Abstract
Background and aims
Single-operator cholangioscopy (SOC) can help diagnose biliopancreatic conditions. The impact of SOC on patient outcome has never been specifically addressed.
Patients and methods
Consecutive patients bearing indeterminate biliary strictures (IDBS), or primary sclerosing cholangitis (PSC) with suspected cholangiocarcinoma, were included. Patients with IDBS had at least one previous inconclusive endoscopic retrograde cholangio pancreatography (ERCP) + cytology. Primary endpoint was the difference in adequacy of management planned before and after SOC with regard to final diagnosis obtained after surgery or 24 months follow-up.
Design
Prospective open-label multicentre trial.
Results
61 patients were included (IDBS: 48; PSC: 13); 70.5% had a benign lesion (IDBS 66.7%, PSC 84.6%). The management adequacy rate was significantly higher after SOC than before SOC overall (p<10–5), in IDBS (p<0.001) and PSC (p<0.05) patients. SOC induced changes in the management of the majority of patients in all groups (60.3%). The overall sensitivity of combined visual impression and biopsy ranged from 52% to 63.6% depending on investigator or independent expert rating (κ 0.92–0.96), whereas specificity, positive and negative predictive values of SOC were, respectively, 100%, 100% and 83.6%. Patient management observed at the end of follow-up was consistent with that anticipated after SOC in 88.5% overall.
Conclusion
Despite a moderate sensitivity for the diagnosis of malignancy, SOC has a dramatic impact on the management of patients with IDBS and PSC with suspected carcinoma. Cholangioscopy might be implemented in the workup of selected patients with challenging diagnosis, when a significant impact on outcome (essentially resection vs conservative management) is to be expected.
Keywords: biliary strictures, primary sclerosing cholangitis, endoscopic procedures, pancreatic tumours, endoscopic retrograde pancreatography
Introduction
A majority of biliary strictures are malignant, mostly due to the extrinsic compression of the distal bile duct by a pancreatic head cancer, less commonly to the intrinsic development of a cholangiocarcinoma or to the ductal extension of a gallbladder carcinoma or lymphatic metastases from a distant tumour. Benign biliary strictures, accounting for up to 30% of cases,1 often have an obvious cause, be it a swollen pancreatic head during the course of chronic pancreatitis, an ischaemic duct-to-duct anastomosis after liver transplantation, sequelae of bile duct injury within months of a cholecystectomy, and so on. However, the standard workup of a biliary stenosis, including blood tests, abdominal ultrasound, CT scan, MRI and endoscopic ultrasonography (guided) - fine needle aspiration (EUS-FNA), fails in some patients to identify the aetiology of the stricture, dictating the need to perform ERCP with cytology. Due to the poor cytological yield of ERCP techniques (including bile and brush cytology and fluoroscopy-guided biopsies, which even in combination, barely reach 50% sensitivity),2 3 a substantial contingent of strictures remain indeterminate. It is recognised that 5%–24% of patients undergoing surgery for suspected but not preoperatively proven malignancies do indeed have a benign condition and could have been spared such complex and sometimes high-risk operations.4 5 It is therefore highly desirable to investigate more accurately so-defined indeterminate biliary strictures (IDBS) in order to reduce the rate of unnecessary surgeries and apply a treatment fit to the patient’s condition. Primary sclerosing cholangitis (PSC) may raise similarly difficult diagnostic issues.6 Cholangioscopy, as a method with an ability to visualise strictures endoscopically and to target biopsies, has been proposed to further advance investigation in such difficult cases, particularly after single-use, single-operator devices were made available in 2007.
Several studies found single-operator cholangioscopy (SOC) to bear high-sensitivity and negative predictive value for the diagnosis of malignancy in case of IDBS and PSC. However, most of those works, later discussed in this article, did not study the impact of SOC on the patient’s outcome. It was the primary aim of the present study to assess to what extent SOC could affect patient management in case of IDBS and PSC with suspected cholangiocarcinoma.
Patients and methods
The scope of this study encompassed two categories of biliary conditions in which to assess the impact of SOC, namely IDBS and PSC. Over a 3-year period, patients from nine French academic tertiary referral centresi fulfilling selection criteria were included, then prospectively followed during 24 months. Selection criteria were adapted for each of the three subgroups. A definitive diagnosis was obtained either after surgical resection or after follow-up. Observed managements and outcomes after SOC were compared for each patient to the initially planned management (ie, before SOC) as well as to currently recommended management after final observations were disclosed, under the authority of independent experts.
