ICUs with moderate to high antibiotic resistance prevalence, use of CHX mouthwash, SOD, or SDD was not associated with reductions in ICU-acquired bloodstream infections caused by MDRGNB compared with standard care.
Trial Registration
ClinicalTrials.gov Identifier: NCT02208154
This cluster randomized trial compares associations between commonly used decontamination strategies (chlorhexidine 2% mouthwash, selective oropharyngeal decontamination, and selective digestive tract decontamination) and occurrence of intensive care unit (ICU)–acquired bloodstream infections with multidrug-resistant gram-negative bacteria in ICUs with moderate to high levels of antibiotic resistance.
Introduction
Care of patients in intensive care units (ICUs) is frequently complicated by infections, which are associated with increased morbidity, mortality, and health care costs.1,2 Selective digestive tract decontamination (SDD) and selective oropharyngeal decontamination (SOD) consist of topical antimicrobial agents targeting aerobic gram-negative pathogens, Staphylococcus aureus, and yeasts in the gastrointestinal tract (SDD) and oropharynx (SDD/SOD), and they aim to prevent infections. In ICUs with low levels of antibiotic resistance, SDD and SOD have been associated with improved patient outcomes,3,4 with SDD being more efficacious than SOD.5,6 Currently, SDD and SOD are routinely used in ICUs in the Netherlands, but their use has not been widely adopted in other countries,7 mainly because of limited efficacy data in settings with higher levels of antibiotic resistance and concern about emergence of antibiotic resistance, although the latter is not supported by meta-analyses.8 In contrast, chlorhexidine (CHX) mouthwash is widely used in ICU patients and its use has been associated with a lower incidence of ventilator-associated pneumonia,9,10 with CHX 2% being more efficacious than lower concentrations.9 Yet, in meta-analyses, CHX mouthwash was associated with higher mortality in ICU patients.11,12 SDD and SOD have never been compared head to head with CHX mouthwash in ICU patients.
Given the equipoise on the effectiveness and ecological safety of these decontamination strategies in ICUs with moderate to high levels of antibiotic resistance, a randomized trial was conducted in 6 European countries to quantify the association between CHX mouthwash, SOD, and SDD and ICU-acquired bloodstream infections (BSIs) with multidrug-resistant gram-negative bacteria (MDRGNB), patient mortality, and unitwide prevalence of antibiotic resistance.
Methods
Study Design
A nonblinded multicenter trial with cluster randomization and crossover of interventions was conducted in 13 ICUs from Belgium, Spain, Portugal, Italy, Slovenia, and the United Kingdom between December 1, 2013, and May 31, 2017. The full trial protocol and statistical analysis plans are in Supplement 1. The characteristics of the participating centers are in eTable 1 in Supplement 2. Institutional review board approval for data collection was obtained prior to study start, and, where required, national regulatory authorities approved the study protocol prior to randomization of interventions. All hospitals obtained a waiver for individual patient informed consent because interventions aimed to achieve ward-level ecologic effects (and patient-based randomization might lead to contamination of effects) and interventions were considered to have minimal risks of harm.
Only ICUs with an extended-spectrum β-lactamase prevalence of at least 5% among Enterobacteriacea-causing BSI were eligible (study protocol in Supplement 1). ICUs with endemic levels of carbapenem-resistant Enterobacteriaceae, multidrug-resistant Pseudomonas or Acinetobacter species or with vancomycin-resistant enterococci (all defined as >10% of ICU-acquired bacteremia with that species) were excluded from participation.
All hospitals started with a baseline period of at least 6 months, which included daily CHX-digluconate 2% body washing (CHX-BW) for all ICU patients until ICU discharge and implementation of the World Health Organization hand hygiene program, including weekly observations.13 CHX mouthwash (0.12% or 0.20%) was allowed as part of standard care if this was part of regular care before the study. Universal CHX-BW and monitoring of hand hygiene continued throughout the 3 following intervention periods. After the baseline period, the 3 study interventions (CHX mouthwash, SOD, and SDD) were implemented in a sequential computer-generated randomized order in each participating center. Randomization of the order of interventions aimed to reduce effects of changes in time in antibiotic resistance or clinical practice that might affect study outcomes. All study periods were intended to last 6 months and were separated by a 1-month washout/in period.
Patients
Patients with an expected duration of invasive mechanical ventilation of at least 24 hours were eligible. Exclusion criteria included age younger than 18 years, pregnancy, and allergy to any study intervention component. Eligible patients admitted during the first 2 weeks of the washout/in period received the new intervention but were not part of the study population; patients admitted during the second 2 weeks received the new intervention and were analyzed as such.
Interventions
CHX 2% mouthwash, SOD, and SDD were manufactured by the pharmacy of the University Medical Center Utrecht, the Netherlands. CHX 2% mouthwash was replaced by CHX 1% oral gel in March 2015 after the reporting of oral mucosal adverse effects in 29 of 295 patients (9.8%) treated in 2 hospitals.14 The oropharyngeal paste used during SOD and SDD contained 0.19 million units of colistin sulfate, 10 mg of tobramycin sulfate, and 0.1 million units of nystatin per dosage (0.5 g) and the gastrointestinal suspension contained 1.9 million units of colistin sulfate, 80 mg of tobramycin sulfate, and 2.0 million units of nystatin per dosage (10 mL through nasogastric tube). Although the SDD regimen, where used routinely (eg, the Netherlands),3,4,5 usually includes a 4-day course of intravenous cephalosporin, prophylactic use of these antibiotics was not considered appropriate in settings with a moderate to high prevalence of antibiotic resistance, and was therefore not part of the study protocol. CHX mouthwash, SOD, and SDD were initiated after study inclusion and applied 4 times daily after regular oral care until mechanical ventilation was stopped. Adherence to decontamination strategies was monitored with monthly adherence measurements and recording of interruptions in individual patients.
Rectum and respiratory surveillance samples (endotracheal aspirate, when possible, or throat swabs) were obtained twice weekly from study patients, and once monthly from all patients present in the unit on that day for point prevalence surveys. Microbiology methods are described in eAppendix 1 in Supplement 2. A safety committee consisting of 3 independent experts reviewed the results of monthly point prevalence samples at 3-month intervals, but not clinical outcomes. The committee members were blinded to the interventions applied and could recommend interruption of the study in a participating ICU if an increase in antibiotic resistance was apparent.
Outcomes
The primary outcome was the incidence of ICU-acquired BSI with MDRGNB in study patients during use of CHX, SOD, or SDD compared with standard care. Secondary outcomes were ICU-acquired BSI with highly resistant microorganism (HRMO), defined as MDRGNB or methicillin-resistant S aureus or vancomycin-resistant enterococci; mortality at day 28 from ICU admission, at ICU discharge, and at hospital discharge (all prespecified); and ICU-acquired BSI with any pathogen (post hoc). Other secondary outcomes are subject to future analyses and not reported in this article: cross-transmission rates of MDRGNB, the occurrence of ICU-acquired rectum and respiratory tract MDRGNB colonization, and associations between colonization and BSI. Ward-level exploratory outcomes included the unitwide prevalence of HRMO measured by monthly point prevalence surveys of the rectum and respiratory tract of all patients in the ICU to monitor ecologic safety, and the unitwide use of systemic antibiotics (descriptive analyses), expressed as defined daily doses per patient day. As a post hoc exploratory analysis, carriage rates with antibiotic-resistant GNB in the rectum and respiratory tract were determined based on the results of surveillance cultures plated on extended-spectrum β-lactamase selective media and obtained twice weekly from study patients.
