Key Points
Question
What is the effect of linagliptin compared with placebo on risk of major cardiovascular (CV) events in type 2 diabetes at high CV risk?
Findings
In this randomized noninferiority trial that included 6979 patients followed up for a median 2.2 years, use of linagliptin compared with usual care resulted in an incidence of the primary composite outcome (CV death, nonfatal myocardial infarction, or nonfatal stroke) of 12.4% vs 12.1%. The hazard ratio had a 1-sided 97.5% confidence limit of 1.17, which met the criterion for noninferiority (upper confidence limit <1.3).
Meaning
Among patients with type 2 diabetes and high CV risk, linagliptin, compared with placebo, demonstrated noninferiority with regard to risk of major CV events over a median of 2.2 years.
Abstract
Importance
Type 2 diabetes is associated with increased cardiovascular (CV) risk. Prior trials have demonstrated CV safety of 3 dipeptidyl peptidase 4 (DPP-4) inhibitors but have included limited numbers of patients with high CV risk and chronic kidney disease.
Objective
To evaluate the effect of linagliptin, a selective DPP-4 inhibitor, on CV outcomes and kidney outcomes in patients with type 2 diabetes at high risk of CV and kidney events.
Design, Setting, and Participants
Randomized, placebo-controlled, multicenter noninferiority trial conducted from August 2013 to August 2016 at 605 clinic sites in 27 countries among adults with type 2 diabetes, hemoglobin A1c of 6.5% to 10.0%, high CV risk (history of vascular disease and urine-albumin creatinine ratio [UACR] >200 mg/g), and high renal risk (reduced eGFR and micro- or macroalbuminuria). Participants with end-stage renal disease (ESRD) were excluded. Final follow-up occurred on January 18, 2018.
Interventions
Patients were randomized to receive linagliptin, 5 mg once daily (n = 3494), or placebo once daily (n = 3485) added to usual care. Other glucose-lowering medications or insulin could be added based on clinical need and local clinical guidelines.
Main Outcomes and Measures
Primary outcome was time to first occurrence of the composite of CV death, nonfatal myocardial infarction, or nonfatal stroke. Criteria for noninferiority of linagliptin vs placebo was defined by the upper limit of the 2-sided 95% CI for the hazard ratio (HR) of linagliptin relative to placebo being less than 1.3. Secondary outcome was time to first occurrence of adjudicated death due to renal failure, ESRD, or sustained 40% or higher decrease in eGFR from baseline.
Results
Of 6991 enrollees, 6979 (mean age, 65.9 years; eGFR, 54.6 mL/min/1.73 m2; 80.1% with UACR >30 mg/g) received at least 1 dose of study medication and 98.7% completed the study. During a median follow-up of 2.2 years, the primary outcome occurred in 434 of 3494 (12.4%) and 420 of 3485 (12.1%) in the linagliptin and placebo groups, respectively, (absolute incidence rate difference, 0.13 [95% CI, −0.63 to 0.90] per 100 person-years) (HR, 1.02; 95% CI, 0.89-1.17; P < .001 for noninferiority). The kidney outcome occurred in 327 of 3494 (9.4%) and 306 of 3485 (8.8%), respectively (absolute incidence rate difference, 0.22 [95% CI, −0.52 to 0.97] per 100 person-years) (HR, 1.04; 95% CI, 0.89-1.22; P = .62). Adverse events occurred in 2697 (77.2%) and 2723 (78.1%) patients in the linagliptin and placebo groups; 1036 (29.7%) and 1024 (29.4%) had 1 or more episodes of hypoglycemia; and there were 9 (0.3%) vs 5 (0.1%) events of adjudication-confirmed acute pancreatitis.
Conclusions and Relevance
Among adults with type 2 diabetes and high CV and renal risk, linagliptin added to usual care compared with placebo added to usual care resulted in a noninferior risk of a composite CV outcome over a median 2.2 years.
Trial Registration
ClinicalTrials.gov Identifier: NCT01897532
This randomized clinical trial compares the effects of the dipeptidyl peptidase 4 inhibitor linagliptin vs placebo plus usual care on cardiovascular (CV) and renal outcomes in adults with type 2 diabetes and high CV and renal risk.
Introduction
Type 2 diabetes is a major risk factor for cardiovascular (CV) and kidney disease.1,2 Because of a number of CV safety concerns associated with some glucose-lowering agents,3,4,5 since 2008 evaluation of the CV safety of new glucose-lowering medications, by conducting large noninferiority studies compared with placebo, has been a requirement of both US and European regulators.6,7 Three CV outcome trials of dipeptidyl peptidase 4 (DPP-4) inhibitors have previously demonstrated a noninferior risk of a composite CV outcome vs placebo but not incremental CV efficacy.8,9,10 One concern has been the within-class heterogeneity observed for risk of hospitalization for heart failure, ranging from no effect of sitagliptin10 to increased risk with saxagliptin.8 Previous CV outcome trials evaluating glucose-lowering medications for type 2 diabetes enrolled limited numbers of patients with concomitant chronic kidney disease, a group of patients with very high CV risk.2
Linagliptin is a selective, once-daily, DPP-4 inhibitor approved for glycemic management of type 2 diabetes.11,12,13 Preliminary early phase 3 clinical data demonstrated that linagliptin had glucose-lowering efficacy and hypothesized potential CV12 and kidney benefits.13 The Cardiovascular and Renal Microvascular Outcome Study With Linagliptin (CARMELINA) was designed to evaluate the CV safety and kidney outcomes of linagliptin in patients with type 2 diabetes at high cardiorenal risk.
Methods
The study protocol was approved by the institutional review board or independent ethics committee from each site and all patients provided written informed consent; the trial protocol and statistical analysis plan are available in the online supplement (Supplement 2 and Supplement 3).
The trial was conducted in accordance with the principles of the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization and was approved by local authorities.
Study Design
The study design has previously been described.14 In brief, this was a randomized, double-blind, placebo-controlled clinical trial conducted in 605 centers across 27 countries that aimed to continue until at least 611 participants had an adjudication-confirmed primary outcome event.
Study Participants
Adults with type 2 diabetes, hemoglobin A1c (HbA1c) values of 6.5% to 10.0% inclusive, and high CV and renal risk were eligible for inclusion. High CV risk was defined as a history of coronary artery disease, stroke or peripheral vascular disease, and microalbuminuria or macroalbuminuria, defined as urinary albumin:creatinine ratio (UACR) higher than 30 mg/g or equivalent; high renal risk was defined as (1) estimated glomerular filtration rate (eGFR) of 45 to 75 mL/min/1.73 m2 and UACR higher than 200 mg/g or equivalent or (2) eGFR of 15 to 45 mL/min/1.73 m2 regardless of UACR. Participants with end-stage renal disease, defined as an eGFR less than 15 mL/min/1.73 m2 or requiring maintenance dialysis, were excluded. Full eligibility criteria are provided in Supplement 1.