Patient selection
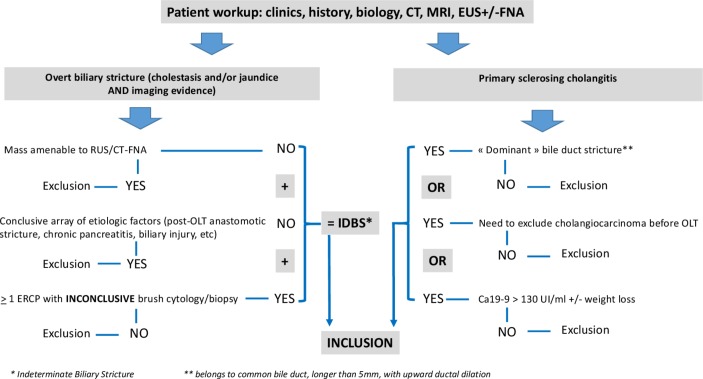
Patients were aged >18 years, were covered by social security insurance and signed informed consent of participation. All the patients had undergone a standard workup including biology, CT scan or MRI, EUS with or without FNA. All patients were rated I or II under the American Society of Anesthesiology classification and deemed fit for surgery. In the IDBS group, only patients with clinically overt strictures (ie, with biological cholestasis and/or jaundice, with or without clinical symptoms of biliary obstruction) were included. Strictures could involve the common bile duct, the main confluence/hilum and/or the peri-hilar part of major intrahepatic ducts. At least one ERCP with inconclusive brush cytologyii was required for the stricture to be deemed as IDBS. The last ERCP had to have been performed <4 weeks before inclusion. Patients with a conclusive array of aetiological factors (ie, orthotopic liver transplantation (OLT) patients with anastomotic stricture developed <1 year before transplantation, recently complicated biliary surgery, and swollen and compressive chronic pancreatitis) as well as those with a pancreatic head or lymphatic mass amenable to EUS-FNA (even after a first inconclusive FNA) were excluded. In the PSC group, patients had to present one of the following criteria: (a) a ‘dominant’ bile duct stricture, defined as a stricture comprised between the sectoral confluences and the papilla, longer than 5 mm, with upward ductal dilation; (b) multi disciplinary team meeting (MDT) request for exclusion of cholangiocarcinoma before OLT waiting list registration; and (c) newly observed Ca19-9 serum elevation above 130 UI/mL with or without weight loss (figure 1).
Figure 1.
Diagram of selection criteria for patient enrolment. IDBS, indeterminate biliary strictures.
SOC procedure, diagnostic workup and follow-up
The contemplated management strategy, would SOC be inconclusive or provide no additional information, had to be consigned at inclusion and before SOC. Procedures were performed in supine position under general anaesthesia with airway intubation, using a standard large channel duodenoscope and the Spyglass-Legacy platform (Boston Scientific, Marlborough, Massachusetts, USA). Most patients had a previous endoscopic sphincterotomy, which could be enlarged at the time of SOC when needed. Cholangioscopy was done under saline irrigation and all patients received prophylactic antibiotics. SOC procedure always included an observational phase, with visual impression being described with as much detail as possible, although no specific terminology was required. It was however required to classify the visual impression as malignant, benign or still indeterminate. Intraductal targeted biopsies were obtained using the Spybite (Boston Scientific) miniforceps as often as possible, with at least four macroscopically visible samples, although it was accepted that no biopsy was taken when visual impression was strictly normal (ie, absence of surface or colour anomalies). Since all participating centres had their own pathology department with expertise in biliopancreatic diseases, pathological sample analyses were not centralised, but toughest cases were discussed, when necessary, with the study’s referee pathologist (BT). A plastic stent was generally, but not systematically, inserted after removing the cholangioscope. The investigator was required to report whether their visual impression was likely or not to change the previously planned management. Patient management decided after SOC and pathology results by the investigator and/or during MDT meetings were recorded. SOC-induced changes within a given type of management (eg, a different type of resection, suggested by the observed extent of lesions) were also reported. Patients were followed after SOC until surgery in case of resection or during 24 months in case of conservative management, with visits scheduled at 3, 6, 9, 12, 18 and 24 months after SOC, including clinical examination, liver blood tests and imaging when needed. Definitive diagnosis and observed management were reported at the end of FU.
Objectives and endpoints
The main objective of the study was to determine the percentage of patients in whom SOC would change outcomes in a favourable manner, that is, by providing the evidence needed to make ad hoc decisions on patient management (ie, essentially surgical vs conservative). To achieve that goal, two endpoints were defined, for which the patient was his own control: (a) planned patient management before SOC versus management recommended after definitive diagnosis and (b) planned patient management after SOC versus management recommended after definitive diagnosis.
The following criteria defined the adequacy between diagnosis and management, depending on the subgroup of biliopancreatic condition: (a) In cases of IDBS, a benign stricture had to be treated by instrumental means, endoscopic or percutaneous, including dilations and (removable) stents, whereas malignant lesions should come under carcinologic resection, with explorations expected to define the extent of lesions to help the surgeon optimise the resection strategy; and (b) In the PSC group, patients with evidence of cholangiocarcinoma should have been excluded from transplantation and instead treated by oncological therapy adapted to their case, whereas transplantation could be confirmed, if needed, or conservative therapy be resumed, when carcinoma was precluded.