ICU-acquired BSI was defined as bacteremia or candidemia diagnosed from day 2 of ICU stay onwards, with the initial day of ICU admission being designated as day 0. Only the first episode per patient was used in the analyses. Microorganisms excluded from the definition of BSI are listed in eTable 2 in Supplement 2. Definitions of MDRGNB and HRMO are listed in eTable 3 in Supplement 2 and mainly include Enterobacteriaceae resistant to third-generation cephalosporins and GNB resistant to carbapenems, colistin, or 3 or more antibiotics.15
Sample Size and Statistical Analyses
To determine the effects of CHX, SOD, and SDD as if these were implemented in ICUs in addition to standard care, each intervention was compared with standard care (baseline period) for all outcomes. Study funding was obtained from a grant call that specifically asked for evaluation of interventions in ICUs that could reduce the incidence of ICU-acquired BSI with MDRGNB. We, therefore, used this as the primary outcome, but based the sample size calculation on 28-day mortality, considered to be a more clinically relevant outcome. A 10% (relative) reduction in 28-day mortality and a 50% relative reduction in the incidence of ICU-acquired MDRGNB BSI were considered clinically relevant.4 To demonstrate a 10% relative difference in 28-day mortality for each intervention compared with baseline, 10 800 patients were required (using a baseline 28-day mortality of 27.5%; α = .05; 80% power), including a margin of 600 patients per study arm to include cluster effects and differences in baseline characteristics. However, an error in the calculation of variance between study groups was discovered after study completion, which had led to lower patient numbers than required for the power of 80%. Details of the sample size calculation are in eAppendix 2 in Supplement 2.
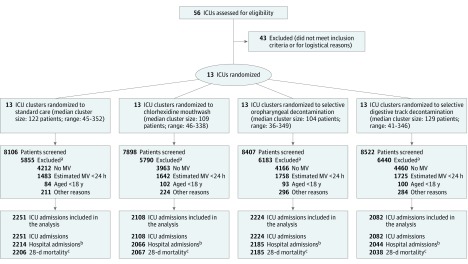
Three cohorts were created for the analyses of clinical outcomes: unique ICU admissions for ICU mortality and ICU-acquired BSI (with MDRGNB, HRMO, and any pathogen), unique hospital admissions for hospital mortality, and unique ICU admissions with no prior ICU admission within 30 days for 28-day mortality (Figure). All analyses were performed on cases without missing covariates or outcomes. To adjust for differences in patient characteristics between study periods, propensity scores were calculated using generalized boosted methods,16 and inverse probability weighting was used to balance the distribution of the confounders center, age, sex, Charlson Comorbidity Index score,17 disease severity, admission type (medical or surgical), antibiotic use on ICU admission, and location before ICU admission (same hospital, other hospital or long-term care facility, or home). Because ICUs used different disease severity scoring systems, separate propensity score models were made for ICUs using either Acute Physiology and Chronic Health Evaluation (APACHE) II or Simplified Acute Physiology Score (SAPS II) scores, and the derivative weights used in the final models. ICU-acquired BSI and ICU and hospital mortality were analyzed with Cox-proportional hazard analyses stratified for center, with discharge and death as competing events where applicable. The Schoenefeld Goodness of Fit test was used to test the proportionality assumption and there was no evidence to reject the proportional hazard assumption at 5% significance level.
Figure. Flowchart and Cohorts for Analyses.
Abbreviations: ICU, intensive care unit; MV, mechanical ventilation.
aSome patients had multiple reasons for exclusion.
bThe cohort for hospital mortality included 8509 unique hospital admissions, 37 with missing hospital mortality status.
cThe cohort for 28-day mortality included 8496 unique ICU admissions with no prior ICU admission within 30 days, 56 with missing 28-day mortality status.
For the analysis of 28-day mortality, a mixed-effects logistic regression model was used with a fixed effect for center and a random effect for the 52 center-period combinations (4 period orders [A-B-C-D] × 13 ICUs). All models were adjusted for the confounders and mean hand hygiene compliance per study period per center. A sensitivity analysis was performed on the mortality outcomes excluding patients who stayed fewer than 3 days in the ICU because they might have been overrepresented in the baseline period. Based on the study findings, an additional post hoc sensitivity analysis was performed to explore potential consequences of not including prophylaxis with third-generation cephalosporins in the SDD regimen and of stopping SDD at the end of mechanical ventilation (rather than at ICU discharge), as had been performed in previous Dutch studies.3,4,5 In this analysis, all SDD-treated patients with ICU-acquired BSI caused by a pathogen susceptible to third-generation cephalosporins during the first 4 days and/or with ICU-acquired BSI with any pathogen after the end of mechanical ventilation were considered alive for all mortality outcomes, thereby maximizing the potentially missed effects of both changes to previous protocols. As a third post hoc sensitivity analysis, head-to-head comparisons between the randomized intervention groups were performed for all patient-level outcomes.
The unitwide prevalence of HRMO carriage based on point prevalence surveys was analyzed separately for rectum and respiratory tract, with binomial models (log link) for each outcome; these specific models included correction for underlying time trends per ICU and estimated a mean time trend per study period (as an exploratory analysis). Because the potential for type I error due to multiple comparisons was not addressed, secondary analyses were considered exploratory.
A 2-sided significance level of .05 was used for all analyses. SPSS (IBM, version 21) and R software, version 3.3.2 (R Project for Statistical Computing) were used for data preparation and statistical analyses, respectively.
Results
Between December 1, 2013, and May 31, 2017, 32 933 ICU admissions were screened, of which 8665 were included, yielding 8509 unique hospital admissions and 8496 inclusions for 28-day mortality (Figure; see eTable 4 in Supplement 2 for baseline characteristics of screened patients). The median durations of study periods were 6 months (range, 6-14.5) for baseline and 6 (range, 4.6-6), 6 (range, 5-8.5), and 6 (range 5-7) months for the CHX, SOD, and SDD periods, respectively (Table 1). Proportions of BSI caused by HRMO and Enterobacteriaceae resistant to third-generation cephalosporins, both among all BSI episodes, were 25.5% and 15.1%, respectively. Per study period, 26.7% to 29.7% of screened patients were eligible and 91% to 94% of these patients were enrolled. Of the 8665 included patients, 5561 were male (64.2%) and their median age was 64.1 years (range, 18-98). Patient characteristics differing between baseline and intervention periods included the mean APACHE II and SAPS II scores and the proportion of patients receiving antibiotics at ICU admission (Table 1; eTable 5 in Supplement 2).