Information on race and ethnicity was based on self-classification by study participants following written informed consent as reported in the electronic case record form (fixed categories) by investigators to allow for outcome subgroup analysis. This information was included partly because of regulatory requests to assess drug effects across different races and ethnicities.
Study Procedures
Eligible individuals were randomized 1:1 using an interactive telephone/web–based system in a block size of 8 to receive once-daily double-blind oral linagliptin, 5 mg, or matching placebo. Treatment assignment was determined by computer-generated random sequence with stratification by geographical region (North America, Latin America, Europe [plus South Africa], and Asia). Following randomization, participants returned for study visits after 12 weeks and then every 24 weeks until study end. A final follow-up visit was scheduled 30 days after the end of treatment. In an attempt to maintain glycemic equipoise, investigators were encouraged to monitor and use additional medication for glycemic control (except DPP-4 inhibitors, glucagon-like peptide 1 receptor agonists, and sodium-glucose cotransporter 2 inhibitors) according to applicable standard of care throughout the trial, independent of study treatment assignment that remained masked. Treatment of other CV risk factors was encouraged in accordance with applicable guidelines and current standards of care. Patients who prematurely discontinued study medication were followed up for ascertainment of CV and secondary kidney outcome events, and attempts were made to collect vital status information on every randomized patient at study completion, in compliance with local law and regulations.
Outcome
The primary outcome was defined as the time to first occurrence of CV death, nonfatal myocardial infarction, or nonfatal stroke (3-point major adverse CV event [MACE]). The original protocol included hospitalization for unstable angina pectoris in the primary outcome (a 4-point MACE). However, this was changed by the steering committee in a protocol amendment in 2016 based on emerging evidence that a primary outcome definition based on 3-point MACE was preferred by regulators and consistent with other CV outcome trials.15,16
The secondary outcome was defined as time to first occurrence of a composite of adjudication-confirmed ESRD, death due to renal failure, or a sustained decrease of at least 40% in eGFR from baseline. The eGFR criterion was changed from the original decrease of at least 50% in eGFR in accord with recommendations emerging from a workshop convened by the National Kidney Foundation and the US Food and Drug Administration (FDA).17,18 Use of the originally planned decrease of at least 50% in eGFR in the kidney composite was evaluated as a tertiary outcome.
Multiple tertiary or exploratory outcomes also were assessed. Among these were time to hospitalization for heart failure, all-cause death, the composite of renal death or ESRD, and a microvascular composite outcome that included albuminuria, sustained ESRD, sustained decrease of at least 50% in eGFR, death due to renal failure, and major ocular events. Additional tertiary outcomes were progression in albuminuria category and change from baseline in HbA1c. Included in Supplement 1 are definitions of all clinical outcomes assessed as well as a complete list of all predefined end points detailed in the statistical analyses plans.
Adverse events were assessed based on reported events, coded using the Medical Dictionary for Drug Regulatory Activities, version 20.1. Adverse events prespecified as being of special interest were hypersensitivity reactions, skin lesions, kidney adverse events, pancreatitis, pancreatic cancer, benign thyroid neoplasms, thyroid cancer, hepatic events, and hypoglycemia.
Cardiovascular outcome events (including hospitalization for heart failure), deaths, secondary kidney outcomes, and pancreatitis were prospectively captured and centrally adjudicated by clinical events committees masked to treatment assignment. Solid cancer cases were assessed for potential relatedness to therapy by independent oncology experts. An independent, unmasked data monitoring committee regularly reviewed trial data throughout the study.
Statistical Analysis
The primary aim was to establish noninferiority of linagliptin compared with placebo for time to 3-point MACE, defined by the upper limit of the 2-sided 95% confidence interval for the hazard ratio (HR) of linagliptin relative to placebo being less than 1.3.6 A sequentially rejective multiple test procedure was applied, first testing the primary hypothesis of noninferiority for linagliptin, and, only if this first test was significant, followed by 2 parallel confirmatory superiority tests of (1) 3-point MACE at a 1-sided α level of 0.5% (ie, 0.2 × 2.5%) and (b) the secondary kidney outcome at a 1-sided α level of 2.0% (ie, 0.8 × 2.5%).14 If either had demonstrated superiority, then the other would be tested at a 1-sided α level of 2.5%.
A total of 611 individuals with an adjudication-confirmed 3-point MACE would provide 90% power to demonstrate noninferiority of linagliptin vs placebo at the overall 1-sided α level of 2.5%, assuming an HR of 1.0, and result in a power of 79% to test for superiority at a 1-sided α level of 2.5%, assuming an HR of 0.80. A total of 432 individuals with an adjudication-confirmed secondary kidney composite outcome would provide 85% power to demonstrate superiority of linagliptin vs placebo at a 1-sided α level of 2.5%, assuming an HR of 0.75.
Outcomes were analyzed in all randomized patients treated with at least 1 dose of study drug (treated set) using the intention-to-treat principle. Patients were analyzed according to their randomized treatment group. Additional sensitivity analyses are described in Supplement 1 and included different censoring approaches depending on treatment exposure; eg, excluding patients with important protocol violations or patients with a minimum treatment duration. Subgroup analyses included treatment × subgroup interaction terms and testing. Handling of missing data is described in the statistical analysis plan (Supplement 3). For time-to-event analyses, censoring was applied the day a patient was last known to be free of the specific outcome event. The change from baseline over time was evaluated with a restricted maximum likelihood–based mixed-model repeated-measures approach.
Time-to-event outcomes were analyzed using Cox proportional hazards regression models, with randomized treatment group and geographical region (North America, Latin America, Europe [plus South Africa], and Asia) as factors. Proportional hazards assumptions were explored for the primary and secondary outcomes by plotting log(−log [survival function]) against the log of time by treatment group and checked for parallelism. The interaction of treatment and geographic region with log of time was included in the model described above for an exploratory analysis. Furthermore, Schoenfeld residuals were plotted against time and log(time). For all Cox proportional hazards analyses, the proportional hazard assumption was met.
Adverse event assessments were conducted using descriptive statistics.
There was no adjustment for multiple comparisons; therefore, the results of the subgroup analyses and the analyses for tertiary and exploratory end points should be interpreted as exploratory.
Analyses were conducted with SAS software, version 9.4 (SAS Institute Inc).