Secondary endpoints were (a) diagnostic performances of SOC, for which combined visual impression and pathology results were taken into accountiii; (b) comparison of actually observed management vs management suggested after SOC; (c) comparison of actually observed management versus currently recommended management with regard to definitive diagnosis; and (d) proportion of patients in whom SOC has modified patients’ management. Procedure duration and side effects were also reported.
The above-described adequacy endpoints (planned management after ERCP or SOC vs definitive diagnosis, actually observed management vs post-SOC suggested management) and classification of true and false negatives and positives for diagnostic performance were first assessed jointly by the principal investigators (FP, SL) for each individual patient. The same analysis was done subsequently from the same database by two experts blinded to the investigators’ assessment. Those two assessors (one gastroenterologist (AL) and one surgeon (SG)), selected for their expertise in biliopancreatic disorders only after the end of data collection, were independent from the participating centres.
Number of subjects and statistics
Sample size calculation, with 80% power and a 5% significance level and discordant proportion of 50%, resulted in a total of 50 patients (25 patients per subgroup). The calculation was based on an expected adequate management (with respect to definitive diagnosis) before SOC of 40% versus 80% after SOC.
Statistical analyses were performed using ad hoc routines implemented in the R V.3.3.1 software (http://www.r-project.org). Discrete variables are presented as counts (percentage) and continuous variables as mean ±SD for normally distributed variables and median (range) for those that did not have a normal distribution. Comparisons of the adequacy between planned management and definitive diagnosis before and after SOC were performed using McNemar tests for paired data. The inter-rater reliability (concordance between separate evaluators) regarding the classification of true and false negatives and positives for diagnostic performance was assessed by the Cohen’s kappa coefficient using the PSY package.7 Concordance was evaluated as follows: no agreement if <0, slight if 0–0.20, fair if 0.21–0.40, moderate if 0.41–0.60, substantial if 0.61–0.80 and almost perfect if 0.81–1.8 A bootstrap method was used to calculate the 95% CI of the kappa’s coefficients. All tests were two-sided at the 5% level of significance.
Results
Sixty-seven patients were included overall, but six were excluded after two early non-procedure-related deaths 2 weeks and 2 months after inclusion and four patients lost at 24-month FU. Sixty-one patients, aged 58.7±14.6 years (median 61, range 21–89, with a sex ratio of 1.34), were analysed. In total, 48 belonged to the IDBS group (21 women, 27 men, aged 64.5±14 years) and 13 to the PSC group (5 women, 8 men, 48.2±16.7 years). There was no failure of cholangioscope insertion; balloon dilatation (up to 6 mm in diameter) was needed in two cases and repeat sphincterotomy in one case to allow for easy passage of the device. Exploration of the biliary tract above the stricture was possible in 77% (37/48) of patients in the IDBS group and 85% (11/13) in the PSC group. Quality of vision was deemed excellent/satisfactory in 85% of SOC procedures, fair or poor in 15%. Spybite biopsies were obtained from 57 patients, whereas 4 with cholangioscopically normal ducts had no biopsies, including 2 patients with unexpected bile duct stones. Two procedure-related cases of cholangitis (3.2%) occurred and resolved with antibiotics. The recorded time of cholangiopancreatoscopy per se (from Spyglass insertion to removal) was 31.1± 1.4 min (median 25 min, range 5–90). The definitive diagnosis after surgery or 24-month FU was a benign disease in 43 patients (70.5%) and a malignancy in 18 (29.5%), as shown and broken down per group in table 1.
Table 1.
Relative numbers and proportions of benign and malignant diseases overall and per subgroup after definitive diagnosis was obtained (ie, after surgery or 24 months follow-up)
| Overall | Indeterminate biliary strictures | Primary sclerosing cholangitis | ||||
| N | % | N | % | N | % | |
| Benign | 43 | 70.5 | 32 | 66.7 | 11 | 84,6 |
| Malignant | 18 | 29.5 | 16 | 33.3 | 2 | 15,4 |
| Total | 61 | 100 | 48 | 100 | 13 | 100 |
Table 2 shows that the adequacy between patient management as anticipated by investigators and the definitive diagnosis was significantly higher after SOC was performed than before, both for the overall casemix (p<0.0001), in patients with IDBS (p<0.001) and, although less significantly, in patients with PSC (p<0.05).
Table 2.
