Key Points
Question
In immunocompromised patients with acute hypoxemic respiratory failure, is high-flow nasal oxygen therapy superior to standard oxygen therapy with respect to mortality at day 28?
Findings
In this randomized clinical trial that included 776 critically ill immunocompromised patients receiving at least 6 L/min of oxygen, high-flow oxygen therapy compared with standard oxygen therapy did not significantly reduce day-28 mortality (35.6% vs 36.1%, respectively).
Meaning
Among immunocompromised patients with acute respiratory failure, high-flow oxygen therapy did not significantly reduce mortality compared with standard oxygen therapy.
Abstract
Importance
High-flow nasal oxygen therapy is increasingly used for acute hypoxemic respiratory failure (AHRF).
Objective
To determine whether high-flow oxygen therapy decreases mortality among immunocompromised patients with AHRF compared with standard oxygen therapy.
Design, Setting, and Participants
The HIGH randomized clinical trial enrolled 776 adult immunocompromised patients with AHRF (Pao2 <60 mm Hg or Spo2 <90% on room air, or tachypnea >30/min or labored breathing or respiratory distress, and need for oxygen ≥6 L/min) at 32 intensive care units (ICUs) in France between May 19, 2016, and December 31, 2017.
Interventions
Patients were randomized 1:1 to continuous high-flow oxygen therapy (n = 388) or to standard oxygen therapy (n = 388).
Main Outcomes and Measures
The primary outcome was day-28 mortality. Secondary outcomes included intubation and mechanical ventilation by day 28, Pao2:Fio2 ratio over the 3 days after intubation, respiratory rate, ICU and hospital lengths of stay, ICU-acquired infections, and patient comfort and dyspnea.
Results
Of 778 randomized patients (median age, 64 [IQR, 54-71] years; 259 [33.3%] women), 776 (99.7%) completed the trial. At randomization, median respiratory rate was 33/min (IQR, 28-39) vs 32 (IQR, 27-38) and Pao2:Fio2 was 136 (IQR, 96-187) vs 128 (IQR, 92-164) in the intervention and control groups, respectively. Median SOFA score was 6 (IQR, 4-8) in both groups. Mortality on day 28 was not significantly different between groups (35.6% vs 36.1%; difference, −0.5% [95% CI, −7.3% to +6.3%]; hazard ratio, 0.98 [95% CI, 0.77 to 1.24]; P = .94). Intubation rate was not significantly different between groups (38.7% vs 43.8%; difference, −5.1% [95% CI, −12.3% to +2.0%]). Compared with controls, patients randomized to high-flow oxygen therapy had a higher Pao2:Fio2 (150 vs 119; difference, 19.5 [95% CI, 4.4 to 34.6]) and lower respiratory rate after 6 hours (25/min vs 26/min; difference, −1.8/min [95% CI, −3.2 to −0.2]). No significant difference was observed in ICU length of stay (8 vs 6 days; difference, 0.6 [95% CI, −1.0 to +2.2]), ICU-acquired infections (10.0% vs 10.6%; difference, −0.6% [95% CI, −4.6 to +4.1]), hospital length of stay (24 vs 27 days; difference, −2 days [95% CI, −7.3 to +3.3]), or patient comfort and dyspnea scores.
Conclusions and Relevance
Among critically ill immunocompromised patients with acute respiratory failure, high-flow oxygen therapy did not significantly decrease day-28 mortality compared with standard oxygen therapy.
Trial Registration
clinicaltrials.gov Identifier: NCT02739451.
This randomized clinical trial compares the effects of high-flow nasal vs standard oxygen therapy on 28-day mortality among immunocompromised patients with acute hypoxemic respiratory failure.
Introduction
Survival with immune deficiencies is increasingly common,1 owing to the increasing life expectancy after cancer2 and expanding use of transplantation3 and immunosuppressant drugs.4 In immunocompromised patients, intensive treatments improve survival2 but only at the cost of life-threatening events, chiefly affecting the lungs.5 Acute hypoxemic respiratory failure (AHRF) in immunocompromised patients, the first reason for intensive care unit (ICU) admission,6,7 is still associated with high mortality rates.5 Need for invasive mechanical ventilation (IMV) is a key prognostic factor in immunocompromised patients, and avoiding IMV has become a major treatment goal. However, no survival benefit of noninvasive ventilation (NIV) compared with standard oxygen therapy was reported from a multicenter randomized clinical trial (RCT),8 in contrast to an earlier single-center study.9
High-flow nasal oxygen therapy, which delivers warm and humidified oxygen through a nasal cannula, has shown conflicting results regarding its benefit over standard oxygen therapy in RCTs. Although high-flow oxygen therapy significantly increased the number of ventilator-free days and decreased day-90 mortality in patients with AHRF,10 this was not confirmed in immunocompromised patients, based on 2 post hoc analyses of RCTs.11,12 Moreover, high-flow oxygen therapy failed to improve comfort, dyspnea, or thirst compared with a Venturi mask in a pilot multicenter RCT.13 Thus, uncertainty remains about whether benefits can be expected from high-flow oxygen therapy in immunocompromised patients with AHRF.
The HIGH multicenter RCT was designed to test the hypothesis that high-flow oxygen therapy, compared with standard oxygen therapy, decreases all-cause day-28 mortality in critically ill immunocompromised patients with AHRF.14
Methods
Study Design and Oversight
From May 19, 2016, to December 31, 2017, this randomized, parallel-group trial was conducted in 32 hospitals in France (24 university-affiliated and 8 non–university-affiliated) belonging to the Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique (GRRR-OH). The study protocol was approved by the CPP Ile de France IV St-Louis ethics committee (March 3, 2016, #N IRB00003835/2016/08) and French health authorities (Agence Nationale de Sécurité du Médicament et des Produits de Santé, EudraCT2016-A00220-51). The protocol and statistical analysis plan have been published14 and are also available in Supplement 1. The trial was overseen by an independent data and safety monitoring board. Written informed consent was obtained from all patients or their proxies.