Results
Study Participants
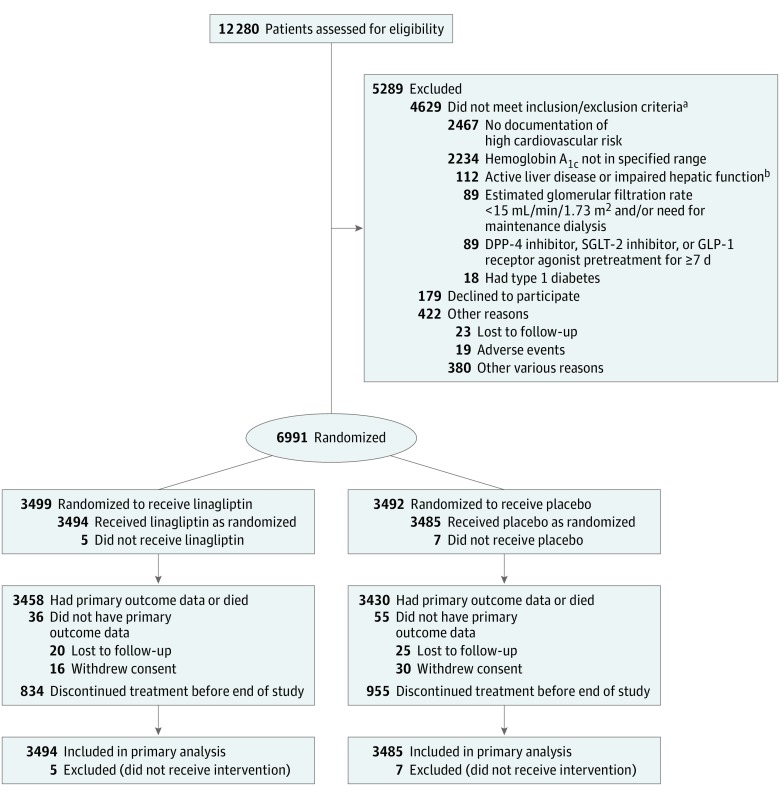
Between August 2013 and August 2016, 6991 patients were randomized in 605 centers, of whom 6979 received at least 1 dose of study drug and were included in the primary analysis (Figure 1). Overall, 98.7% of participants completed the study, with 25.6% of patients prematurely discontinuing study drug, producing 6766 patient-year exposures in the linagliptin group vs 6586 patient-year exposures in the placebo group. Vital status was available for 99.7% of patients at study completion, data for the primary MACE outcome were available for 98.7%, and data for the key secondary kidney outcome were available for 88.0% (Figure 1).
Figure 1. Flow of Participants in the CARMELINA Trial of Linagliptin.
DPP-4 indicates dipeptidyl peptidase 4; SGLT-2, sodium-glucose cotransporter 2; GLP-1, glucagon-like peptide 1.
aPatients could have more than 1 reason, so numbers sum to more than 4692.
bDefined as serum levels of alanine aminotransferase, aspartate aminotransferase, or alkaline phosphatase at least 3 times the upper limit of normal.
Baseline clinical characteristics were balanced between groups (Table 1) and patients’ CV and kidney disease risk factors were well managed overall: 57% had established CV disease, 74% had prevalent kidney disease (defined as eGFR <60 mL/min/1.73 m2 and/or UACR >300 mg/g creatinine), 33% had both CV and kidney disease, and 15.2% had an eGFR less than 30 mL/min/1.73 m2. Median treatment duration was 1.9 years in both the linagliptin group and the placebo group, and the median observation time was 2.2 years in both groups.
Table 1. Baseline Participant Characteristicsa.
| Characteristics | Linagliptin (n = 3494) | Placebo (n = 3485) |
|---|---|---|
| Age, y | 66.1 (9.1) | 65.6 (9.1) |
| Sex, No. (%) | ||
| Male | 2148 (61.5) | 2242 (64.3) |
| Female | 1346 (38.5) | 1243 (35.7) |
| Race, No. (%) | ||
| White | 2827 (80.9) | 2769 (79.5) |
| Asian | 307 (8.8) | 333 (9.6) |
| Black/African American | 194 (5.6) | 217 (6.2) |
| Otherb | 166 (4.8) | 166 (4.8) |
| Region, No. (%) | ||
| Europe (plus South Africa) | 1473 (42.2) | 1461 (41.9) |
| Latin America | 1156 (33.1) | 1154 (33.1) |
| North America | 593 (17.0) | 587 (16.8) |
| Asia | 272 (7.8) | 283 (8.1) |
| Smoking status, No. (%) | ||
| Never smoker | 1897 (54.3) | 1856 (53.3) |
| Ex-smoker | 1231 (35.2) | 1276 (36.6) |
| Current smoker | 362 (10.4) | 350 (10.0) |
| Missing data | 4 (0.1) | 3 (0.1) |
| History of heart failure, No. (%) | 952 (27.2) | 921 (26.4) |
| Ischemic heart disease, No. (%) | 2029 (58.1) | 2052 (58.9) |
| History of hypertension, No. (%) | 3171 (90.8) | 3178 (91.2) |
| Atrial fibrillation, No. (%) | 319 (9.1) | 354 (10.2) |
| eGFR (MDRD), mL/min/1.73 m2 | 54.7 (25.1) | 54.5 (24.9) |
| No. (%) | ||
| ≥90 | 363 (10.4) | 365 (10.5) |
| ≥60 | 1294 (37.0) | 1337 (38.4) |
| ≥45 to <60 | 690 (19.7) | 658 (18.9) |
| ≥30 to <45 | 994 (28.4) | 944 (27.1) |
| <30 | 516 (14.8) | 546 (15.7) |
| UACR, median (IQR), mg/g | 162 (43-700) | 162 (44-750) |
| No. (%)c | ||
| <30 | 696 (20.0) | 696 (20.0) |
| 30-300 | 1463 (41.9) | 1431 (41.1) |
| >300 | 1333 (38.2) | 1357 (38.9) |
| Body mass indexd | 31.4 (5.3) | 31.3 (5.4) |
| Hemoglobin A1c, % | 7.9 (1.0) | 8.0 (1.0) |
| Fasting plasma glucose, mg/dL | 151.2 (45.0) | 151.2 (45.0) |
| Diabetes duration, y | 15.0 (9.6) | 14.5 (9.3) |
| Systolic blood pressure, mm Hg | 140.4 (17.7) | 140.6 (18.0) |
| Diastolic blood pressure, mm Hg | 77.8 (10.5) | 77.9 (10.4) |
| Heart rate, /min | 69.8 (12.2) | 69.8 (12.3) |
| Total cholesterol, mg/dL | 173 (49) | 171 (47) |
| Low-density lipoprotein cholesterol, mg/dL | 92 (40) | 91 (39) |
| High-density lipoprotein cholesterol, mg/dL | 45 (13) | 44 (13) |
| Triglycerides, mg/dL | 190 (136) | 187 (130) |
| ≥1 Glucose-lowering medication, No. (%) | 3378 (96.7) | 3376 (96.9) |
| Metformin | 1881 (53.8) | 1927 (55.3) |
| Sulfonylurea | 1102 (31.5) | 1140 (32.7) |
| Insulin | 2056 (58.8) | 1995 (57.2) |
| No. of background glucose-lowering therapies, No. (%) | ||
| 1 | 1756 (50.3) | 1769 (50.8) |
| 2 | 1424 (40.8) | 1420 (40.7) |
| 3 | 192 (5.5) | 180 (5.2) |
| ≥4 | 6 (0.2) | 7 (0.2) |
| ≥1 Antihypertensive medication, No. (%) | 3337 (95.5) | 3354 (96.2) |
| ACE inhibitors or ARBs | 2860 (81.9) | 2798 (80.3) |
| β-Blockers | 2080 (59.5) | 2073 (59.5) |
| Diuretics | 1892 (54.1) | 1936 (55.6) |
| Calcium antagonists | 1433 (41.0) | 1446 (41.5) |
| Aspirin, No. (%) | 2166 (62.0) | 2178 (62.5) |
| Statins, No. (%) | 2495 (71.4) | 2523 (72.4) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; eGFR estimated glomerular filtration rate; IQR, interquartile range; MDRD, Modification of Diet in Renal Disease study equation; UACR, urinary albumin:creatinine ratio.