Comparison of the adequacy between planned management and definitive diagnosis before and after single-operator cholangioscopy (SOC) for (a) all groups, (b) indeterminate biliary strictures (IDBS) and (c) primary sclerosing cholangitis (PSC)
| (a) Overall (p<10–5) |
After SOC (%) | ||
| Inadequate | Adequate | ||
| Before SOC (%) | |||
| Inadequate | 6 | 32 | 38 (62.3) |
| Adequate | 5 | 18 | 23 (37.7) |
| 11 (18.0) | 50 (82.0) | 61 (100) | |
| (b) IDBS (p<0.001) |
After SOC (%) | ||
| Inadequate | Adequate | ||
| Before SOC (%) | |||
| Inadequate | 3 | 24 | 27 (43.8) |
| Adequate | 4 | 17 | 21 (56.2) |
| 7 (14.6) | 41 (85.4) | 48 (100) | |
| (c) PSC (p<0.05) |
After SOC (%) | ||
| Inadequate | Adequate | ||
| Before SOC (%) | |||
| Inadequate | 3 | 8 | 11 (84.6) |
| Adequate | 1 | 1 | 2 (15.4) |
| 4 (30.8) | 9 (69.2) | 13 (100) | |
This is reflected in the rate of changes in planned management after SOC, which was estimated between 58.3% in patients with IDBS and 69.2% in patients with PSC (table 3).
Table 3.
Single-operator cholangioscopy-induced changes in planned management
| Planned management modified | Overall | Indeterminate biliary strictures | Primary sclerosing cholangitis | |||
| N | % | N | % | N | % | |
| No | 24 | 39.3 | 20 | 41.7 | 4 | 30.8 |
| Yes | 37 | 60.7 | 28 | 58.3 | 9 | 69.2 |
| Total | 61 | 100 | 48 | 100 | 13 | 100 |
Agreement between PIs and independent experts was perfect for this endpoint in all groups and overall (κ=1). Since most SOC procedures (57 out of 61) had been undertaken with an intent to operate for resection in case malignancy was confirmed, surgery was precluded after SOC in 58% of patients (33/57). More specifically, in patients with IDBS, surgery was confirmed as initially planned in 20/48 patients whereas ‘active’ management was changed to conservative in 26 and only 2 were changed to a more active treatment (surgery in one patient and chemotherapy in another). In patients with PSC, SOC has allowed to confirm the registration of three patients on the OLT waiting list and exclude one other from prospective OLT, whereas seven others were avoided unnecessary hepatobiliary resection.
Diagnostic performance results (table 4) have been presented only for the overall casemix and for IDBS group because the small size of the PSC group, with very large CIs, made a correct interpretation of results uncertain (data available as online supplementary material). The overall and IDBS results demonstrate high specificity and positive predictive values of SOC at 100%, high negative predictive value but moderate sensitivity, the latter being slightly higher when visual impression is considered than when pathology results are introduced. Although up to five instances of disagreement in classification between principal investigator (PI) and experts have been noted, kappa agreement indices remained in the near perfect range at >0.88.
Table 4.
Diagnostic performance of single-operator cholangioscopy (SOC) (overall and indeterminate biliary strictures (IDBS))*
| Performance criteria (95% CI) | Overall | IDBS |
| (a) SOC visual impression (PI) | ||
| Se | 63.6 (40.7 to 82.8) | 64.7 (38.3 to 85.8) |
| Sp | 100 (93 to 100) | 100 (88.8 to 100) |
| PPV | 100 (76.8 to 100) | 100 (71.5 to 100) |
| NPV | 86.4 (75 to 94) | 83.8 (68.0 to 93.8) |
| (b) SOC visual impression + pathology (PI) | ||
| Se | 54.5 (32.2 to 75.6) | 56.3 (29.9 to 80.2) |
| Sp | 100 (93 to 100) | 100 (89.1 to 100) |
| PPV | 100 (73.5 to 100) | 100 (66.4 to 100) |
| NPV | 83.6 (71.9 to 91.8) | 82.1 (66.5 to 92.5) |
| (c) Expert review 1 (AL) for SOC visual impression + pathology | ||
| Kappa (CI95%) PI vs AL overall: 0.94 (0.84–1.0)/IDBS: 0.92 (0.80–1.00) | ||
| Se | 57.1 (34.0 to 78.2) | 56.3 (29.9 to 80.2) |
| Sp | 100 (93 to 100) | 100 (89.1 to 100) |
| PPV | 100 (73.5 to 100) | 100 (66.4 to 100) |
| NPV | 85.0 (73.4 to 92.9) | 82.1 (66.5 to 92.5) |
| (d) Expert review 2 (SG) SOC visual impression + pathology | ||
| Kappa (CI95%) PI vs SG overall: 0.97 (0.90–1.0)/IDBS: 0.96 (0.86–100) | ||
| Se | 52.0 (29.8 to 74.3) | 50.0 (24.7 to 75.3) |
| Sp | 100 (93 to 100) | 100 (89.1 to 100) |
| PPV | 100 (71.5 to 100) | 100 (63.1 to 100) |
| NPV | 83.6 (71.9 to 91.8) | 80 (64.4 to 90.9) |
*Indeterminate visual impression is classified as negative for malignancy.
PI, classification by principal investigators (SL, FP).