Table 1. Baseline Characteristics of the Study Population.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Baseline (n = 2251) | CHX (n = 2108) | SOD (n = 2224) | SDD (n = 2082) | |
| Patient Characteristics | ||||
| Age, mean (SD), y | 62.0 (15.6) | 61.4 (15.7) | 61.6 (15.7) | 62.8 (15.5) |
| Sex | ||||
| Male | 1420 (63.1) | 1358 (64.4) | 1439 (64.7) | 1344 (64.6) |
| Female | 831 (36.9) | 750 (35.6) | 785 (35.3) | 738 (35.4) |
| APACHE II scores for 5 hospitals, mean (SD)a | 20.3 (8.6) | 19.8 (8.2) | 20.5 (9.3) | 21.8 (8.7) |
| SAPS II scores for 8 hospitals, mean (SD)b | 53.0 (18.0) | 54.8 (17.9) | 54.4 (17.5) | 55.0 (18.0) |
| Type of ICU admission | ||||
| Medical | 1464 (65.3) | 1323 (63.0) | 1442 (64.9) | 1385 (66.6) |
| Trauma with surgery | 138 (6.2) | 142 (6.8) | 156 (7.0) | 115 (5.5) |
| Trauma, no surgery | 113 (5.0) | 88 (4.2) | 104 (4.7) | 88 (4.2) |
| Surgical, scheduled | 198 (8.8) | 173 (8.2) | 173 (7.8) | 178 (8.6) |
| Surgical, unscheduled | 328 (14.6) | 374 (17.8) | 346 (15.6) | 314 (15.1) |
| Surgical, unspecified | 10 (0.4) | 8 (0.4) | 3 (0.1) | 2 (0.1) |
| Location before ICU admission | ||||
| Same hospital | 1020 (45.3) | 1032 (49.0) | 1025 (46.1) | 1035 (49.7) |
| Another hospital or long term care facility | 400 (17.8) | 312 (14.8) | 316 (14.2) | 301 (14.5) |
| Home (directly or via emergency department) | 831 (36.9) | 764 (36.2) | 883 (39.7) | 746 (35.8) |
| Antibiotic at the time of ICU admission | 943 (41.9) | 832 (39.5) | 992 (44.6) | 744 (35.8) |
| Sites of organ failure | ||||
| Respiratory illness | 1023 (45.5) | 990 (47.0) | 998 (44.9) | 985 (47.3) |
| Cardiovascular illness | 828 (36.8) | 811 (38.5) | 835 (37.5) | 792 (38.0) |
| Neurologic illness | 686 (30.5) | 674 (32.0) | 615 (27.7) | 603 (29.0) |
| Other illness (renal, hepatic, metabolic, hematologic, and/or other) | 633 (28.1) | 617 (29.3) | 742 (33.4) | 676 (32.5) |
| Charlson Comorbidity Index score, mean (SD)c | 2.15 (2.42) | 2.38 (2.49) | 2.35 (2.42) | 2.42 (2.56) |
| 0 | 738 (32.8) | 631 (29.9) | 653 (29.4) | 626 (30.1) |
| 1-2 | 759 (33.7) | 674 (32.0) | 718 (32.3) | 654 (31.4) |
| 3-4 | 399 (17.7) | 398 (18.9) | 461 (20.7) | 410 (19.7) |
| >4 | 355 (15.8) | 405 (19.2) | 392 (17.6) | 392 (18.8) |
| ICU Characteristics (Type, No. of beds)d | Order of Study Arms per ICU (Duration, mo) [No. of Study Patients]e | |||
| ICU 1 (mixed, 36 beds) | A (6) [212] | B (5.6) [214]f | C (6) [245] | D (6) [229] |
| ICU 9 (mixed, 42 beds) | A (6) [333] | B (6) [338] | C (5) [309]h | D (6) [317] |
| ICU 2 (mixed, 24 beds) | A (6) [77] | B (4.6) [59]f | D (6) [101] | C (6) [80] |
| ICU 11 (mixed, 8 beds) | A (9) [63]g | B (6) [50] | D (6) [70] | C (5) [54]k |
| ICU 5 (mixed, 30 beds) | A (6) [169] | C (6) [277] | B (8.5) [349]g | D (6) [248] |
| ICU 12 (mixed, 22 beds) | A (8) [352]i | C (6) [272] | B (6) [248] | D (6) [237] |
| ICU 4 (mixed, 42 beds) | A (6) [266] | C (6) [285] | D (6) [334] | B (7) [346]g |
| ICU 7 (mixed, 10 beds) | A (14.5) [297]i | C (6) [109] | D (6) [104] | B (6) [129] |
| ICU 8 (mixed, 15 beds) | A (6) [85] | D (6) [92] | B (6) [85] | C (6) [75] |
| ICU 3 (medical, 12 beds) | A (6) [45] | D (6) [46] | B (6) [36] | C (6) [41] |
| ICU 10 (medical, 24 beds) | A (8) [113]j | D (6) [85] | C (6) [85] | B (6) [92] |
| ICU 6 (mixed, 12 beds) | A (6) [122] | D (6) [177] | C (3.5 + 2.5) [155]h | B (6) [144] |
| ICU 13 (mixed, 9 beds) | A (7) [117]i | D (6) [104] | C (6) [103] | B (6) [90] |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; CHX, chlorhexidine mouthwash; ICU, intensive care unit; SAPS, Simplified Acute Physiology Score; SDD, selective digestive tract decontamination; SOD, selective oropharyngeal decontamination.
The APACHE II disease severity score ranges from 0 to 71, with higher scores indicating increased severity and an increased probability of in-hospital death. A patient with an APACHE II score of 20 would have an estimated probability of in-hospital death ranging from 6.3% to 71%, depending on the reason for ICU admission and the need for emergency surgery.18
The SAPS II disease severity score ranges from 0 to 163, with higher scores indicating increased severity and an increased probability of in-hospital death. A patient with a SAPS II score of 52 would have an estimated probability of in-hospital death of 50%.19
The Charlson Comorbidity Index ranges from ranges from 0 to 37, with higher scores associated with a higher probability of 1-year mortality.17
ICUs are numbered in in order of study start date (eTable 1 in Supplement 2).
A, B, C, and D represent the first, second, third, and fourth study periods, respectively.
Suspension of CHX 2% intervention period due to oromucosal adverse effects.
Prolongation of study period, pending approval for the amendment for the switch from CHX 2% mouthwash to CHX 1% oral gel.
Interruption of SOD period due to increase in antibiotic-resistant bacteria.
Prolongation of baseline period, pending approval from the regulatory agencies for the introduction of study interventions.
Prolongation of baseline period for local logistic reasons.
Shortened study period for logistical reasons.
Among study patients, the mean proportions receiving decontamination according to protocol, determined by monthly compliance measurements, were 92.5%, 92.4%, and 94.2% during the CHX, SOD, and SDD periods, respectively (eTable 6 in Supplement 2). There were 23 ICU admissions with missing covariates and 1, 37, and 56 patients with a missing ICU, hospital, and 28-day mortality status, respectively. Average hand hygiene compliance was 64.1% during the baseline period and ranged from 72.2% to 72.5% during the intervention periods (eTable 7 in Supplement 2). Five ICUs used CHX 0.12% and 6 used 0.20% mouthwash as part of standard care. The intracluster correlation coefficient was 0.001.
Deviations From Study Protocol
The study was temporarily interrupted in 2 centers. In one center, an increased prevalence of colistin-resistant Klebsiella pneumoniae was identified by the safety committee, which led to the identification of a clonal outbreak after SOD had been used for 3.5 months. After a 7-month period of outbreak containment, SOD was reintroduced. In another center, the hospital infection control committee interrupted the study after SOD had been used for 5 months, pending evaluations of an increased prevalence of carbapenem-resistant Enterobacteriaceae. Further investigation revealed that the outbreak was polyclonal and occurring in multiple hospital wards simultaneously. After an interruption of 7 months, the next randomized study phase (being SDD) was introduced after institutional review board approval. During both interruptions, SOD was not applied and patients included in the intervals were not included in the analyses.