SI conversions: To convert glucose to mmol/L, multiply by 0.0555; to convert total, low-density lipoprotein, and high-density lipoprotein cholesterol to mmol/L, multiply by 0.0259; to convert triglycerides to mmol/L, multiply by 0.0113.
Data are expressed as mean (SD) unless otherwise specified.
American Indian/Alaska Native or Native Hawaiian/other Pacific Islander.
Data were missing for 3 patients: 2 (0.1%) in the linagliptin group and 1 (<0.1%) in the placebo group.
Calculated as weight in kilograms divided by height in meters squared.
Primary Outcome
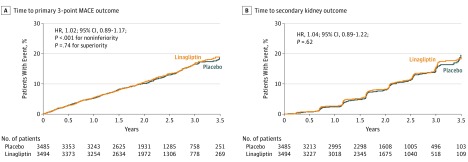
The primary composite 3-point MACE outcome occurred in 434 (12.4%) of 3494 patients randomized to linagliptin (5.77 per 100 person-years) and 420 (12.1%) of 3485 patients randomized to placebo (5.63 per 100 person-years), for an absolute incidence rate difference of 0.13 (95% CI, −0.63 to 0.90) per 100 person-years (HR, 1.02; 95% CI, 0.89-1.17; P<.001 for noninferiority), meeting the criterion for noninferiority (Figure 2A). The subsequent testing for superiority according to the prespecified testing procedure was not statistically significant (P = .74).
Figure 2. Time to Primary and Secondary Outcomes.
Hazard ratio (HR) based on Cox regression analyses in patients treated with at least 1 dose of study drug. A, Time to 3-point major adverse cardiovascular event (MACE) primary outcome (first cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke). Median observation time was 2.1 (interquartile range [IQR], 1.5-2.9) years for linagliptin and 2.1 (IQR, 1.5-2.8) years for placebo. B, Time to secondary kidney outcome (first sustained end-stage renal disease, death due to renal failure, or sustained decrease of ≥40% in estimated glomerular filtration rate from baseline). Median observation time was 1.9 (IQR, 1.2-2.6) years for linagliptin and 1.7 (IQR, 1.2-2.5) years for placebo.
Secondary Outcome
The risk of the secondary kidney composite outcome was not significantly different between the groups randomized to linagliptin (9.4%; 4.89 per 100 person-years) and placebo (8.8%; 4.66 per 100 person-years) (absolute incidence rate difference, 0.22 [95% CI, −0.52 to 0.97] per 100 person-years), and the test for superiority did not achieve statistical significance (HR, 1.04; 95% CI, 0.89-1.22; P = .62) (Figure 2B). Prespecified sensitivity and subgroup analyses demonstrated similar results (Supplement 1), except for some indication of heterogeneity for duration of type 2 diabetes (P = .04 for interaction).
Exploratory CV Analyses
Prespecified sensitivity analyses of the primary outcome yielded consistent results (Supplement 1). Overall, the estimate of effect for the primary outcome was consistent across prespecified subgroups, except for some indication of heterogeneity for subgroups of hemoglobin A1c (P = .04 for interaction) and use of calcium channel blockers (P = .04 for interaction), but no adjustment for multiple comparisons was made. No significant differences between randomized groups were observed for the risk of individual components of the primary MACE outcome, including CV death (Table 2).
Table 2. Primary, Secondary, and Exploratory Outcomes.