Patients
Patients were recruited in 32 ICUs having experience and expertise with immunocompromised patients and respiratory care strategies.8,15 Eligibility criteria were ICU admission; age 18 years or older; AHRF with Pao2 less than 60 mm Hg or oxygen saturation by pulse oximetry (Spo2) less than 90% on room air, or tachypnea greater than 30/min or labored breathing or respiratory distress; need for oxygen flow of 6 L/min or greater; known immunosuppression, defined as use of long-term (>3 months) or high-dose (>0.5 mg/kg/d) steroids, use of other immunosuppressant drugs, solid organ transplantation, solid tumor requiring chemotherapy in the last 5 years, hematologic malignancy regardless of time since diagnosis and received treatments, or primary immune deficiency; and written informed consent from the patient or proxy. Patients with AIDS were not eligible.
Exclusion criteria were imminent death; refusal of study participation by the patient; anatomical factors precluding the use of a nasal cannula; hypercapnia indicating NIV (Paco2 ≥50 mm Hg); isolated cardiogenic pulmonary edema indicating NIV; pregnancy or breastfeeding; absence of coverage by the French statutory health care insurance system; and surgery within the last 6 days.
Randomization
Eligible patients were included by investigators in each ICU, then randomly assigned in a 1:1 ratio to either high-flow oxygen therapy or standard oxygen therapy throughout the ICU stay. Randomization was stratified on study center, oxygen flow rate at randomization (>9 L/min vs ≤9 L/min), need for vasopressors, and time since ICU admission (≤2 vs ≥3 days), based on preestablished lists with permutation blocks having a fixed size of 4; block size was concealed. Randomization was achieved using an electronic system incorporated in the electronic case report form to ensure allocation concealment. The nature of the intervention precluded blinding of patients and health care staff. Baseline was defined as time of randomization.
Treatments
All management decisions other than oxygen therapy were made by the managing physicians according to standard practice in each ICU. All patients in both groups received the best standard of care according to local management protocols. The randomly allocated treatment (high-flow oxygen therapy or standard oxygen therapy) was started within 15 minutes after randomization.
In the intervention group, oxygen was delivered only by continuous high-flow oxygen therapy, initiated at 50 L/min and 100% fraction of inspired oxygen (Fio2), with a subsequent flow rate increase to achieve Spo2 of 95% or greater, up to at least 50 L/min within the first 3 days then up to 60 L/min as needed. Fraction of inspired oxygen was tapered as possible while maintaining Spo2 of 95% or greater. In patients who required IMV, high-flow oxygen therapy was used during laryngoscopy and immediately after extubation. Patients with discomfort from high-flow oxygen therapy had their flow rate decreased until the discomfort resolved. Standard oxygen therapy was used in this group only if the nasal cannula generated significant discomfort or skin breakdown, in which case a Venturi mask was used until high-flow oxygen therapy could be tolerated again. Criteria for weaning off high-flow oxygen therapy were improvement in clinical signs of respiratory distress, Pao2:Fio2 ratio greater than 300, and ability to maintain Spo2 of 95% or greater with less than 6 L/min of standard oxygen therapy. After weaning, patients whose oxygen flow was 6 L/min or greater at any time were returned to high-flow oxygen therapy.
In the standard oxygen therapy (control) group, oxygen was delivered via any device or combination of devices used for standard care (nasal prongs or mask with or without a reservoir bag and with or without a Venturi system). Oxygen flow was set to achieve Spo2 of 95% or greater. High-flow oxygen therapy could be used only for patients with do-not-intubate orders for whom standard oxygen therapy had failed. ICU discharge was considered when patients maintained Spo2 values of 95% or greater with less than 6 L/min oxygen.
Noninvasive ventilation has been found either nonbeneficial8 or harmful10,11,16 and was therefore used only when, and as long as, hypercapnia or pulmonary edema were present.
In both groups, intubation decisions were based on the therapeutic response, clinical status (including Spo2, respiratory rate, signs of respiratory distress, and bronchial secretion volume). Ventilator settings for IMV complied with the best standard of care.17
Study Outcomes
The primary end point was overall mortality within 28 days after randomization.
The secondary end points were the proportion of patients requiring IMV by day 28, respiratory rate (normal values, 12-20), lowest Pao2:Fio2 ratio (normal values, 500-600; values <300 indicate severe dysfunction of gas exchange in the lungs), patient comfort score (range, 0 [severe discomfort] to 10 [perfect comfort]), dyspnea score (range, 0 [anchor; “no dyspnea”] to 10 [“severe dyspnea”]), ICU and hospital lengths of stay, and incidence of ICU-acquired infections. Minimal clinically important differences were not established.
Data reported in the tables and figures were collected prospectively using an electronic case report form. No blinding of adjudication was performed for outcome assessments.
Statistical Analysis
The protocol first submitted for the grant application was for a noninferiority RCT with a 9% noninferiority margin and with different secondary end points. However, based on the results of the FLORALI trial10 and as a condition of awarding the grant, the jury requested that the study be changed to a superiority trial. The revised protocol submitted to the institutional review board has been published.14 Based on an expected 30% day-28 mortality rate in the standard oxygen therapy group with a decrease to 20% in the high-flow oxygen therapy group11 and with α set at 5%, 779 patients (389 in each group) were required to obtain 90% power for demonstrating an decrease in day-28 mortality.