Adverse Events
CHX 2% mouthwash was replaced by CHX 1% oral gel after adverse events, mainly consisting of oromucosal lesions, recorded in a total of 29 (9.8%) of 295 patients treated with CHX 2% in the 2 centers that first implemented CHX 2%.14 No serious adverse events were reported during the use of CHX 1%, SOD, and SDD.
ICU-Acquired BSIs
ICU-acquired BSI with MDRGNB (primary outcome) occurred in 144 patients (154 episodes), most frequently with K pneumoniae (n = 56), Enterobacter cloacae (n = 20), Pseudomonas aeruginosa (n = 17), and Escherichia coli (n = 15) (Table 2). These occurred in 2.1%, 1.8%, 1.5%, and 1.2% of the patients included in the baseline, CHX, SOD, and SDD periods, respectively. Absolute risk reductions were 0.3% (95% CI, −0.6% to 1.1%), 0.6% (95% CI, −0.2% to 1.4%), and 0.8% (95% CI, 0.1% to 1.6%) for CHX, SOD, and SDD, respectively, compared with the baseline period. Corresponding adjusted hazard ratios (aHRs) of ICU-acquired MDRGNB BSI, compared with baseline, were 1.13 (95% CI, 0.68 to 1.88), 0.89 (95% CI, 0.55 to 1.45), and 0.70 (95% CI, 0.43 - 1.14) during the CHX, SOD, and SDD periods, respectively (Table 3). Incidences per center can be found in eTable 8 in Supplement 2.
Table 2. ICU-Acquired Bloodstream Infections per Study Group.
| Study Group | Baseline (n = 2251) | CHX (n = 2108) | SOD (n = 2224) | SDD (n = 2082) | ||||
|---|---|---|---|---|---|---|---|---|
| No. of Episodes | Proportion of BSI Episodes, % | No. of Episodes | Proportion of BSI Episodes, % | No. of Episodes | Proportion of BSI Episodes, % | No. of Episodes | Proportion of BSI Episodes, % | |
| Primary Outcome: ICU-Acquired BSIs With Multidrug-Resistant Gram-Negative Bacteria (MDRGNB)a,b | ||||||||
| BSI with MDRGNB, No. of episodes, (No. of patients) | 52 (47) | 41 (38) | 34 (33) | 27 (26) | ||||
| Enterobacteriaceae | 39 | 75.0 | 29 | 70.7 | 26 | 76.5 | 24 | 88.9 |
| Resistant to third-generation cephalosporins | 35 | 25 | 24 | 24 | ||||
| Resistant to colistin | 2 | 2 | 5 | 5 | ||||
| Glucose nonfermenting gram-negative bacteria | 9 | 17.3 | 10 | 24.4 | 5 | 14.7 | 3 | 11.1 |
| Pseudomonas species | 4 | 9 | 3 | 2 | ||||
| Other glucose nonfermenting gram-negative bacteriac | 4 | 7.7 | 2 | 4.9 | 3 | 8.8 | 0 | 0.0 |
| Secondary Outcomes: ICU-Acquired BSIs With Highly Resistant Microorganisms (HRMOs)a,d | ||||||||
| BSI with HRMO, No. of episodes (No. of patients) | 58 (53) | 49 (44)e | 40 (38)e | 35 (34) | ||||
| MDRGNB, No. of episodes (No. of patients) | 52 (47) | 89.7 | 41 (38) | 83.7 | 34 (33) | 85.0 | 27 (26) | 77.1 |
| Highly resistant Gram-positive bacteria, No. of episodes (No. of patients) | 6 (6) | 10.3 | 8 (8) | 16.3 | 6 (6) | 15.0 | 8 (8) | 22.9 |
| Vancomycin-resistant enterococci | 3 | 4 | 3 | 0 | ||||
| Methicillin-resistant Staphylococcus aureus | 3 | 4 | 3 | 8 | ||||
| ICU-Acquired BSIs With Any Pathogena,f | ||||||||
| BSI with any pathogen, No. of episodes (No. of patients) | 199 (154) | 201 (156) | 172 (140) | 141 (123) | ||||
| Enterobacteriaceae | 99 | 49.7 | 90 | 44.8 | 77 | 44.8 | 51 | 36.2 |
| Intrinsic colistin resistant | 30 | 13 | 14 | 10 | ||||
| Glucose nonfermenting gram-negative bacteria | 31 | 15.6 | 19 | 9.5 | 20 | 11.6 | 15 | 10.6 |
| Pseudomonas species | 21 | 16 | 15 | 9 | ||||
| Gram-positive bacteria | 43 | 21.6 | 61 | 30.3 | 47 | 27.3 | 50 | 35.5 |
| Enterococcus faecium/faecalis | 27 | 32 | 34 | 32 | ||||
| Staphylococcus aureus | 13 | 25 | 12 | 17 | ||||
| Yeasts | 15 | 7.5 | 22 | 10.9 | 23 | 13.4 | 18 | 12.8 |
| Otherg | 11 | 5.5 | 9 | 4.5 | 5 | 2.9 | 7 | 5.0 |
Abbreviations: BSI, bloodstream infection; CHX, chlorhexidine mouthwash; ICU, intensive care unit; SDD, selective digestive tract decontamination; SOD, selective oropharyngeal decontamination.
BSI defined as first occurrence of unique species on day 2 of ICU stay onwards, with the initial day of ICU admission being designated as day 0. ICU-acquired BSIs with any pathogen was a post hoc outcome.
In brief, MDRGNB include Enterobacteriaceae resistant to third-generation cephalosporins, gram-negative bacteria resistant to carbapenems, colistin, or 3 or more antibiotics from separate classes (complete definition in eTable 3 in Supplement 2).
Stenotrophomonas spp, Burkholderia spp, and Achromobacter spp.
HRMOs include MDRGNB, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant enterococci (complete definition in eTable 3 in Supplement 2).
Two patients in the CHX period and 1 patient in the SOD period had a BSI both with MDRGNB and gram-positive HRMO.
Excluding coagulase-negative Staphylococcus, Micrococcus, and Clostridium species and nonpneumococcal Streptococci (eTable 2 in Supplement 2), also including HRMO.
These included BSI with Bacteroides spp (18), Parabacteroides spp (2), Haemophilus influenzae (3), and Streptococcus pneumoniae (9).