| Outcomes | Linagliptin (n = 3494)a | Placebo (n = 3485)a | Incidence Rate Difference, Linagliptin − Placebo (95% CI) | Hazard Ratio (95% CI)b | P Value | ||
|---|---|---|---|---|---|---|---|
| No. (%) | Rate per 100 Patient-Years | No. (%) | Rate per 100 Patient-Years | ||||
| Primary Outcome (3-Point MACE) | |||||||
| Cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke | 434 (12.4) | 5.77 | 420 (12.1) | 5.63 | 0.13 (−0.63 to 0.90) | 1.02 (0.89-1.17) | <.001c; .74d |
| Cardiovascular deathe | 221 (6.3) | 225 (6.5) | |||||
| Nonfatal myocardial infarctione | 154 (4.4) | 132 (3.8) | |||||
| Nonfatal strokee | 59 (1.7) | 63 (1.8) | |||||
| Secondary Kidney Composite Outcome | |||||||
| Sustained ESRD, death due to kidney failure, or sustained decrease of ≥40% in eGFR from baseline | 327 (9.4) | 4.89 | 306 (8.8) | 4.66 | 0.22 (−0.52 to 0.97) | 1.04 (0.89-1.22) | .62 |
| ESRDe | 63 (1.8) | 64 (1.8) | |||||
| Death due to renal failuree | 1 (0.03) | 1 (0.03) | |||||
| Sustained decrease of ≥40% in eGFRe | 263 (7.5) | 241 (6.9) | |||||
| Exploratory Cardiovascular and Death Outcomes | |||||||
| All-cause death | 367 (10.5) | 4.69 | 373 (10.7) | 4.80 | −0.11 (−0.79 to 0.58) | 0.98 (0.84-1.13) | .74 |
| Cardiovascular death | 255 (7.3) | 3.26 | 264 (7.6) | 3.40 | −0.14 (−0.71 to 0.44). | 0.96 (0.81-1.14) | .63 |
| Noncardiovascular death | 112 (3.2) | 1.43 | 109 (3.1) | 1.40 | 0.03 (−0.34 to 0.40) | 1.02 (0.78-1.33) | .89 |
| Fatal myocardial infarction | 11 (0.3) | 0.14 | 14 (0.4) | 0.18 | −0.04 (−0.17 to 0.09) | 0.78 (0.36-1.72) | .54 |
| Nonfatal myocardial infarction | 156 (4.5) | 2.06 | 135 (3.9) | 1.80 | 0.27 (−0.18 to 0.71) | 1.15 (0.91-1.45) | .23 |
| Fatal or nonfatal myocardial infarction | 165 (4.7) | 2.18 | 146 (4.2) | 1.94 | 0.24 (−0.22 to 0.70) | 1.12 (0.90-1.40) | .30 |
| Fatal stroke | 17 (0.5) | 0.22 | 16 (0.5) | 0.21 | 0.01 (−0.13 to 0.16) | 1.05 (0.53-2.09) | .88 |
| Nonfatal stroke | 65 (1.9) | 0.85 | 73 (2.1) | 0.96 | −0.12 (−0.42 to 0.19) | 0.88 (0.63-1.23) | .45 |
| Fatal or nonfatal stroke | 81 (2.3) | 1.06 | 88 (2.5) | 1.16 | −0.11 (−0.44 to 0.23) | 0.91 (0.67-1.23) | .53 |
| 4-point MACE (3-point MACE plus hospitalization for unstable angina) | 463 (13.3) | 6.20 | 459 (13.2) | 6.21 | −0.02 (−0.82 to 0.79) | 1.00 (0.88-1.13) | .96 |
| Hospitalization for unstable angina | 42 (1.2) | 0.55 | 48 (1.4) | 0.63 | −0.09 (−0.33 to 0.16) | 0.87 (0.57-1.31) | .50 |
| Coronary revascularization procedure | 160 (4.6) | 2.12 | 149 (4.3) | 1.99 | 0.13 (−0.33 to 0.59) | 1.07 (0.85-1.33) | .57 |
| Hospitalization for heart failure | 209 (6.0) | 2.77 | 226 (6.5) | 3.04 | −0.27 (−0.82 to 0.28) | 0.90 (0.74-1.08) | .26 |
| Exploratory Kidney and Microvascular Outcomes | |||||||
| Sustained ESRD, death due to kidney failure, or sustained decrease of ≥50% in eGFR from baseline | 230 (6.6) | 3.39 | 227 (6.5) | 3.42 | −0.03 (−0.65 to 0.60) | 0.98 (0.82-1.18) | .87 |
| Death due to renal failure or sustained ESRD | 136 (3.9) | 1.78 | 154 (4.4) | 2.04 | −0.26 (−0.70 to 0.18) | 0.87 (0.69-1.10) | .24 |
| Albuminuria progression | 763 (35.3) | 21.36 | 819 (38.5) | 24.54 | −3.18 (−5.44 to −0.92) | 0.86 (0.78-0.95) | .003 |
| Composite microvascular end pointf | 785 (36.3) | 22.14 | 843 (39.6) | 25.42 | −3.28 (−5.59 to −0.97) | 0.86 (0.78-0.95) | .003 |
| Composite ocular end pointg | 36 (1.0) | 0.47 | 49 (1.4) | 0.65 | −0.18 (−0.41 to 0.01) | 0.73 (0.47-1.12) | .15 |
Abbreviations: eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; MACE, major adverse cardiovascular events.
The mean observation time and cumulative time in study, respectively, were 2.2 years and 7829.1 person-years for linagliptin and 2.2 years and 7781.6 patient years for placebo.
Hazard ratio based on Cox regression analyses in patients treated with at least 1 dose of study drug.
P value for noninferiority.
P value for superiority.
Events for individual components of composite outcomes were counted only when they were the first event in the composite.
Time to first ESRD, death due to renal failure, sustained decrease of at least 50% in eGFR, albuminuria progression, retinal photocoagulation, anti–vascular endothelial growth factor injection therapy for diabetic retinopathy, vitreous hemorrhage, and diabetes-related blindness.
Time to first use of retinal laser coagulation therapy or treatment with intravitreal injection(s) of an anti–vascular endothelial growth factor therapy for diabetic retinopathy or vitreous hemorrhage or diabetes-related blindness.
There was no significant difference between the groups in the incidence of death due to any cause with linagliptin (10.5%; 4.69 per 100 person-years) and placebo (10.7%; 4.80 per 100 person-years), for an absolute incidence rate difference of −0.11 (95% CI, −0.79 to 0.58) (HR, 0.98; 95% CI, 0.84-1.13; P = .74) (Table 2). The 4-point MACE outcome occurred in 463 (13.3%) of 3493 patients in the linagliptin group vs 459 (13.2%) of 3485 patients in the placebo group, for an absolute incidence rate difference of −0.02 (95% CI, −0.82 to 0.79) (HR, 1.00; 95% CI, 0.88-1.13; P = .96) (Table 2 and Supplement 1).
Hospitalization for heart failure occurred in 209 of 3494 patients randomized to linagliptin (6.0%; 2.77 per 100 person-years) and in 226 of 3485 patients randomized to placebo (6.5%; 3.04 per 100 person-years), for an absolute incidence rate difference of −0.27 (95% CI, −0.82 to 0.28), with no significant difference between the 2 treatment groups (HR, 0.90; 95% CI, 0.74-1.08; P = .26) (Table 2 and Supplement 1).
Exploratory Kidney and Microvascular Analyses
The exploratory composite of sustained ESRD, death due to renal failure, or sustained decrease of 50% or more in eGFR showed results similar to the secondary kidney composite outcome (Table 2). An additional exploratory outcome of the components of the composite outcome comprising a composite of sustained ESRD or death due to renal failure was also not statistically different (linagliptin, 3.9%; 1.78 per 100 person-years vs placebo, 4.4%; 2.04 per 100 person-years; absolute incidence rate difference, −0.26; 95% CI, −0.70 to 0.18) (HR, 0.87; 95% CI, 0.69-1.10; P = .24). Progression of albuminuria category (ie, change from normoalbuminuria to microalbuminuria/macroalbuminuria or change from microalbuminuria to macroalbuminuria) occurred less frequently in the linagliptin group (763/2162 [35.3%]; 21.4 per 100 person-years) than in the placebo group (819/2129 [38.5%]; 24.5 per 100 person-years; absolute incidence rate difference, −3.18; 95% CI, −5.44 to −0.92) (HR, 0.86; 95% CI, 0.78-0.95; P = .003) (Table 2 and Supplement 1). Another prespecified microvascular composite outcome including both kidney and major ocular events occurred less frequently in linagliptin-treated participants (Table 2 and Supplement 1).