NPV, negative predictive value; PPV, positive predictive value; SE, sensitivity; SP, specificity
flgastro-2018-100985supp001.docx (19.3KB, docx)
Finally, we tried to determine whether the actually observed management was in conformity with what SOC results suggested (table 5).
Table 5.
Conformity between patient management anticipated after single-operator cholangioscopy (SOC) and actually observed management
| Actual management consistent with SOC findings |
Overall | Indeterminate biliary strictures | Primary sclerosing cholangitis | |||
| N | % | N | % | N | % | |
| Yes | 54 | 88.5 | 43 | 89.6 | 11 | 84.6 |
| No | 7 | 11.5 | 5 | 10.4 | 2 | 15.4 |
| Total | 61 | 100 | 48 | 100 | 13 | 100 |
In seven patients (11.4%) overall the actual treatment was not found in accordance with SOC findings. These inconsistencies resulted from an error in cholangioscopic interpretation or false negative biopsy, an unexpected change in the patient’s clinical course or a decision from the patient’s or the doctor’s part independently from objective findings. Details of each case are presented as online supplementary material.
Discussion
In this study, we show that not only SOC improves the diagnosis of biliopancreatic lesions compared with ERCP with brush cytology—a previously accepted notion, especially for PSC and IDBS,9–12 but we also show for the first time that SOC can favourably change disease management in a large proportion of patients. The diagnostic workup of biliary disease is straightforward when a mass, generally a malignant one, is amenable to EUS-FNA, or when history and imaging are typical of a specific aetiology, often a ‘benign’ one. Much more difficult is diagnosis when such a presentation is lacking. In the case of PSC, the identification of a malignant stricture among many others, inflammatory ones, is particularly challenging.
More than 10 years ago, peroral cholangioscopy had been shown to improve diagnostic performance in patients with IDBS or PSC with a dominant stricture.9 13 In the landmark study by Fukuda et al, sensitivity for malignancy compared with ERCP + brush cytology increased from 58% to 100% and accuracy from 78% to 93% in a series including 38 cholangiocarcinomas and 38 benign lesions.13 These early works were achieved with ‘mother and baby’ systems using a reprocessable cholangioscope, a technique which did not reach wide acceptance for at least four reasons, namely the high cost of the investment in a cholangioscope for a limited number of uses, the brittleness of the device with high repair costs, the need for two operators and the non-sterile device to be introduced in a supposedly sterile cavity. The advent of SOC in 2007 has made cholangioscopy a much easier, although still costly procedure, with a single-use sterile device, operated by a single endoscopist and requiring a more affordable investment in a relatively low-tech endoscopy platform. Since the initial report by Chen and Pleskow showing the feasibility of the Spyglass procedure in 35 patients,14 a large number of studies, both retrospective and prospective, but none randomised, have been published. In 2015, two systematic reviews and meta-analyses were published, with slightly different methodologies and criteria for study inclusion: the review by Navaneethan et al including 10 retrospective and prospective studies (456 patients) aimed to determine the value of cholangioscopy-guided biopsies, whereas that by Xi Sun including 8 studies (335 patients), aimed at differentiating the value of visual impression from that of guided-biopsies.15 16 In the first meta-analysis, a pooled sensitivity of 60.1% (95% CI 54.9% to 65.2%) was found for the diagnosis of malignant strictures against 69% (95% CI 57% to 79%) in the second one, whereas specificity of Spybite biopsy was 98% in both studies. However, Xi Sun et al calculated that visual impression had a higher sensitivity for malignancy at 90% (95% CI 73% to 97%), with specificity at 87%, but low positive and negative likelihood ratios at 7.1 (95% CI 3.8 to 13.3) and 0.12 (95% CI 0.04 to 0.33), respectively.15 16 It is noteworthy that only four out of the studies on which both meta-analyses were based included patients with inconclusive ERCP and brushing,17–20 meaning that many of the strictures explored in the other studies were ‘indeterminate’ insofar as CT and MRI were inconclusive, but possibly EUS-FNA and/or ERCP and brushings might have provided the information needed. Naveenathan et al calculated a sensitivity of SOC in those four studies of 74.7% (95% CI 63.3% to 84.0%), a specificity of 93.3% (95% CI 85.1% to 97.8%) and a pooled diagnostic odds ratio (DOR) of 46.0 (95% CI 15.4 to 138.1).15 Perhaps even more interestingly, Siddiqui et al, in a retrospective analysis of 30 patients with eventually proven cholangiocarcinoma but negative ERCP and EUS-FNA findings, found that SOC had an accuracy of 77% for the diagnosis of malignancy.19 In our study, prospectively including patients from nine referral centres, enrolment was limited to patients with strictly defined criteria, in whom no mass was easily amenable to EUS-FNA, no history suggested a definite diagnosis and at least one ERCP + brushing and/or fluoroscopy-guided biopsies were inconclusive. As in the above-mentioned reviews, we found sensitivity for malignancy to be moderate, with marginal differences between investigators and independent experts classifications of true and false cases. However, as also previously observed, visual impression provided slightly better sensitivity figures, both overall and in the IDBS group, but it is widely accepted that tumour boards do not decide oncological therapy on such subjective information. On the contrary, a visual impression of normality or mild inflammation tends to suggest upholding conservative management. Moreover, a visual impression of malignancy should not be overstated because, as shown by Sethi et al,21 interobserver agreement on cholangioscopic features is at best