Table 3. Associations Between Interventions and ICU-Acquired BSI and Patient Mortality.
| Crude Analyses | Adjusted Analyses, Adjusted Hazard Ratio (95% CI)a | ||||||
|---|---|---|---|---|---|---|---|
| Baseline (n = 2251) | CHX (n = 2108) | SOD (n = 2224) | SDD (n = 2082) | CHX vs Baseline | SOD vs Baseline | SDD vs Baseline | |
| Primary Outcome | |||||||
| Patients with ICU-acquired BSI with MDRGNB | |||||||
| Incidence, No. (%) | 47 (2.1) | 38 (1.8) | 33 (1.5) | 26 (1.2) | |||
| Absolute risk reduction vs baseline, % (95% CI) | 0.3 (−0.6 to 1.1) | 0.6 (−0.2 to 1.4) | 0.8 (0.1 to 1.6) | ||||
| Rate (per 1000 patient days at risk) | 1.62 | 1.34 | 1.14 | 0.94 | 1.13 (0.68 to 1.88) |
0.89 (0.55 to 1.45) |
0.70 (0.43 to 1.14) |
| Secondary Outcomes | |||||||
| Patients with ICU-acquired BSI with HRMOb | |||||||
| Incidence, No. (%) | 53 (2.4) | 44 (2.1) | 38 (1.7) | 34 (1.6) | |||
| Absolute risk reduction vs baseline, % (95% CI) | 0.3 (−0.6 to 1.2) |
0.6 (−0.2 to 1.5) |
0.7 (−0.1 to 1.6) |
||||
| Rate (per 1000 patient days at risk) | 1.84 | 1.56 | 1.32 | 1.24 | 1.07 (0.58 to 1.99) |
0.83 (0.46 to 1.51) |
0.77 (0.38 to 1.52) |
| Patients with ICU-acquired BSI (any pathogen) | |||||||
| Incidence, No. (%) | 154 (6.8) | 156 (7.4) | 140 (6.3) | 123 (5.9) | |||
| Absolute risk reduction vs baseline, % (95% CI) | −0.6 (−2.1 to 1.0) |
0.5 (−0.9 to 2.0) |
0.9 (−0.5 to 2.4) |
||||
| Rate (per 1000 patient days at risk) | 5.69 | 5.95 | 5.12 | 4.67 | 1.08 (0.85 to 1.39) |
0.94 (0.76 to 1.17) |
0.79 (0.60 to 1.05) |
| Mortality in ICUc | |||||||
| Incidence, no./No. (%) | 691/2251 (30.7) | 664/2107 (31.5) | 685/2224 (30.8) | 645/2082 (31.0) | |||
| Absolute risk reduction vs baseline, % (95% CI) | −0.8 (−3.6 to 1.9) |
−0.1 (−2.8 to 2.6) |
−0.3 (−3.0 to 2.5) |
1.03 (0.92 to 1.16) |
1.00 (0.89 to 1.14) |
0.95 (0.81 to 1.11) |
|
| Mortality in hospitald | |||||||
| Incidence, no./No. (%) | 839/2206 (38.0) |
782/2055 (38.1) |
845/2184 (38.7) |
816/2027 (40.3) |
|||
| Absolute risk reduction vs baseline, % (95% CI) | 0.0 (−2.9 to 2.9) |
−0.7 (−3.5 to 2.2) |
−2.2 (−5.2 to 0.7) |
0.97 (0.85 to 1.11) |
1.00 (0.87 to 1.14) |
0.96 (0.82 to 1.12) |
|
| Mortality at 28 d from ICU admissione | |||||||
| Incidence, no./No. (%) | 701/2198 (31.9) |
675/2049 (32.9) |
703/2171 (32.4) |
689/2022 (34.1) |
|||
| Absolute risk reduction vs baseline, % (95% CI) | −1.1 (−3.9 to 1.8) |
−0.5 (−3.3 to 2.3) |
−2.2 (−5.0 to 0.7) |
1.07 (0.86 to 1.32)f |
1.05 (0.85 to 1.29)f |
1.03 (0.80 to 1.32)f |
|
| Other outcomes, median (IQR), d | |||||||
| ICU | 10 (5 to 18) | 10 (6 to 19) | 10 (6 to 18) | 11 (6 to 18) | |||
| In hospital | 23 (11 to 45) | 24 (12 to 45) | 23 (12 to 43) | 24 (12 to 44) | |||
| On mechanical ventilation in ICU | 6 (3 to 13) | 7 (4 to 13) | 6 (3 to 12) | 7 (3 to 12) | |||
Abbreviations: BSI, bloodstream infection; CHX, chlorhexidine mouthwash; HRMO, highly resistant microorganism; ICU, intensive care unit; IQR, interquartile range; MDRGNB, multidrug-resistant gram-negative bacteria; SDD, selective digestive tract decontamination; SOD, selective oropharyngeal decontamination.
All models accounted for clustering using a fixed effect on ICU and a random effect on study period (13 ICUs × 4 study periods) and were adjusted for age, sex, Charlson Comorbidity Index score, APACHE II or SAPS II score, admission type, antibiotic use on ICU admission, location before ICU admission (in both propensity score and final models), and mean hand hygiene compliance per study period (only in final models).
Includes MDRGNB and highly resistant gram-positive microorganisms (methicillin-resistant S aureus and vancomycin-resistant E faecium/E faecalis), according to definitions in eTable 3 in Supplement 2.
One missing outcome.
The cohort included 8509 unique hospital admissions, of which 37 were missing hospital mortality status.
The cohort for 28-day mortality included 8496 unique ICU admissions with no prior ICU admission within 30 days, of which 56 were missing 28-day mortality status.
Adjusted odds ratio (95% CI).
ICU-acquired BSI with HRMO occurred in 169 patients (182 episodes) (Table 2). Risks for ICU-acquired BSI with HRMO were 2.4%, 2.1%, 1.7%, and 1.6% during the baseline, CHX, SOD, and SDD periods, respectively. Absolute risk reductions were 0.3% (95% CI, −0.6% to 1.2%), 0.6% (95% CI, −0.2% to 1.5%), and 0.7% (95% CI, −0.1% to 1.6%) for CHX, SOD, and SDD, compared with baseline, respectively. Corresponding aHRs of HRMO BSI during study interventions, compared with baseline, were 1.07 (95% CI, 0.58 to 1.99), 0.83 (95% CI, 0.46 to 1.51), and 0.77 (0.38 to 1.52) during CHX, SOD, and SDD, respectively (Table 3).
Mortality
The risk rates for mortality on day 28 were 31.9%, 32.9%, 32.4%, and 34.1% during the baseline, CHX, SOD, and SDD periods, respectively. Absolute risk reductions were −1.1% (95% CI, −3.9% to 1.8%), −0.5% (95% CI, −3.3% to 2.3%), −2.2% (95% CI, −5.0% to 0.7%) for CHX, SOD, and SDD, respectively, compared with baseline. Corresponding adjusted odds ratios for 28-day mortality were 1.07 (95% CI, 0.86 to 1.32), 1.05 (95% CI, 0.85 to 1.29), and 1.03 (95% CI, 0.80 to 1.32) during CHX, SOD, and SDD, respectively (Table 3). The risk rates for ICU mortality were 30.7%, 31.5%, 30.8%, and 31.0% during the baseline, CHX, SOD, and SDD periods, respectively. Absolute risk reductions were −0.8% (95% CI, −3.6% to 1.9%), −0.1% (95% CI, −2.8% to 2.6%), and −0.3% (95% CI, −3.0% to 2.5%) for CHX, SOD, and SDD, respectively, compared with baseline. Corresponding aHRs were 1.03 (95% CI, 0.92 to 1.16), 1.00 (95% CI, 0.89 to 1.14), and 0.95 (95% CI, 0.81 to 1.11) during the CHX, SOD, and SDD periods, respectively. The risk rates for hospital mortality were 38.0%, 38.1%, 38.7%, and 40.3% during the baseline, CHX, SOD, and SDD periods, respectively. Absolute risk reductions were 0.0% (95% CI, −2.9% to 2.9%), −0.7% (95% CI, −3.5% to 2.2%), and −2.2% (95% CI, −5.2% to 0.7%) for CHX, SOD, and SDD, respectively, compared with baseline. Corresponding adjusted odds ratios were 0.97 (95% CI, 0.85-1.11), 1.00 (95% CI, 0.87-1.14), and 0.96 (95% CI, 0.82-1.12) during the CHX, SOD, and SDD periods, respectively.
Antibiotic Use and Resistance
The unitwide consumption of systemic antibiotics was 1.1, 1.0, 1.0, and 1.1 defined daily doses per patient day during the baseline, CHX, SOD, and SDD periods, respectively (eTable 9 in Supplement 2).
In total, 5536 respiratory and 5441 rectal samples were obtained from 5706 survey participants during 329-point prevalence surveys (Table 4; eTable 10 in Supplement 2). Completeness of susceptibility testing was greater than 95% (eTable 11 in Supplement 2). Based on the point prevalence surveys, the overall prevalence of carriage with MDRGNB ranged from 17.1% to 25.3% in rectum samples and of carriage with MDRGNB from 10.2% to 15.2% in respiratory tract samples, without statistically significant differences between study groups (Table 5). The prevalence of colistin resistance did not increase during the intervention periods (Table 5; eTables 10 and 12 in Supplement 2).
Table 4. Descriptive Statistics of Point Prevalence Surveys for Unitwide Carriage of Antibiotic-Resistant Microorganisms in the Rectum and Respiratory Tract.
| Descriptive Statistics Point Prevalence Surveys | Baseline | CHX | SOD | SDD | |
|---|---|---|---|---|---|
| Proportion of patients in the unit screened, % | 93.1 | 94.3 | 92.2 | 92.3 | |
| No. of patients sampled | 1456 | 1424 | 1469 | 1407 | |
| Included in study population, % of patients sampled | 63.0 | 61.7 | 60.7 | 59.8 | |
| No. of rectal samples (% of patients sampled) | 1392 (95.6) | 1370 (96.2) | 1419 (96.6) | 1355 (96.3) | |
| No. of respiratory samples (% of patients sampled) | 1381 (94.8) | 1333 (93.6) | 1408 (95.8) | 1319 (93.7) | |
Abbreviations: CHX, chlorhexidine mouthwash; SDD, selective digestive tract decontamination; SOD, selective oropharyngeal decontamination.
Table 5. Prevalence of Unitwide Carriage of Antibiotic-Resistant Microorganisms in the Rectum and Respiratory Tract (Exploratory Outcome).
| Baseline | CHX | SOD | SDD | ||||
|---|---|---|---|---|---|---|---|
| Prevalence, % | Prevalence, % | aRR (95% CI)a | Prevalence, % | aRR (95% CI)a | Prevalence, % | aRR (95% CI)a | |
| Rectum | |||||||
| HRMO enterobacteriaceae | 16.1 | 21.7 | 1.07 (0.99-1.16) | 19.7 | 1.04 (0.96-1.13) | 13.9 | 1.05 (0.95-1.16) |
| Third-generation cephalosporin resistance | 15.8 | 21.5 | 1.07 (0.99-1.16) | 19.2 | 1.04 (0.96-1.13) | 13.7 | 1.07 (0.97-1.18) |
| Carbapenem resistance | 3.2 | 3.1 | 0.68 (0.54-0.86) | 2.9 | 0.85 (0.71-1.03) | 2.6 | 0.80 (0.64-1.01) |
| Resistance to ≥3 antibiotics (or classes) | 10.8 | 15.5 | 1.07 (0.97-1.19) | 14.2 | 1.06 (0.96-1.17) | 10.0 | 1.10 (0.97-1.24) |
| Colistin resistanceb | 0.5 | 1.6 | 0.81 (0.54-1.21) | 1.8 | 0.97 (0.65-1.45) | 1.3 | 0.96 (0.60-1.54) |
| HRMO glucose nonfermenting GNB | 3.2 | 3.2 | 0.77 (0.62-0.95) | 3.3 | 0.93 (0.76-1.14) | 2.3 | 0.81 (0.63-1.04) |
| MDRGNB, regardless of antibiotic susceptibility | 1.0 | 1.5 | 0.80 (0.50-1.27) | 1.1 | 0.80 (0.49-1.30) | 1.6 | 1.01 (0.64-1.58) |
| Any MDRGNB (aggregate) | 19.3 | 25.3 | 1.03 (0.96-1.11) | 23.0 | 1.03 (0.96-1.11) | 17.1 | 1.04 (0.96-1.14) |
| VRE | 2.2 | 1.5 | 0.96 (0.74-1.24) | 1.8 | 0.94 (0.73-1.21) | 4.2 | 1.03 (0.84-1.27) |
| Respiratory Tract | |||||||
| HRMO Enterobacteriaceae | 6.6 | 7.6 | 0.94 (0.81-1.09) | 4.2 | 0.93 (0.80-1.09) | 4.7 | 0.94 (0.78-1.13) |
| Third-generation cephalosporin resistance | 6.4 | 7.4 | 0.95 (0.82-1.10) | 4.2 | 0.93 (0.80-1.09) | 4.5 | 0.94 (0.78-1.13) |
| Carbapenem resistance | 1.4 | 1.1 | 0.71 (0.47-1.07) | 0.9 | 0.68 (0.48-0.94) | 0.5 | 0.59 (0.37-0.97) |
| Resistance to ≥3 antibiotics (or classes) | 4.0 | 5.2 | 1.02 (0.84-1.23) | 3.3 | 0.92 (0.76-1.12) | 3.5 | 1.04 (0.83-1.31) |
| Colistin resistanceb | 0.1 | 0.8 | 0.57 (0.29-1.14) | 0.9 | 0.66 (0.36-1.21) | 0.3 | 0.61 (0.30-1.22) |
| HRMO glucose nonfermenting GNB | 3.4 | 2.9 | 0.80 (0.64-1.00) | 3.8 | 0.84 (0.70-1.00) | 2.7 | 0.75 (0.58-0.96) |
| MDRGNB, regardless of antibiotic susceptibility | 3.8 | 5.2 | 1.16 (0.94-1.44) | 3.2 | 0.97 (0.77-1.22) | 3.6 | 1.04 (0.83-1.31) |
| Any MDRGNB (aggregate) | 12.9 | 15.2 | 0.98 (0.88-1.08) | 10.3 | 0.93 (0.84-1.04) | 10.2 | 0.94 (0.83-1.06) |
| MRSA | 1.7 | 1.1 | 0.95 (0.66-1.36) | 1.3 | 0.77 (0.59-1.00) | 1.7 | 0.73 (0.54-0.97) |
Abbreviations: aRR, adjusted relative risk; CHX, chlorhexidine mouthwash; GNB, gram-negative bacteria; HRMO, highly resistant microorganism; MDRGNB, multidrug-resistant gram-negative bacteria (eTable 3 in Supplement 2); MRSA, methicillin-resistant S aureus; SDD, selective digestive tract decontamination; SOD, selective oropharyngeal decontamination; VRE, vancomycin-resistant E faecium/E faecalis.
aRR per month, all models were corrected for underlying time trends per center.
Excluding Enterobacteriaceae with intrinsic colistin resistance (Proteus spp, Morganella spp, Serratia spp, Providencia spp, and Hafnia alvei).
Sensitivity Analyses
Post hoc sensitivity analyses in which BSIs were assumed to have been prevented by third-generation cephalosporins and SDD treatment until the end of ICU stay yielded similar results for SDD (eTable 13 in Supplement 2). Sensitivity analyses excluding patients who stayed in an ICU fewer than 3 days led to similar results for all mortality outcomes (eTable 13 in Supplement 2).
Post Hoc Outcomes
Overall, 573 patients had 713 episodes of ICU-acquired BSI with any pathogen, most frequently caused by Enterococcus spp (n = 125), Klebsiella spp (n = 121), Candida spp (n = 69), S aureus (n = 67), and Pseudomonas spp (n = 61) (Table 2). These occurred in 6.8%, 7.4%, 6.3%, and 5.9% during the baseline, CHX, SOD, and SDD periods, respectively. Absolute risk reductions were −0.6% (95% CI, −2.1% to 1.0%), 0.5% (95% CI, −0.9% to 2.0%), and 0.9% (95% CI, −0.5% to 2.4%) for CHX, SOD, and SDD, respectively, compared with baseline. As compared with baseline, the aHRs were 1.08 (95% CI, 0.85 to 1.39), 0.94 (95% CI, 0.76 to 1.17), and 0.79 (95% CI, 0.60 to 1.05) for CHX, SOD and SDD, respectively (Table 3). SDD was associated with lower risk of ICU-acquired MDRGNB BSI compared with CHX (aHR, 0.62; 95% CI, 0.39 - 0.98) (eTable 14 in Supplement 2). There were no statistically significant differences in any of the mortality outcomes in the post hoc head-to-head comparisons between interventions (eTable 14 in Supplement 2). There were no statistically significant associations between interventions and competing end points in any of these analyses (eTable 15 in Supplement 2).
In an exploratory analysis based on the results of surveillance cultures plated on extended-spectrum β-lactamase selective media and obtained twice weekly from study patients, carriage rates with antibiotic-resistant GNB in the rectum during SDD and in the respiratory tract during SDD/SOD appeared to remain stable, in comparison with other study groups where there appeared to be a gradual increase in colonization during ICU stay (eFigure in Supplement 2). On day 14 of ICU stay, the proportion of rectal cultures growing GNB from selective media was 14.8% during SDD and 28.3% during the baseline period.
Discussion
In this cluster randomized multicenter study in 13 European ICUs, decontamination strategies with either antibiotics (SDD or SOD) or CHX mouthwash were not associated with reductions in ICU-acquired BSI with MDRGNB, nor mortality, in ventilated ICU patients when compared with standard care, which included universal daily BWs with CHX during ICU stay and a hand hygiene program. Furthermore, the unitwide prevalence of carriage with antibiotic-resistant bacteria did not change during the interventions, which is consistent with results obtained in all large SDD trials of the last 20 years.8
The strengths of this study include participation of ICUs in 6 European countries, with resistance rates that better reflect the average European or American setting than Dutch ICUs, thereby improving external validity and generalizability of findings, as well as the detailed unitwide resistance monitoring with monthly point prevalence studies.
The findings of the current study differ in several aspects from those obtained in similar studies in Dutch centers.3,4,5 First, the current study aimed to test decontamination regimens in ICUs with higher prevalence of antibiotic resistance. Indeed, the observed 17.6% unitwide rectal carriage rate of third-generation cephalosporin-resistant Enterobacteriaceae and an overall proportion of 25.5% of ICU-acquired BSIs caused by HRMO are considerably higher than in previous Dutch studies.3,4,5 Decontamination strategies using conventional SDD or SOD regimens may be less effective in this context, especially in areas with high prevalence of resistance to aminoglycosides or colistin among GNB. The unitwide prevalence of colonization with gentamicin-resistant GNB was 8.3% in the rectum and 4.5% in the respiratory tract, which is twice as high as in a previous Dutch study performed between 2004 and 2006,4 but comparable with the more recent Dutch study performed between 2009 and 2013.5
Second, SDD did not include a 4-day course of intravenous third-generation cephalosporins, which might have reduced the effects of SDD. During SDD, there were 48 episodes of ICU-acquired BSIs occurring within the first 4 days of inclusion, 17 of which involved pathogens susceptible to third-generation cephalosporins. Absence of cefotaxime during SDD cannot explain the discrepant findings for SOD, which was also associated with a reduction in mortality and ICU-acquired BSI in a previous Dutch study.4
Third, interventions were discontinued at the end of mechanical ventilation, instead of at ICU discharge. In a previous Dutch study, SDD and SOD were administered during more than 95% of patients’ days,4,5 whereas in the current study, mechanical ventilation days accounted for 69.2% of ICU days in study patients, reflecting the maximum proportion of time during which patients received study interventions. In fact, during CHX, SOD, and SDD, there were 32, 23, and 33 ICU-acquired BSI episodes that occurred on days without mechanical ventilation. A post hoc sensitivity analysis in which BSIs were assumed to have been prevented by third-generation cephalosporins and SDD treatment until the end of ICU stay yielded similar results for SDD. It is, therefore, unlikely that these protocol variations explain the discrepant findings with regard to SDD efficacy for patient outcome compared with previous studies.
Fourth, standard care in the current study included strategies that may have influenced carriage and transmission of HRMO and were not implemented in previous Dutch studies, such as oral care with antiseptics (CHX mouthwash 0.12% or 0.20%) in 11 of 13 centers, implementation of the World Health Organization hand hygiene program and daily CHX 2% BWs for all patients in the ICU until discharge. Although the effects of these strategies on colonization and infection with GNB cannot be assessed within the current study, they may have reduced the potential of the 3 interventions to offer additional benefits.20
Limitations
This study has several limitations. First, its design involves the inherent risk of (selection) bias due to cluster randomization and the fixed start with the baseline period, precluding adjustment for changes in ICU organization, ecology, or unmeasured patient characteristics over time. The study was also designed to compare each intervention with standard care, but not with each other. The head-to-head comparisons of the 3 interventions for primary and secondary outcomes, as reported, were based on a post hoc analysis.
Second, the originally targeted sample size of 10 800 patients was not reached, and accordingly, the study may have been underpowered to detect a clinically relevant difference in the primary outcome. However, post hoc power calculation revealed that this study had 80% power to detect an absolute reduction in hospital mortality of 4.2%, which is within the 2.9% to 5.3% range that was suggested by meta-analyses,12 and 78.7% power to detect a 50% relative reduction in ICU-acquired BSI caused by MDRGNB. The confidence intervals for the primary outcome, BSI, do leave room for a potential effect of SDD in a larger study. Yet, as most hazard rates for the mortality outcomes were close to or even above 1, a larger study population would probably not have resulted in a statistically significant association for any of the mortality outcomes. For example, the aHR of 0.96 for hospital mortality during SDD corresponds to a relative risk reduction of 2.25% and an absolute risk reduction of 0.95% compared with baseline (with 38% hospital mortality).
Third, monitoring of carriage with MDRGNB ended at ICU discharge, precluding evaluation of long-term effects of the interventions.
Conclusions
Among patients receiving mechanical ventilation in ICUs with moderate to high antibiotic resistance prevalence, use of CHX 1% mouthwash, SOD, or SDD was not associated with reductions in ICU-acquired bloodstream infections caused by MDRGNB as compared with standard care.
Trial Protocol
eAppendix 1. eMicrobiology Methods
eAppendix 2. Sample Size Calculation
eFigure. Proportion of Surveillance Samples Positive for Antibiotic-Resistant Gram-Negative Bacteria on Day 1-14 of ICU Admission (ESBL-Selective Media)
eTable 1. Characteristics of Participating Centers
eTable 2. Micro-organisms in Positive Blood Cultures That are Not Included in the Study Definition of ICU-Acquired Bloodstream Infection
eTable 3. Antibiotic Susceptibility Testing and Definition of Highly Resistant Micro-organism
eTable 4. Baseline Characteristics of Screened Population
eTable 5. All Baseline Characteristics (Study Population)
eTable 6. Compliance Measures
eTable 7. Average Hand Hygiene Compliance per Study Period
eTable 8. Incidence of ICU-Acquired Bloodstream Infection With Multidrug Resistant Gram-Negative Bacteria (Primary Outcome) per ICU
eTable 9. Systemic Antibiotics Used
eTable 10. Prevalence of Unit-Wide Carriage of Antibiotic Resistant Microorganisms in Rectum and Respiratory Tract (Complete Results)
eTable 11. Compliance With Antibiotic Susceptibility Testing in Point Prevalence Samples
eTable 12. Prevalence of Colistin-Resistant Gram-Negative Bacteria in 3 Monthly Point Prevalence Surveys (10/13 Centers)
eTable 13. Results of Sensitivity Analyses
eTable 14. Head-to-Head Comparisons Between Study Interventions; Primary and Secondary Outcomes
eTable 15. Results for Competing End Points for Primary and Secondary Outcomes
Data Sharing Statement
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Vincent JL, Rello J, Marshall J, et al. ; EPIC II Group of Investigators . International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323-2329. doi: 10.1001/jama.2009.1754 [DOI] [PubMed] [Google Scholar]
- 2.Scott RD. The direct medical costs of healthcare-associated infections in US hospitals and the benefits of prevention. Centers for Disease Control and Prevention. https://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf. Published March 2009. Accessed January 4, 2017.
- 3.de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet. 2003;362(9389):1011-1016. doi: 10.1016/S0140-6736(03)14409-1 [DOI] [PubMed] [Google Scholar]
- 4.de Smet AM, Kluytmans JA, Cooper BS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360(1):20-31. doi: 10.1056/NEJMoa0800394 [DOI] [PubMed] [Google Scholar]
- 5.Oostdijk EAN, Kesecioglu J, Schultz MJ, et al. Notice of retraction and replacement: Oostdijk et al. effects of decontamination of the oropharynx and intestinal tract on antibiotic resistance in ICUs: a randomized clinical trial. JAMA. 2014;312(14):1429-1437. JAMA. 2017;317(15):1583-1584. doi: 10.1001/jama.2017.1282 [DOI] [PubMed] [Google Scholar]
- 6.Plantinga NL, de Smet AMGA, Oostdijk EAN, et al. Selective digestive and oropharyngeal decontamination in medical and surgical ICU patients: individual patient data meta-analysis. [Published online September 6, 2017]. Clin Microbiol Infect. 2018;24(5):505-513. doi: 10.1016/j.cmi.2017.08.019 [DOI] [PubMed] [Google Scholar]
- 7.Duncan EM, Cuthbertson BH, Prior ME, et al. ; SuDDICU International Study Group . The views of health care professionals about selective decontamination of the digestive tract: an international, theoretically informed interview study. J Crit Care. 2014;29(4):634-640. doi: 10.1016/j.jcrc.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 8.Daneman N, Sarwar S, Fowler RA, Cuthbertson BH; SuDDICU Canadian Study Group . Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(4):328-341. doi: 10.1016/S1473-3099(12)70322-5 [DOI] [PubMed] [Google Scholar]
- 9.Labeau SO, Van de Vyver K, Brusselaers N, Vogelaers D, Blot SI. Prevention of ventilator-associated pneumonia with oral antiseptics: a systematic review and meta-analysis. Lancet Infect Dis. 2011;11(11):845-854. doi: 10.1016/S1473-3099(11)70127-X [DOI] [PubMed] [Google Scholar]
- 10.Hua F, Xie H, Worthington HV, Furness S, Zhang Q, Li C. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev. 2016;10(10):CD008367. doi: 10.1002/14651858.CD008367.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klompas M, Speck K, Howell MD, Greene LR, Berenholtz SM. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and meta-analysis. JAMA Intern Med. 2014;174(5):751-761. doi: 10.1001/jamainternmed.2014.359 [DOI] [PubMed] [Google Scholar]
- 12.Price R, MacLennan G, Glen J; SuDDICU Collaboration . Selective digestive or oropharyngeal decontamination and topical oropharyngeal chlorhexidine for prevention of death in general intensive care: systematic review and network meta-analysis. BMJ. 2014;348:g2197. doi: 10.1136/bmj.g2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization WHO guidelines on hand hygiene in health care: first global patient safety challenge: clean care is safer care. http://apps.who.int/iris/bitstream/10665/44102/1/9789241597906_eng.pdf. Published 2009. Accessed January 4, 2017. [PubMed]
- 14.Plantinga NL, Wittekamp BHJ, Leleu K, et al. Oral mucosal adverse events with chlorhexidine 2% mouthwash in ICU. Intensive Care Med. 2016;42(4):620-621. doi: 10.1007/s00134-016-4217-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268-281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 16.McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388-3414. doi: 10.1002/sim.5753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818-829. doi: 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 19.Le Gall JR, Neumann A, Hemery F, et al. Mortality prediction using SAPS II: an update for French intensive care units. Crit Care. 2005;9(6):R645-R652. doi: 10.1186/cc3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derde LPG, Cooper BS, Goossens H, et al. ; MOSAR WP3 Study Team . Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Lancet Infect Dis. 2014;14(1):31-39. doi: 10.1016/S1473-3099(13)70295-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. eMicrobiology Methods
eAppendix 2. Sample Size Calculation
eFigure. Proportion of Surveillance Samples Positive for Antibiotic-Resistant Gram-Negative Bacteria on Day 1-14 of ICU Admission (ESBL-Selective Media)
eTable 1. Characteristics of Participating Centers
eTable 2. Micro-organisms in Positive Blood Cultures That are Not Included in the Study Definition of ICU-Acquired Bloodstream Infection
eTable 3. Antibiotic Susceptibility Testing and Definition of Highly Resistant Micro-organism
eTable 4. Baseline Characteristics of Screened Population
eTable 5. All Baseline Characteristics (Study Population)
eTable 6. Compliance Measures
eTable 7. Average Hand Hygiene Compliance per Study Period
eTable 8. Incidence of ICU-Acquired Bloodstream Infection With Multidrug Resistant Gram-Negative Bacteria (Primary Outcome) per ICU
eTable 9. Systemic Antibiotics Used
eTable 10. Prevalence of Unit-Wide Carriage of Antibiotic Resistant Microorganisms in Rectum and Respiratory Tract (Complete Results)
eTable 11. Compliance With Antibiotic Susceptibility Testing in Point Prevalence Samples
eTable 12. Prevalence of Colistin-Resistant Gram-Negative Bacteria in 3 Monthly Point Prevalence Surveys (10/13 Centers)
eTable 13. Results of Sensitivity Analyses
eTable 14. Head-to-Head Comparisons Between Study Interventions; Primary and Secondary Outcomes
eTable 15. Results for Competing End Points for Primary and Secondary Outcomes
Data Sharing Statement