Exploratory Glycemic and CV Risk Factor Analyses
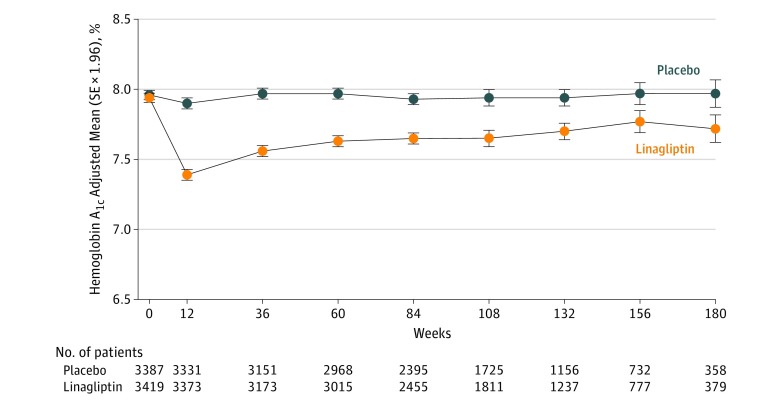
After 12 weeks of treatment, the adjusted mean difference in hemoglobin A1c with linagliptin vs placebo was −0.51% (95% CI, −0.55% to −0.46%) (Figure 3), with an overall difference over the full study duration of −0.36% (95% CI, −0.42% to −0.29% based on least-square means), without an increase in overall hypoglycemia observed (linagliptin group, 19.9 per 100 person-years compared with placebo group, 20.2 per 100 person-years) and in the context of a higher use of additional glucose-lowering medications in the placebo group (HR, 0.76; 95% CI, 0.69-0.84; P < .001); notably fewer patients in the linagliptin group initiated or increased doses of preexisting insulin therapy (HR, 0.72; 95% CI, 0.65-0.81; P < .001). There were no significant differences in new introductions of blood pressure–lowering medications, anticoagulants, or low-density lipoprotein cholesterol–lowering drugs between the linagliptin and placebo groups (Supplement 1), and overall, changes in weight, systolic and diastolic blood pressure, and low- and high-density lipoprotein cholesterol were not different between groups (Supplement 1).
Figure 3. Hemoglobin A1c Measurements Over Time by Treatment Group.
Median time in study was 2.0 (interquartile range [IQR], 1.3-2.6) years for linagliptin and 2.0 (IQR, 1.2-2.6) years for placebo. Baseline values are descriptive; postbaseline data from mixed-model repeated-measures analysis are adjusted for treatment, region, baseline hemoglobin A1c value, week, treatment × week interaction, and baseline hemoglobin A1c value × week interaction.
Adverse Events
The frequency of occurrence of adverse events, serious adverse events, and adverse events leading to study drug discontinuation for patients treated with linagliptin vs placebo were 77.2% vs 78.1%, 37.0% vs 38.5%, and 10.3% vs 11.5%, respectively (Table 3). Numerical imbalances for pemphigoid events (linagliptin, n=7 [0.2%] vs placebo, n=0], skin lesions (linagliptin, n=5 [0.2%] vs placebo, n=1 [<0.1%)]), and adjudication-confirmed acute pancreatitis events (linagliptin, n=9 [0.3%] vs placebo, n=5 [0.1%]) were observed.
Table 3. Adverse Events, Including Hypoglycemic Events, by Treatment Groupa.
| Adverse Events | No. (%) | |
|---|---|---|
| Linagliptin (n = 3494) | Placebo (n = 3485) | |
| Any adverse events | 2697 (77.2) | 2723 (78.1) |
| Serious adverse events | 1293 (37.0) | 1343 (38.5) |
| Adverse events leading to study drug discontinuation | 359 (10.3) | 402 (11.5) |
| Hypersensitivity reactionsb | 114 (3.3) | 109 (3.1) |
| Angioedema events with concomitant ACE inhibitor/ARB use at baseline | 13 (0.5) | 16 (0.6) |
| Pemphigoid | 7 (0.2) | 0 (0.0) |
| Skin lesions | 5 (0.1) | 1 (<0.1) |
| Acute pancreatitis (adjudication confirmed) | 9 (0.3)c | 5 (0.1) |
| Chronic pancreatitis (adjudication confirmed) | 2 (0.1) | 3 (0.1) |
| All cancers | 116 (3.3) | 134 (3.8) |
| Colon cancer | 6 (0.2) | 8 (0.2) |
| Pancreatic cancer (adjudication confirmed) | 11 (0.3) | 4 (0.1) |
| Gastric cancer | 0 | 3 (0.1) |
| Hypoglycemic events | ||
| Investigator-reported hypoglycemia | 1036 (29.7) | 1024 (29.4) |
| Confirmed hypoglycemic adverse events with plasma glucose <54 mg/dL (<3.0 mmol/L) or severe eventsd | 557 (15.9) | 572 (16.4) |
| Severe eventsd | 106 (3.0) | 108 (3.1) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Adverse events are classified based on MedDRA version 20.1 and include adverse events in patients treated with at least 1 dose of study drug until less than 7 days after the last intake of study medication, with the exception of pancreatitis and cancers, which include all events in patients treated with at least 1 dose of study drug until study end.
Based on 276 MedDRA version 20.1 preferred terms.
There were 2 fatal cases of pancreatitis (0.1%).
Requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions.
The frequency of malignancies was 3.3% (n = 116) in the linagliptin group and 3.8% (n = 134) in the placebo group. Overall, reports of pancreatic cancers were rare but were numerically higher in the linagliptin group (n=11 [0.3%]) than in the placebo group (n=4 [0.1%]). The oncology expert assessment committee deemed 1 case in each treatment group to be possibly related to study drug treatment.
The frequency of confirmed hypoglycemic adverse events (including severe hypoglycemia) was 15.9% in the linagliptin group and 16.4% in the placebo group. A numerically higher rate of hypoglycemia was observed with linagliptin compared with placebo in patients taking sulfonylurea at baseline (15.5 per 100 person-years compared with placebo, 13.7 per 100 person-years) but not in other subgroups at increased risk of hypoglycemia (Supplement 1).
Discussion
In this large, multicenter, randomized clinical trial involving a population of patients with type 2 diabetes at high risk of CV events and with a high prevalence of kidney disease, linagliptin added to usual care was noninferior to placebo added to usual care for the primary outcome of 3-point MACE and did not demonstrate evidence of CV benefit. Similarly, there was no significant benefit of linagliptin compared with placebo for the incidence of the secondary kidney composite outcome. The MACE and composite kidney findings were consistent across all prespecified sensitivity analyses and most subgroups.