slight in the absence of standardised, consensus-based terminology. We did not consider spyglass biopsies to be mandatory when visual impression (VI) was obviously benign since biopsies have a low negative predictive value and likelihood ratio. However, because VI remains subjective and there is no currently available consensual classification of SOC visual features, SOC-guided biopsies of any abnormal finding should therefore remain the rule. The sensitivity figures in patients with IDBS, relatively lower than in some previous studies, can be explained by the lower prevalence of malignant lesions, with two out of three patients eventually found to have a benign condition. This lower prevalence is also the consequence of strict criteria for enrolment, with all patients having a mass undergoing EUS-FNA and excluded from the study and some of the study patients having undergone more than one inconclusive ERCP. The study by Nguyen et al in 2013 showing that EUS-FNA can avoid the need for SOC in 60% of cases with difficult bile duct strictures strongly supports this approach.22 Another possible contributor to decreased SOC diagnostic performance results from the methodology of the study, in which nearly all patients had a stent in situ at the time of enrolment, with the associated and potentially confounding inflammatory changes. However, this is also the ‘real-life’ situation, in which patients are often referred for SOC after one or several ERCPs in which prophylactic stenting is recommended.
Despite the moderate sensitivity of SOC for malignancy, our results, as analysed by investigators and independent experts, show that SOC has a major influence on patient management, an observation confirmed both overall and in both study subgroups. The benign findings in a majority of the patients have led to downgrade the initial diagnosis and preclude surgery in more than half of patients overall. Importantly, follow-up showed that false negative SOCs did not impair outcomes because further findings (including repeat SOC and brushings) redressed diagnosis before disease progression (see additional material). However, the potential to delay a diagnosis of cancer is clearly a limitation to keep in mind when discussing post-SOC indeterminate cases.
As important as changes in management suggested by SOC results in a given patient can be, they are irrelevant if these suggestions are not followed by consistent treatment adaptation, for example, if the MDT proposal sticks to an initially planned resection although SOC suggests a benign lesion with no need for surgery. The 11% rate of inconsistencies between patient management anticipated after SOC and the actually observed management can be deemed acceptable for a relatively new technique whose results may not be regarded as beyond any question.
We acknowledge that our study failed to reach the targets assigned to the PSC group, thus weakening the findings and precluding accurate measurement of diagnostic performance criteria for this subgroup. However, with regard to our main study endpoint, this underpowered group did not prevent finding a significant difference in the adequacy of suggested management to the definitive diagnosis between pre- and post-SOC assessment. In the PSC group, although SOC remained inconclusive in nearly one-third of patients, the proportion of inadequate managements was reduced by 63%, from 84.6% to 30.8%, and planned management was modified in more than two out of three patients. In the IDBS group, the inadequacy rate fell by 73%, and in the whole casemix by 71%.
Starting from these findings, two important issues should be raised: first, could the technique of SOC be improved in order to further reduce the number of inadequacies and more confidently guide professionals in their decision-making? Second, could the diagnostic strategy of biliopancreatic conditions and particularly IDBS, and the place of SOC in that strategy be modified to further improve outcomes? To address the first issue, the recently launched digital SOC system (Spyglass-DS) is certainly one significant step forward, with encouraging results from recent retrospective and prospective series.23 24 Most recent cases of SOC performed in some of our centres with this new device were not included in our study, which may not fully reflect the current possibilities of this evolving technology, but it was methodologically necessary to base our assessment of SOC on the same technology for all the patients included. Even if this new generation brings more comfort of use and image reliability than fundamentally new technology, the only fact of feeling comfortable with taking a—still to be defined—sufficiently large number of biopsies and not be bothered by poor image quality and broken optic fibre is yet a significant progress. Better biopsies and easier targeting are certainly key factors in improving SOC diagnostic yield. The quality of Spybite biopsies has been compared with biopsies obtained with paediatric forceps with controversial results.25 26 However, although cholangioscopic biopsies are necessarily small, one challenge will be to achieve deeper biopsy sampling in order to collect tumour cells buried within the thick fibrous stroma of most cholangiocarcinomas.27 The second issue requires a better understanding of the optimal timing for SOC. In particular, it must be determined whether SOC must be contemplated after a first inconclusive ERCP with brushing, as is most commonly done currently, or should we rather perform SOC at the same time as a first ERCP, when other imaging modalities, especially EUS-FNA, remained inconclusive. The latter option would save a potentially precious time in the case of an invasive carcinoma and possibly allow for more clearcut distinction of malignant and inflammatory changes in bile ducts unaltered by weeks of stenting, but could also induce additional undue costs and morbidity. A valid answer to this question will require a randomised trial.
In conclusion, this study shows that single-operator and single-use peroral cholangiopancreatoscopy unveils a new era in the exploration of the most difficult biliopancreatic conditions, particularly IDBS, with a dramatic and positive impact on disease management. However, much remains to be done to improve the diagnostic performance of direct visualisation of intraductal diseases, with the implementation of a standardised semiology and specific terminology to describe anomalies, as well as a clarification of the optimal timing of SOC in the diagnostic workup.
Significance of this study.
What is already known on this topic
ERCP guided cytology/biopsy has low diagnostic yield.
Biliary strictures without a mass and primary sclerosing cholangitis-dominant strictures are difficult diagnoses.
Cholangioscopy can visualise lesions and target biopsies.
Actual impact of cholangioscopy on patient outcome is unknown.
What this study adds
Cholangioscopy induces changes in management in the majority of patients and avoids unnecessary surgeries.
Although sensitivity for malignancy is moderate, cholangioscopy provides information necessary to optimise management in most patients with indeterminate biliary strictures.
How might it impact on clinical practice in the foreseeable future
Cholangioscopy might be implemented in the workup of selected patients with challenging diagnosis, when a significant impact on outcome (essentially resection versus conservative management) is to be expected.
Acknowledgments
The authors wish to acknowledge the Clinical Research Unit (URC-Cochin) and its staff for their contribution throughout the study, as well as Prof Benoit Terris (BT), head of the Dept of pathology at Cochin hospital, Paris, for his expertise in most difficult cases.
Footnotes
All tertiary referral endoscopy centres performed >400 ERCPs per year.
Inconclusive cytology=acellular, inflammatory cells, atypia. The presence of high-grade dysplasia was considered suggestive of neoplasia, and thus conclusive.
Visual impression was deemed sufficient when suggesting a benign stenosis, that is, with no colour or surface anomaly; presence of malignant cells or high-grade dysplasia was required, whatever the visual impression, to diagnose malignancy; a visual impression of malignancy with no malignant or dysplastic cells, as well as cellular atypia with benign or malignant visual impression, were considered indeterminate.
Contributors: FP designed and planned the study, contributed patients and data analysis, wrote and submitted the study. SL contributed patients and analysed data. FF was the methodologist and statistician for the study. TP, RéL, PB, FééM, DC, AC, BV and IB contributed patients. SC formalised the idea of a multi-centre prospective study of SOC. NK monitored patient inclusion and study quality. AL and SG played the role of external independent reviewers with regard to patient outcome classification.
Funding: The study was funded through a grant from the National Cancer Institute (INCA).
Competing interests: FP, RL and TP have received consultancy fees from Boston Scientific before, during or after the study.
Patient consent: Obtained.
Ethics approval: IRB approval from CPP Ile de France-III under N°Am5042-2-S.C.2564 and registered under EUDRACT N°2008-A00672-53.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Tummala P, Munigala S, Eloubeidi MA, et al. Patients with obstructive jaundice and biliary stricture ± mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. J Clin Gastroenterol 2013;47:532–7. 10.1097/MCG.0b013e3182745d9f [DOI] [PubMed] [Google Scholar]
- 2. Jailwala J, Fogel EL, Sherman S, et al. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc 2000;51:383–90. 10.1016/S0016-5107(00)70435-4 [DOI] [PubMed] [Google Scholar]
- 3. Schoefl R, Haefner M, Wrba F, et al. Forceps biopsy and brush cytology during endoscopic retrograde cholangiopancreatography for the diagnosis of biliary stenoses. Scand J Gastroenterol 1997;32:363–8. [DOI] [PubMed] [Google Scholar]
- 4. Clayton RA, Clarke DL, Currie EJ, et al. Incidence of benign pathology in patients undergoing hepatic resection for suspected malignancy. Surgeon 2003;1:32–8. 10.1016/S1479-666X(03)80006-9 [DOI] [PubMed] [Google Scholar]
- 5. Gerhards MF, Vos P, van Gulik TM, et al. Incidence of benign lesions in patients resected for suspicious hilar obstruction. Br J Surg 2001;88:48–51. 10.1046/j.1365-2168.2001.01607.x [DOI] [PubMed] [Google Scholar]
- 6. Bangarulingam SY, Bjornsson E, Enders F, et al. Long-term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology 2010;51:174–80. 10.1002/hep.23277 [DOI] [PubMed] [Google Scholar]