A scheduled interim analysis was performed when 100 deaths had occurred, using the Haybittle-Peto boundary, ie, a P value threshold of .001 for the interim analysis (because of the risk of inflation of the type I error rate). The interim analysis was reviewed by the independent data and safety monitoring board. To assess the between-group difference in terms of futility or efficacy, the Bayesian posterior probabilities of the day-28 mortality rate and of the log odds ratio were computed, using a uniform noninformative prior; no specific stopping rules were prespecified.
The analysis used the intent-to-treat approach, ie, all patients were analyzed in the group allocated by randomization, with no exclusion after randomization except exclusions for withdrawn consent according to the French regulation at the time. Continuous variables were described as medians (interquartile ranges) and categorical variables as proportions. No participants were excluded from analyses because of missing or incomplete data.
Overall mortality was estimated using the Kaplan-Meier method, with administrative censoring of patients alive in the ICU on day 28. The effect size was evaluated by computing the absolute risk difference with its 95% CI and the hazard ratio (HR) with 95% CI as estimated from univariable Cox regression models; the proportional hazards assumption was checked (P = .72), based on weighted residuals.18 The cumulative incidence of IMV (with death without IMV as a competing risk) in each group was estimated using a nonparametric estimator and compared using the Gray test19; effect size was measured using a univariable cause-specific Cox model. The proportions of ICU-acquired infections in the 2 groups were compared by χ2 test. The Wilcoxon rank-sum test was chosen for comparisons of the visual analog scale scores for comfort and dyspnea, respiratory rate, and ICU length of stay. Relative risk was estimated as a measure of treatment effect in terms of ICU and hospital mortality.
Effect of high-flow oxygen therapy vs standard oxygen therapy was measured using HRs estimated from Cox regression models in subgroups defined by stratification variables, then displayed in forest plots. The Gail and Simon interaction test was then applied to assess whether these estimates were homogeneous across subsets ie, to test for quantitative interactions between the study treatment and stratification variables (baseline oxygen flow rate, need for vasopressors, and time from ICU admission to randomization).
Because there was no handling of the potential for type I error inflation due to multiple comparisons of secondary analyses, those analyses should be considered exploratory. Post hoc analyses included the search for site effect in terms of the primary end point, using frailty model and subset analyses in intubated patients.
All reported P values are 2-sided; P < .05 was considered statistically significant. All analyses were performed using R version 3.1.0 (R Foundation for Statistical Computing [http://www.R-project.org/]).
Results
Patients
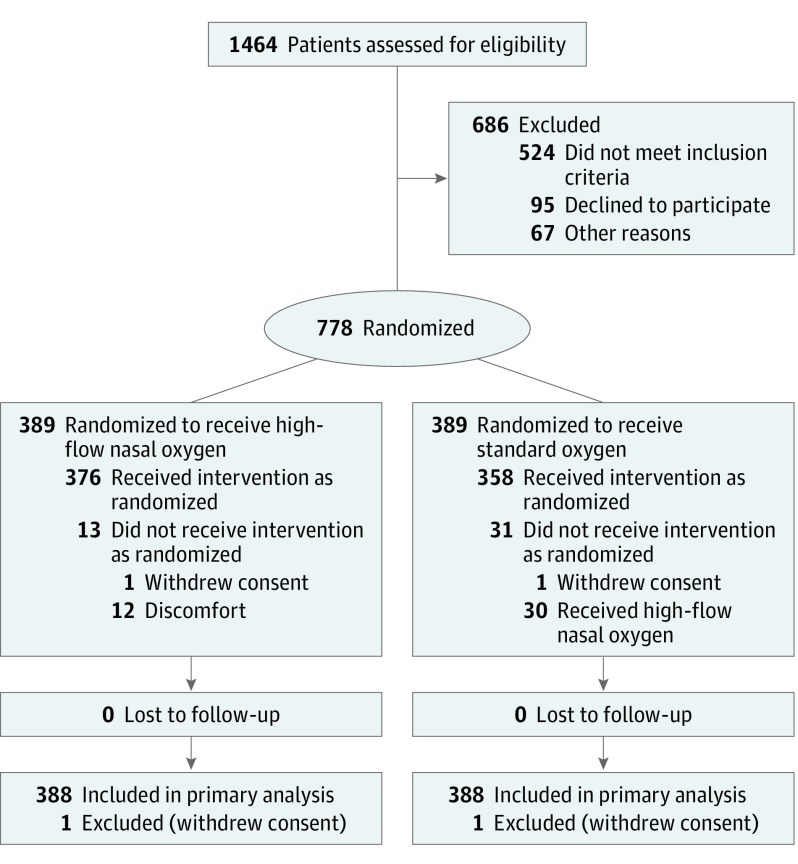
Of 778 patients (median age, 64 [interquartile range {IQR}, 54-71] years; 259 [33.3%] women)randomized to high-flow nasal oxygen therapy (n = 389) and standard oxygen therapy (n= 389), 776 (99.7%; n = 388 in each group) completed the trial (Figure 1). No patient was lost to follow-up. Baseline characteristics were evenly distributed between the 2 groups (Table 1). Malignancies and their treatments were the main causes of immunosuppression.
Figure 1. Flow of Patients Through the HIGH Trial.
The number of patients excluded and the reasons for the exclusions were not available in all centers.
Table 1. Patient Characteristics at Randomization.
| Characteristic | No. (%) | |
|---|---|---|
| High-Flow Oxygen Therapy (n = 388) |
Standard Oxygen Therapy (n = 388) |
|
| Demographics | ||
| Age, median (IQR), y | 64 (55-70) | 63 (56-71) |
| Sex | ||
| Men | 270 (69.6) | 247 (63.6) |
| Women | 118 (30.4) | 141 (36.4) |
| Comorbidities | ||
| Chronic | ||
| Respiratorya | 115 (29.6) | 127 (32.7) |
| Heart failure | 23 (5.9) | 27 (6.9) |
| Liver | 45 (13.3) | 56 (14.4) |
| Kidney disease | 73 (18.8) | 69 (20.4) |
| Charlson Comorbidity Indexb | 5 (4-7) | 5 (3-7) |
| Underlying conditionsc | ||
| Cancer | 294 (75.8) | 319 (82.2) |
| Hematologic malignancies | 167 (43.0) | 181 (46.6) |
| Solid tumors | 127 (32.7) | 138 (35.6) |
| Immunosuppressive drugs | 133 (34.3) | 135 (34.8) |
| Non–transplant-related reasons | 89 (22.9) | 98 (25.2) |
| After solid organ transplantation | 44 (11.3) | 37 (9.5) |
| Time since diagnosis of underlying condition, median (IQR), mo | 6.4 (1-29) | 7.0 (0.8-40.0) |
| Chemotherapy at ICU admission | 221/294 (75.2) | 228/319 (71.5) |
| Autologous stem cell transplantation | 26/167 (15.6) | 22/181 (12.1) |
| Allogeneic stem cell transplantation | 28/167 (16.8) | 33 /181 (18.2) |
| Poor performance status (3 or 4)d | 61 (15.7) | 54 (13.9) |
| Randomization and Other Characteristics | ||
| Randomization | ||
| Day of ICU admission | 244 (62.9) | 251 (64.7) |
| Day after ICU admission | 77 (19.8) | 79 (20.4) |
| Two days after ICU admission | 47 (12.1) | 38 (9.8) |
| ≥3 days after | 20 (5.1) | 20 (5.1) |
| No. randomized in the postextubation period | 14 (4.1) | 18 (5.3) |
| SOFA at randomization, median (IQR)e | 6 (4-8) | 6 (4-8) |
| SAPSII at randomization, median (IQR)f | 36 (28-46) | 37 (28-48) |
| Vasopressors at randomization | 33 (8.5) | 39 (10.0) |
| Goals of care at randomization | ||
| Full code management | 308 (79.4) | 309 (79.6) |
| Do not intubate | 13 (3.3) | 15 (3.9) |
| Do not resuscitate | 3 (0.7) | 1 (0.2) |
| Time-limited trial of intensive care | 35 (9.0) | 36 (9.3) |
| Unknown | 29 (7.5) | 27 (6.9) |
| Respiratory status immediately before randomization | ||
| Respiratory rate, median (IQR), /min | 33 (28-39) | 32 (27-38) |
| Pao2:Fio2 ratio, median (IQR) | 136 (96-187) | 128 (92-164) |
| Received standard oxygen therapy before randomization | 311 (80.1) | 334 (86.1) |
| Oxygen flow, median (IQR), L/min | 10 (6-15) | 10 (6-15) |
| Pao2 with standard oxygen, median (IQR) | 81 (65-111) | 75 (65-93) |
| Estimated Pao2:Fio2 ratio (on oxygen), median (IQR) | 120 (86-164) | 114 (82-149) |
| Received NIV or high-flow oxygen therapy before randomization | ||
| NIV | 25 (6.4) | 18 (4.6) |
| High-flow oxygen therapy | 52 (13.4) | 36 (9.3) |
| Pao2:Fio2 ratio, median (IQR) | 117 (87-173) | 108 (76-167) |
Abbreviations: Fio2, fraction of inspired oxygen; ICU, intensive care unit; IQR, interquartile range; NIV, noninvasive ventilation; SOFA, Sequential Organ Failure Assessment; SAPSII, Simplified Acute Physiology Score version II.
Chronic respiratory insufficiency includes obstructive and restrictive chronic respiratory disease.
Contains 19 categories of comorbidities and predicts the 10-year mortality for a patient who may have a range of comorbid conditions. The physician assigns each condition a score of 1, 2, 3, or 6, depending on the patient’s risk of dying associated with the condition; higher scores indicate greater comorbidity, resulting in an index ranging from 19 (low risk of death) to 114 (high risk of death). It is measured by physicians.
Main hematologic malignancies were acute myeloid leukemia (n = 123), non-Hodgkin lymphoma (n = 97), and myeloma (n = 41). Solid tumors primarily affected the lung (n = 72), digestive tract (n = 60), and breast (n = 30). Immunosuppressive drugs included steroids in 174 patients; the main transplanted solid organs were the kidney (n = 46) and liver (n = 19).
Indicates patients who are bedridden or dependent.
SOFA score collects information on the presence and intensity of respiratory, coagulation, hemodynamic, neurologic, liver, and kidney failures. Each organ is assessed from 0 (no failure) to 4 (worst possible failure); score range, 0 (no organ failure) to 24 (all organ failures). The highest value was recorded. A score between 7 and 9 indicates a mortality risk of 15% to 20%.
SAPSII score was calculated as previously reported.20 The score ranges from 0 (predicted hospital mortality of 0%) to 163 (predicted hospital mortality of 100%). A score of 36 indicates a mortality risk of 18% to 20%.
At randomization, median respiratory rate was 33 (IQR, 28-39) and 32 (IQR, 27-38) and median Pao2:Fio2 ratio was 136 (IQR, 96-187) and 128 (IQR, 92-164) in the high-flow oxygen therapy and standard oxygen therapy groups, respectively. The median Sequential Organ Failure Assessment score was 6 (IQR, 4-8) in both groups (Table 1). The leading cause of AHRF was bacterial pneumonia (n = 320), followed by invasive fungal infection (n = 91, including 59 cases of Pneumocystis pneumonia) and lung involvement from the underlying disease (n = 80). At randomization, 32 patients (4.1%) had do not intubate/do not resuscitate orders in place (16 in each group) (Table 1). In addition, 37 patients (4.8%) who did not have do not intubate/do not resuscitate orders in place at randomization acquired this status during the ICU stay (20 in the high-flow oxygen therapy group, 17 in the standard oxygen therapy group).
Interventions
All patients in the intervention group received continuous high-flow oxygen therapy starting immediately after randomization, with an oxygen flow of 50 L/min or greater and Fio2 of 100%. Intolerance required switching from high-flow oxygen therapy to a Venturi mask in 12 patients (3%), of whom 3 died. In the standard oxygen therapy group, median oxygen flow was 10 (IQR, 6-15) L/min through a thin nasal cannula (29.5%), mask with no bag (23.5%), mask with a bag (40.6%), or Venturi mask (6.4%). Of the 30 patients (7.7%) in the standard oxygen therapy group with do-not-intubate orders who were switched to high-flow oxygen therapy after failure of standard oxygen therapy, 14 (46.7%) died.
Interim Analysis
Interim analysis, performed as planned after 100 deaths, yielded a P value of .94; the trial was therefore continued.
Primary Outcome
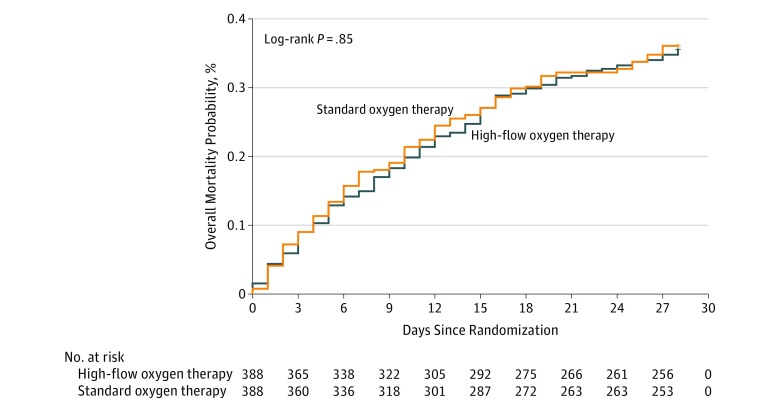
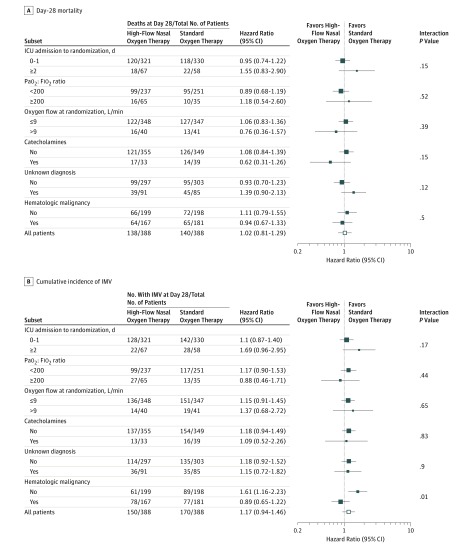
By day 28 after randomization, 138 of 388 patients (35.6%) randomized to high-flow oxygen therapy and 140 of 388 patients (36.1%) randomized to standard oxygen therapy had died, and day-28 mortality was not significantly different between groups (risk difference, −0.5% [95% CI, −7.3% to +6.3%]; HR, 0.98 [95% CI, 0.77-1.24]; P = .94) (Table 2 and Figure 2). There was no significant interaction between the intervention effect and the 3 predefined subgroups (Figure 3).
Table 2. Primary and Secondary End Pointsa.
| End Points | No. (%) | Mean Difference, % (95% CI)b | Relative Difference (95% CI) | P Value | |
|---|---|---|---|---|---|
| High-Flow Oxygen Therapy (n = 388) | Standard Oxygen Therapy (n = 388) | ||||
| Primary | |||||
| All-cause day-28 mortality | 138 (35.6) | 140 (36.1) | −0.5 (−7.3 to 6.3) | HR, 0.98 (0.77 to 1.24) | .94 |
| Secondary | |||||
| Invasive mechanical ventilationc | 150 (38.7) | 170 (43.8) | −5.1 (−12.3 to 2.0) | HR, 0.85 (0.68 to 1.06)d | .17 |
| ICU-acquired infection | 39 (10.0) | 41 (10.6) | −0.6 (−4.6 to 4.1) | HR, 1.01 (0.96 to 1.06)d | .91 |
| ICU mortality | 123 (31.7) | 122 (31.4) | 0.3 (−6.3 to 6.8) | RR, 1.01 (0.82 to 1.24) | .64 |
| Hospital mortality | 160 (41.2) | 162 (41.7) | −0.5 (−7.5 to 6.4) | RR, 0.99 (0.84 to 1.17) | .77 |
| Length of stay, median (IQR), d | |||||
| ICU | 8 (4-14) | 6 (4-13) | 0.6 (−1.0 to 2.2) | NAe | .07 |
| Hospital | 24 (14-40) | 27 (15-42) | −2 (−7.3 to 3.3) | NAe | .60 |
Abbreviations: HR, hazard ratio; ICU, intensive care unit; IQR, interquartile range; NA, not available; RR, relative risk.
No patients were lost to follow-up.
Mean difference was defined across intervention and controls groups by absolute risk difference for binary outcomes (mortality, invasive mechanical ventilation, infections) and difference in means for quantitative outcomes (lengths of stay in ICU and in hospital).
The use of invasive mechanical ventilation was based on the clinical response to oxygen or noninvasive ventilation, clinical status (including oxygen saturation by pulse oximetry [Spo2], respiratory rate, signs of respiratory distress, and bronchial secretion volume), and patient adherence to noninvasive ventilation. Criteria for invasive mechanical ventilation were severe hemodynamic instability (requiring norepinephrine or epinephrine >0.3 μg/kg/min) or cardiorespiratory arrest or ongoing myocardial infarction, severe encephalopathy (Glasgow Coma Scale score <11), severe airway secretion retention or worsening of respiratory distress (SpO2 <92% or respiratory rate >40/min regardless of oxygen flow rate or use of accessory respiratory muscles), inability to maintain Pao2 greater than 65 mm Hg with fraction of inspired oxygen (Fio2) greater than 0.6 or dependency on noninvasive ventilation with inability to remain off noninvasive ventilation for longer than 2 hours, greater than 50% increase in the time on noninvasive ventilation from one day to the next (eg, 6 hours of noninvasive ventilation on day 1, then >9 hours on day 2).
Cause-specific HR.
Effect of high-flow oxygen therapy on length-of-stay measures could not be expressed by HRs.
Figure 2. Probability of Day-28 Mortality in Immunocompromised Patients With Acute Respiratory Failure Receiving High-Flow Oxygen Therapy or Standard Oxygen Therapy.
Median survival was not reached in either group.
Figure 3. Hazard Ratios for Day-28 Mortality (Primary Outcome) and Cumulative Incidence of Mechanical Ventilation, Overall and in Predefined Subgroups, in Immunocompromised Patients With Acute Respiratory Failure Receiving High-Flow Oxygen Therapy or Standard Oxygen Therapy.
Square sides of data markers are proportional to subgroup sizes, with the exception of the open squares in “All patients” rows. Error bars indicate 95% confidence intervals. The Gail and Simon test for interaction was used.
Secondary Outcomes
Need for IMV was not significantly different between groups, required in 150 patients (38.7%) receiving high-flow oxygen therapy and 170 patients (43.8%) receiving standard oxygen therapy (absolute risk difference, −5.1% [95% CI, −12.3% to +2.0%]; cause-specific HR, 0.85 [95% CI, 0.68 to 1.06]; P = .17). The cumulative incidence of intubation was not significantly different between groups (eFigure 1 in Supplement 2). With high-flow oxygen therapy vs standard oxygen therapy, the respiratory rate was significantly lower after 6 hours (25/min vs 26/min; mean difference, −1.8 [95% CI, −3.2 to −0.3]), and Pao2:Fio2 ratio was significantly higher until day 4 (150 vs 119; mean difference, 19.5 [95% CI, 4.4 to 34.6]) (eFigure 2 and eTable in Supplement 2). Comfort and dyspnea scores were not significantly different between groups at any time (eFigure 3 in Supplement 2). There was no significant difference in ICU-acquired infections (10.0% vs 10.6%; absolute risk difference, −0.6% [95% CI, −4.6% to +4.1%]), ICU length of stay (8 vs 6 days; mean difference, 0.6 days[95% CI, −1.0 to +2.2]), or hospital length of stay (24 vs 27 days; mean difference, −2 days [95% CI, −7.3 to +3.3]). None of the other secondary outcomes differed significantly between groups (Table 2).
Post Hoc Outcomes
There was no significant center effect on mortality (P = .33) or intubation rate (P = .07). In the overall population, vasopressors and renal replacement therapy were needed in 153 patients (19.7%) randomized to high-flow oxygen therapy and 31 patients (4%) randomized to standard oxygen therapy, with no statistical difference between groups.
Duration of high-flow oxygen therapy was 2 (IQR, 1-5) days, and all patients were discharged from the ICU with standard oxygen therapy (3 L/min, with no significant difference between groups). In patients who needed IMV, median time from randomization to intubation was 1 (IQR, 0-2) day, and this did not differ significantly between groups (mean difference, −0.5 days [95% CI, −1.2 to 0.1]). Mortality in intubated patients was not significantly different (55.3% with high-flow oxygen therapy vs 52.3% with standard oxygen therapy; absolute risk difference, +3% [95% CI, −8.5% to +14.5%]) (P = .65). Decisions to limit treatment were made for 170 patients (21.9%), of whom 135 (79.4%) died before day 28, with no significant difference between groups. Day-28 mortality was not significantly different in patients with and without cancer as the cause of immunosuppression (absolute risk difference, +1.8% [95% CI, −10.8% to +14.3%]) (P = .50).
Day-90 mortality did not differ significantly between groups (46.9% with high-flow oxygen therapy, 48.2% with standard oxygen therapy).
Discussion
This RCT found no significant survival benefits with high-flow oxygen therapy compared with standard oxygen therapy in immunocompromised patients with AHRF. Neither were significant differences found for intubation requirements, ICU-acquired infections, subjective dyspnea and comfort, or ICU length of stay. These results suggest that attention to oxygenation strategies may not be the best means of improving survival among immunocompromised patients with AHRF.
Improving oxygenation is relevant in all patients with AHRF, but even more so in those who are immunocompromised. These patients are more severely hypoxemic11 and most often require a diagnostic strategy5,21,22 for which high-flow oxygen therapy, which effectively improves oxygenation, can translate into improved outcomes. However, this trial did not find a significantly reduced intubation rate in immunocompromised patients receiving high-flow oxygen therapy. These results agree with those of 2 post hoc analyses showing no significant clinical benefits from high-flow oxygen therapy compared with standard oxygen therapy in immunocompromised patients with AHRF.11,12 Noninvasive ventilation was either neutral8 or harmful11 in that population. Also, both standard oxygen therapy and high-flow oxygen therapy are valid options in immunocompromised patients with AHRF.
Strengths of this trial should be noted. First, to the best of our knowledge, it is the largest trial to date enrolling immunocompromised patients with AHRF. Second, the assumptions made for the sample size estimation were met. Third, the participation of a large number of ICUs in university-affiliated and community hospitals supports external validity. Fourth, the results are consistent with a pilot trial and 2 post hoc studies in smaller numbers of patients.11,12,13
In the current trial, high-flow oxygen therapy compared with standard oxygen therapy failed to decrease the intubation rate, despite producing better oxygenation. Moreover, in agreement with results from a pilot trial in immunocompromised patients, comfort and dyspnea were not improved.13 RCTs in unselected patients with AHRF have produced conflicting results when high-flow oxygen therapy was used for AHRF,23 during intubation,24,25,26 after extubation,27,28,29 or after thoracic surgery.30,31 These apparent contradictions, combined with the lower mortality with high-flow oxygen therapy in 1 trial,10 led to the choice of mortality as the primary end point.
This study has several limitations. First, all participating centers were located in France, raising questions about the general applicability of these findings. Second, the NIV–high-flow oxygen therapy combination was not assessed. However, NIV failed to provide clinical benefits in immunocompromised patients in an RCT,8 and the NIV–high-flow oxygen therapy combination was associated with increased mortality in another RCT.10,11 Third, the lack of blinding may have affected the likelihood of differential treatment or assessments of outcomes. Fourth, a minimal Spo2 of 95% was targeted without having an upper target. However, findings have strongly suggested that a conservative protocol for oxygen therapy vs conventional therapy resulted in lower mortality rates.32,33 Fifth, estimates of treatment effect were not adjusted on stratification factors, and this may have resulted in an overestimation of the P value for the difference between end point rates in treatment groups. Sixth, potential risk of false-positive findings attributable to repeated testing should be taken into account and results of the secondary analyses considered as exploratory.
Conclusions
Among critically ill immunocompromised patients with acute respiratory failure, high-flow oxygen therapy did not significantly decrease day-28 mortality compared with standard oxygen therapy.
Study Protocol and Statistical Analysis Plan
eTable. Oxygenation and Ventilation 6 Hours After Randomization
eFigure 1. Cumulative Incidence of Mechanical Ventilation
eFigure 2. Respiratory Rate and PaO2:FIO2 Ratio
eFigure 3. Patient Comfort and Dyspnea
Data Sharing Statement
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA. 2016;316(23):2547-2548. doi: 10.1001/jama.2016.16477 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 3.Israni AK, Zaun D, Rosendale JD, Schaffhausen C, Snyder JJ, Kasiske BL. OPTN/SRTR 2016 annual data report: deceased organ donation. Am J Transplant. 2018;18(suppl 1):434-463. doi: 10.1111/ajt.14563 [DOI] [PubMed] [Google Scholar]
- 4.Weyand CM, Goronzy JJ. Clinical practice: giant-cell arteritis and polymyalgia rheumatica. N Engl J Med. 2014;371(1):50-57. doi: 10.1056/NEJMcp1214825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azoulay E, Pickkers P, Soares M, et al. ; Efraim Investigators and Nine-I Study Group . Acute hypoxemic respiratory failure in immunocompromised patients: the Efraim multinational prospective cohort study. Intensive Care Med. 2017;43(12):1808-1819. doi: 10.1007/s00134-017-4947-1 [DOI] [PubMed] [Google Scholar]
- 6.Azoulay E, Mokart D, Pène F, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium—a Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique study. J Clin Oncol. 2013;31(22):2810-2818. doi: 10.1200/JCO.2012.47.2365 [DOI] [PubMed] [Google Scholar]
- 7.Canet E, Osman D, Lambert J, et al. Acute respiratory failure in kidney transplant recipients: a multicenter study. Crit Care. 2011;15(2):R91. doi: 10.1186/cc10091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemiale V, Mokart D, Resche-Rigon M, et al. ; Groupe de Recherche en Réanimation Respiratoire du patient d’Onco-Hématologie (GRRR-OH) . Effect of noninvasive ventilation vs oxygen therapy on mortality among immunocompromised patients with acute respiratory failure: a randomized clinical trial. JAMA. 2015;314(16):1711-1719. doi: 10.1001/jama.2015.12402 [DOI] [PubMed] [Google Scholar]
- 9.Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344(7):481-487. doi: 10.1056/NEJM200102153440703 [DOI] [PubMed] [Google Scholar]
- 10.Frat J-P, Thille AW, Mercat A, et al. ; FLORALI Study Group; REVA Network . High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185-2196. doi: 10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 11.Frat J-P, Ragot S, Girault C, et al. ; REVA Network . Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: a post-hoc analysis of a randomised trial. Lancet Respir Med. 2016;4(8):646-652. doi: 10.1016/S2213-2600(16)30093-5 [DOI] [PubMed] [Google Scholar]
- 12.Lemiale V, Resche-Rigon M, Mokart D, et al. High-flow nasal cannula oxygenation in immunocompromised patients with acute hypoxemic respiratory failure: a Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique study. Crit Care Med. 2017;45(3):e274-e280. doi: 10.1097/CCM.0000000000002085 [DOI] [PubMed] [Google Scholar]
- 13.Lemiale V, Mokart D, Mayaux J, et al. The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: a multicenter randomized trial. Crit Care. 2015;19:380. doi: 10.1186/s13054-015-1097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azoulay E, Lemiale V, Mokart D, et al. High-flow nasal oxygen vs. standard oxygen therapy in immunocompromised patients with acute respiratory failure: study protocol for a randomized controlled trial. Trials. 2018;19(1):157. doi: 10.1186/s13063-018-2492-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azoulay E, Pène F, Darmon M, et al. ; Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique (GRR-OH) . Managing critically Ill hematology patients: time to think differently. Blood Rev. 2015;29(6):359-367. doi: 10.1016/j.blre.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 16.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group . Noninvasive ventilation of patients with acute respiratory distress syndrome: insights from the LUNG SAFE study. Am J Respir Crit Care Med. 2017;195(1):67-77. doi: 10.1164/rccm.201606-1306OC [DOI] [PubMed] [Google Scholar]
- 17.Fan E, Del Sorbo L, Goligher EC, et al. ; American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine . An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253-1263. doi: 10.1164/rccm.201703-0548ST [DOI] [PubMed] [Google Scholar]
- 18.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 19.Ruan PK, Gray RJ. Analyses of cumulative incidence functions via non-parametric multiple imputation. Stat Med. 2008;27(27):5709-5724. doi: 10.1002/sim.3402 [DOI] [PubMed] [Google Scholar]
- 20.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957-2963. doi: 10.1001/jama.1993.03510240069035 [DOI] [PubMed] [Google Scholar]
- 21.Azoulay E, Thiéry G, Chevret S, et al. The prognosis of acute respiratory failure in critically ill cancer patients. Medicine (Baltimore). 2004;83(6):360-370. doi: 10.1097/01.md.0000145370.63676.fb [DOI] [PubMed] [Google Scholar]
- 22.Azoulay E, Mokart D, Lambert J, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med. 2010;182(8):1038-1046. doi: 10.1164/rccm.201001-0018OC [DOI] [PubMed] [Google Scholar]
- 23.Jones PG, Kamona S, Doran O, Sawtell F, Wilsher M. Randomized controlled trial of humidified high-flow nasal oxygen for acute respiratory distress in the emergency department: the HOT-ER study. Respir Care. 2016;61(3):291-299. doi: 10.4187/respcare.04252 [DOI] [PubMed] [Google Scholar]
- 24.Vourc’h M, Asfar P, Volteau C, et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med. 2015;41(9):1538-1548. doi: 10.1007/s00134-015-3796-z [DOI] [PubMed] [Google Scholar]
- 25.Jaber S, Monnin M, Girard M, et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial. Intensive Care Med. 2016;42(12):1877-1887. doi: 10.1007/s00134-016-4588-9 [DOI] [PubMed] [Google Scholar]
- 26.Simon M, Wachs C, Braune S, de Heer G, Frings D, Kluge S. High-flow nasal cannula versus bag-valve-mask for preoxygenation before intubation in subjects with hypoxemic respiratory failure. Respir Care. 2016;61(9):1160-1167. doi: 10.4187/respcare.04413 [DOI] [PubMed] [Google Scholar]
- 27.Hernández G, Vaquero C, González P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354-1361. doi: 10.1001/jama.2016.2711 [DOI] [PubMed] [Google Scholar]
- 28.Futier E, Paugam-Burtz C, Godet T, et al. ; OPERA Study Investigators . Effect of early postextubation high-flow nasal cannula vs conventional oxygen therapy on hypoxaemia in patients after major abdominal surgery: a French multicentre randomised controlled trial (OPERA). Intensive Care Med. 2016;42(12):1888-1898. doi: 10.1007/s00134-016-4594-y [DOI] [PubMed] [Google Scholar]
- 29.Corley A, Bull T, Spooner AJ, Barnett AG, Fraser JF. Direct extubation onto high-flow nasal cannulae post-cardiac surgery versus standard treatment in patients with a BMI ≥30: a randomised controlled trial. Intensive Care Med. 2015;41(5):887-894. doi: 10.1007/s00134-015-3765-6 [DOI] [PubMed] [Google Scholar]
- 30.Brainard J, Scott BK, Sullivan BL, et al. Heated humidified high-flow nasal cannula oxygen after thoracic surgery—a randomized prospective clinical pilot trial. J Crit Care. 2017;40:225-228. doi: 10.1016/j.jcrc.2017.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stéphan F, Barrucand B, Petit P, et al. ; BiPOP Study Group . High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. 2015;313(23):2331-2339. doi: 10.1001/jama.2015.5213 [DOI] [PubMed] [Google Scholar]
- 32.Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the Oxygen-ICU randomized clinical trial. JAMA. 2016;316(15):1583-1589. doi: 10.1001/jama.2016.11993 [DOI] [PubMed] [Google Scholar]
- 33.Chu DK, Kim LH-Y, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. doi: 10.1016/S0140-6736(18)30479-3 [DOI] [PubMed] [Google Scholar]
- 34.Vincent JL, Moreno R, Takala J, et al. ; Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine . The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol and Statistical Analysis Plan
eTable. Oxygenation and Ventilation 6 Hours After Randomization
eFigure 1. Cumulative Incidence of Mechanical Ventilation
eFigure 2. Respiratory Rate and PaO2:FIO2 Ratio
eFigure 3. Patient Comfort and Dyspnea
Data Sharing Statement