Patients with advanced chronic kidney disease have largely been excluded from previous CV outcome trials of glucose-lowering drugs, resulting in limited available information about use of these drugs in this particular population.19 This was not the case for the present trial, in which 74% of patients had prevalent chronic kidney disease, 43% had an eGFR below 45 mL/min/1.73 m2, and 15.2% had an eGFR below 30 mL/min/1.73 m2. Overall, the high event rate for 3-point MACE observed (5.63 per 100 person-years) demonstrates that this trial recruited one of the highest-risk cohorts studied to date in CV outcome trials of glucose-lowering medications conducted in accordance with FDA requirements for assessing CV safety of drugs for type 2 diabetes, and underscores the clinical effect of kidney impairment.2 Consequently, rates of CV death in the placebo group were also high at 3.40 per 100 person-years and were considerably higher than in prior DPP-4 inhibitor trials, with corresponding rates ranging from 1.45 to 2.45 per 100 person-years.8,9,10
To our knowledge, this is the first CV outcome trial of a glucose-lowering medication to study the effects on progression of kidney disease in patients with type 2 diabetes in an adequately powered trial. The study results did not demonstrate any benefit of linagliptin for the secondary composite kidney outcome. Patients with kidney impairment commonly have long-term hyperglycemic control, consistent with the baseline HbA1c of 8% in this trial.19,20 This is likely a reflection of the reduced access to an available armamentarium of glucose-lowering therapies due to label restrictions for patients with low eGFR.19,20,21 The modest HbA1c reductions without an increase in hypoglycemia observed with linagliptin in the present trial are therefore important for patients with established kidney disease, particularly because few glucose-lowering agents have been studied in patients with advanced chronic kidney disease.19 The results of exploratory analyses in this study also support the hypothesis that linagliptin may reduce mean albuminuria progression compared with placebo in this population, a hypothesis that emerged from previous studies of other DPP-4 inhibitors, although an effect of glucose control cannot be excluded.22,23 The ADVANCE trial suggested that glucose lowering may have potential to reduce the risk of ESRD24; however, that trial had a longer mean follow-up (5 years vs 2.2 years) and a larger glycemic difference between randomized groups (0.7% vs 0.4%).
Hospitalization for heart failure was one of numerous prespecified exploratory outcomes in this study, with all suspected events captured systematically and undergoing central adjudication, and with a formal heart failure statistical analysis plan prospectively formulated.25 There was no significant difference between the linagliptin and placebo groups in the risk of heart failure hospitalization. This finding is particularly relevant in view of previous reports with saxagliptin (SAVOR-TIMI 53)8,26 and alogliptin (EXAMINE)9,27 that reported significant and numerical increases in this outcome, respectively; whereas sitagliptin (TECOS) showed no effect.10,28
Limitations
This trial has several limitations. First, the study population was at high CV and renal risk, with a lower mean eGFR than typically is studied in CV outcome trials. However, 38% of study participants did not have low eGFR, and therefore, the study results would not necessarily be applicable to individuals without the features of the overall study population. Second, glycemic control was improved with linagliptin despite attempts to maintain glucose equipoise and despite higher use of glucose-lowering medications in the placebo group. Although intensive glucose control per se has not been clearly associated with either CV mortality29 or improvement in ESRD or death due to renal failure,30 the possibility that differences in glucose control might have had a role in the exploratory outcome of albuminuria progression cannot be excluded. Third, a potential limitation in interpreting the kidney effects is the trial duration, which could have been too short to modulate kidney-related clinical outcomes like ESRD. Fourth, while information was available on introduction of therapies after baseline, information was not reported on whether use of linagliptin was related to patients reducing use of other therapies. Fifth, although adverse events were balanced across treatment groups, some adverse events occurred with numerical imbalances, but the trial was not powered to assess effects on cancer risk or individual adverse events. However, the pattern of adverse events aligns with that observed for other incretin therapies,31,32 and further postmarketing studies and future trial data on linagliptin will be informative.33
Conclusions
Among adults with type 2 diabetes and high CV and renal risk, linagliptin added to usual care compared with placebo added to usual care resulted in a noninferior risk of a composite CV outcome over a median 2.2 years.
eAppendix 1. List of Investigators
eAppendix 2. Trial Committees and Independent Statistical Center
eAppendix 3. Inclusion Criteria
eAppendix 4. Exclusion Criteria
eAppendix 5. Definitions of Major Clinical Outcomes
eAppendix 6. Description of Superiority Tests of 3-Point MACE and the Secondary Kidney Outcome And Their Sensitivity Analyses and Subgroup Analyses
eAppendix 7. Treatment and Observation Times
eAppendix 8. Sensitivity and Subgroup Analyses for the Primary Outcome
eAppendix 9. Sensitivity and Subgroup Analyses for the Secondary Kidney Composite Outcome
eAppendix 10. Exploratory CV Outcomes
eAppendix 11. Exploratory Kidney and Microvascular Outcomes
eAppendix 12. Hypoglycemia Incidence Overall and in Subgroups at Elevated Hypoglycaemia Risk
eAppendix 13. Post-baseline Introduction of New Glucose-Lowering and CV Therapies
eAppendix 14. Exploratory CV and Renal Risk Factor Analysis
eAppendix 15. Endpoints Prespecified in the Statistical Analysis Plans of CARMELINA
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376(15):1407-1418. doi: 10.1056/NEJMoa1608664 [DOI] [PubMed] [Google Scholar]
- 2.Wen CP, Chang CH, Tsai MK, et al. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int. 2017;92(2):388-396. doi: 10.1016/j.kint.2017.01.030 [DOI] [PubMed] [Google Scholar]
- 3.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457-2471. doi: 10.1056/NEJMoa072761 [DOI] [PubMed] [Google Scholar]
- 4.Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294(20):2581-2586. doi: 10.1001/jama.294.20.joc50147 [DOI] [PubMed] [Google Scholar]
- 5.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370(9593):1129-1136. doi: 10.1016/S0140-6736(07)61514-1 [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration Guidance for Industry. Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes 2008. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf. Accessed August 31, 2018.
- 7.Committee for Medicinal Products for Human Use Guideline on Clinical Investigation of Medicinal Products in the Treatment or prevention of Diabetes Mellitus London, England: European Medicines Agency; 2012. https://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf. Accessed August 31, 2018.