- 7. Falissard B. psy: Various procedures used in psychometry. R package version 1.1. 2012. http://CRAN.R-project.org/package=psy
- 8. Fleiss JL, Cohen J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educ Psychol Meas 1973;33:613–9. 10.1177/001316447303300309 [DOI] [Google Scholar]
- 9. Tischendorf JJ, Krüger M, Trautwein C, et al. Cholangioscopic characterization of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Endoscopy 2006;38:665–9. 10.1055/s-2006-925257 [DOI] [PubMed] [Google Scholar]
- 10. Kim HJ, Kim MH, Lee SK, et al. Tumor vessel: a valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc 2000;52:635–8. 10.1067/mge.2000.108969 [DOI] [PubMed] [Google Scholar]
- 11. Seo DW, Lee SK, Yoo KS, et al. Cholangioscopic findings in bile duct tumors. Gastrointest Endosc 2000;52:630–4. 10.1067/mge.2000.108667 [DOI] [PubMed] [Google Scholar]
- 12. Itoi T, Osanai M, Igarashi Y, et al. Diagnostic peroral video cholangioscopy is an accurate diagnostic tool for patients with bile duct lesions. Clin Gastroenterol Hepatol 2010;8:934–8. 10.1016/j.cgh.2010.06.029 [DOI] [PubMed] [Google Scholar]
- 13. Fukuda Y, Tsuyuguchi T, Sakai Y, et al. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest Endosc 2005;62:374–82. 10.1016/j.gie.2005.04.032 [DOI] [PubMed] [Google Scholar]
- 14. Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video). Gastrointest Endosc 2007;65:832–41. 10.1016/j.gie.2007.01.025 [DOI] [PubMed] [Google Scholar]
- 15. Navaneethan U, Hasan MK, Lourdusamy V, et al. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc 2015;82:608–14. 10.1016/j.gie.2015.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun X, Zhou Z, Tian J, et al. Is single-operator peroral cholangioscopy a useful tool for the diagnosis of indeterminate biliary lesion? A systematic review and meta-analysis. Gastrointest Endosc 2015;82:79–87. 10.1016/j.gie.2014.12.021 [DOI] [PubMed] [Google Scholar]
- 17. Ramchandani M, Reddy DN, Gupta R, et al. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: a single-center, prospective study. Gastrointest Endosc 2011;74:511–9. 10.1016/j.gie.2011.04.034 [DOI] [PubMed] [Google Scholar]
- 18. Manta R, Frazzoni M, Conigliaro R, et al. SpyGlass single-operator peroral cholangioscopy in the evaluation of indeterminate biliary lesions: a single-center, prospective, cohort study. Surg Endosc 2013;27:1569–72. 10.1007/s00464-012-2628-2 [DOI] [PubMed] [Google Scholar]
- 19. Siddiqui AA, Mehendiratta V, Jackson W, et al. Identification of cholangiocarcinoma by using the Spyglass Spyscope system for peroral cholangioscopy and biopsy collection. Clin Gastroenterol Hepatol 2012;10:466–71. 10.1016/j.cgh.2011.12.021 [DOI] [PubMed] [Google Scholar]
- 20. Nishikawa T, Tsuyuguchi T, Sakai Y, et al. Comparison of the diagnostic accuracy of peroral video-cholangioscopic visual findings and cholangioscopy-guided forceps biopsy findings for indeterminate biliary lesions: a prospective study. Gastrointest Endosc 2013;77:219–26. 10.1016/j.gie.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 21. Sethi A, Widmer J, Shah NL, et al. Interobserver agreement for evaluation of imaging with single operator choledochoscopy: what are we looking at? Dig Liver Dis 2014;46:518–22. 10.1016/j.dld.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 22. Nguyen NQ, Schoeman MN, Ruszkiewicz A. Clinical utility of EUS before cholangioscopy in the evaluation of difficult biliary strictures. Gastrointest Endosc 2013;78:868–74. 10.1016/j.gie.2013.05.020 [DOI] [PubMed] [Google Scholar]
- 23. Brewer Gutierrez OI, Bekkali NLH, Raijman I, et al. Efficacy and safety of digital single-operator cholangioscopy for difficult biliary stones. Clin Gastroenterol Hepatol 2018;16:918–26. 10.1016/j.cgh.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 24. Lenze F, Bokemeyer A, Gross D, et al. Safety, diagnostic accuracy and therapeutic efficacy of digital single-operator cholangioscopy. United European Gastroenterol J 2018;6:902–9. 10.1177/2050640618764943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Draganov PV, Chauhan S, Wagh MS, et al. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointest Endosc 2012;75:347–53. 10.1016/j.gie.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 26. Walter D, Peveling-Oberhag J, Schulze F, et al. Intraductal biopsies in indeterminate biliary stricture: Evaluation of histopathological criteria in fluoroscopy- vs. cholangioscopy guided technique. Dig Liver Dis 2016;48:765–70. 10.1016/j.dld.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 27. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013;145:1215–29. 10.1053/j.gastro.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2018-100985supp001.docx (19.3KB, docx)