- 8.Scirica BM, Bhatt DL, Braunwald E, et al. ; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317-1326. doi: 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- 9.White WB, Cannon CP, Heller SR, et al. ; EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327-1335. doi: 10.1056/NEJMoa1305889 [DOI] [PubMed] [Google Scholar]
- 10.Green JB, Bethel MA, Armstrong PW, et al. ; TECOS Study Group . Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232-242. doi: 10.1056/NEJMoa1501352 [DOI] [PubMed] [Google Scholar]
- 11.Boehringer Ingelheim Tradjenta (linagliptin) tablets prescribing information. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Tradjenta/Tradjenta.pdf. Accessed August 31, 2018.
- 12.Johansen OE, Neubacher D, von Eynatten M, Patel S, Woerle HJ. Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: a pre-specified, prospective, and adjudicated meta-analysis of a phase 3 programme. Cardiovasc Diabetol. 2012;11:3. doi: 10.1186/1475-2840-11-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groop PH, Cooper ME, Perkovic V, Emser A, Woerle HJ, von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. 2013;36(11):3460-3468. doi: 10.2337/dc13-0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenstock J, Perkovic V, Alexander JH, et al. ; CARMELINA Investigators . Rationale, design, and baseline characteristics of the Cardiovascular Safety and Renal Microvascular Outcome Study With Linagliptin (CARMELINA): a randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk. Cardiovasc Diabetol. 2018;17(1):39. doi: 10.1186/s12933-018-0682-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marx N, McGuire DK, Perkovic V, et al. Composite primary end points in cardiovascular outcomes trials involving type 2 diabetes patients: should unstable angina be included in the primary end point? Diabetes Care. 2017;40(9):1144-1151. doi: 10.2337/dc17-0068 [DOI] [PubMed] [Google Scholar]
- 16.Center for Drug Evaluation and Research Meeting expectations to exclude a CV risk margin of 1.3. In: Application Number 204042Orig1s000 Summary Review Page 20. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204042Orig1s000SumR.pdf. Accessed August 31, 2018.
- 17.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64(6):821-835. doi: 10.1053/j.ajkd.2014.07.030 [DOI] [PubMed] [Google Scholar]
- 18.Thompson A, Lawrence J, Stockbridge N. GFR decline as an end point in trials of CKD: a viewpoint from the FDA. Am J Kidney Dis. 2014;64(6):836-837. doi: 10.1053/j.ajkd.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 19.Lo C, Toyama T, Wang Y, et al. Insulin and glucose-lowering agents for treating people with diabetes and chronic kidney disease. Cochrane Database Syst Rev. 2018;9:CD011798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316(6):602-610. doi: 10.1001/jama.2016.10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312(24):2668-2675. doi: 10.1001/jama.2014.15298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornel JH, Bakris GL, Stevens SR, et al. ; TECOS Study Group . Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: outcomes from TECOS. Diabetes Care. 2016;39(12):2304-2310. doi: 10.2337/dc16-1415 [DOI] [PubMed] [Google Scholar]
- 23.Mosenzon O, Leibowitz G, Bhatt DL, et al. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care. 2017;40(1):69-76. doi: 10.2337/dc16-0621 [DOI] [PubMed] [Google Scholar]
- 24.Patel A, MacMahon S, Chalmers J, et al. ; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-2572. doi: 10.1056/NEJMoa0802987 [DOI] [PubMed] [Google Scholar]
- 25.McGuire DK, Alexander JH, Johansen OE, et al. Linagliptin effects on heart failure outcomes in participants with type 2 diabetes at high CV and renal risk in CARMELINA [published online November 11, 2018]. Circulation. doi: 10.1161/CIRCULATIONAHA.118.038352 [DOI] [PubMed] [Google Scholar]
- 26.Scirica BM, Braunwald E, Raz I, et al. ; SAVOR-TIMI 53 Steering Committee and Investigators . Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130(18):1579-1588. doi: 10.1161/CIRCULATIONAHA.114.010389 [DOI] [PubMed] [Google Scholar]
- 27.Zannad F, Cannon CP, Cushman WC, et al. ; EXAMINE Investigators . Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385(9982):2067-2076. doi: 10.1016/S0140-6736(14)62225-X [DOI] [PubMed] [Google Scholar]
- 28.McGuire DK, Van de Werf F, Armstrong PW, et al. ; Trial Evaluating Cardiovascular Outcomes With Sitagliptin Study Group . Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1(2):126-135. doi: 10.1001/jamacardio.2016.0103 [DOI] [PubMed] [Google Scholar]
- 29.Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52(11):2288-2298. doi: 10.1007/s00125-009-1470-0 [DOI] [PubMed] [Google Scholar]
- 30.Ruospo M, Saglimbene VM, Palmer SC, et al. Glucose targets for preventing diabetic kidney disease and its progression. Cochrane Database Syst Rev. 2017;6:CD010137. doi: 10.1002/14651858.CD010137.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knapen LM, de Jong RG, Driessen JH, et al. Use of incretin agents and risk of acute and chronic pancreatitis: a population-based cohort study. Diabetes Obes Metab. 2017;19(3):401-411. doi: 10.1111/dom.12833 [DOI] [PubMed] [Google Scholar]
- 32.Nauck MA, Jensen TJ, Rosenkilde C, Calanna S, Buse JB; LEADER Publication Committee; LEADER Trial Investigators . Neoplasms reported with liraglutide or placebo in people with type 2 diabetes: results from the LEADER randomized trial. Diabetes Care. 2018;41(8):1663-1671. doi: 10.2337/dc17-1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marx N, Rosenstock J, Kahn SE, et al. Design and baseline characteristics of the Cardiovascular Outcome Trial of Linagliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA). Diab Vasc Dis Res. 2015;12(3):164-174. doi: 10.1177/1479164115570301 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. List of Investigators
eAppendix 2. Trial Committees and Independent Statistical Center
eAppendix 3. Inclusion Criteria
eAppendix 4. Exclusion Criteria
eAppendix 5. Definitions of Major Clinical Outcomes
eAppendix 6. Description of Superiority Tests of 3-Point MACE and the Secondary Kidney Outcome And Their Sensitivity Analyses and Subgroup Analyses
eAppendix 7. Treatment and Observation Times
eAppendix 8. Sensitivity and Subgroup Analyses for the Primary Outcome
eAppendix 9. Sensitivity and Subgroup Analyses for the Secondary Kidney Composite Outcome
eAppendix 10. Exploratory CV Outcomes
eAppendix 11. Exploratory Kidney and Microvascular Outcomes
eAppendix 12. Hypoglycemia Incidence Overall and in Subgroups at Elevated Hypoglycaemia Risk
eAppendix 13. Post-baseline Introduction of New Glucose-Lowering and CV Therapies
eAppendix 14. Exploratory CV and Renal Risk Factor Analysis
eAppendix 15. Endpoints Prespecified in the Statistical Analysis Plans of CARMELINA
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement