Abstract
Objective: To investigate the effect of ultrasound in the treatment of osteoarthritis of the knee.
Methods: Eighty‐seven out‐ and in‐patients with osteoarthritis of the knee (15 men and 72 women) underwent ultrasonic therapy from February to October 2010. The patients were randomly assigned to an ultrasound group (Group A) and a placebo group (Group B). Group A was offered ultrasonic therapy while Group B underwent mock treatment. The visual analogue scale (VAS) and Lequesne scores of the two groups before and after treatment were compared. The data were analyzed by normal distribution, Student's t‐test and the rank sum test. The means during and after treatment of the VAS and Lequesne efficacy index of the treatment group were calculated and plotted on a bar graph.
Results: Single sample analysis of Groups A and B showed VAS efficacy index: mean = 0.3640, P= 0.000; Lequesne efficacy index, median = 0.3080, P= 0.000, and mean = 0.1000, P= 0.000; Lequesne efficacy index, mean = 0.0364, P= 0.024, respectively. Independent samples t test and rank sum test showed significant differences between the two groups, namely P= 0.000 for both the VAS and Lequesne curative effect indexes. The means of the VAS efficacy index of the treatment group increased during and after treatment. The means of the Lequesne efficacy index of the treatment group showed no apparent decrease by 28 days after treatment.
Conclusion: Ultrasound treatment significantly alleviates joint symptoms, relieving joint swelling, increasing joint mobility and reducing inflammation, in osteoarthritis patients.
Keywords: Knee, Osteoarthritis, Sonication
Introduction
Osteoarthritis (OA) is a joint disease caused by long‐term wear, which damages articular cartilage and causes intra‐articular fracture and intra‐articular loose bodies, resulting in joint fibrosis, pain and dysfunction. It is characterized by cartilaginous changes, bone hypertrophy and osteophyte formation. It is the commonest chronic progressive joint disease, having a serious impact on the quality of life of patients. Current clinical methods for the treatment of OA are diverse, but both drug therapy and surgical treatment have shortcomings and limitations. Ultrasound, a new treatment method developed in recent years, has been proved to promote repair of full‐thickness articular cartilage defects, result in formation of hyaline cartilage‐like repair tissue at the site of the defect 1 , soften and dissipate condensed fibrous connective tissue and delay the progress of early OA 2 . This study aimed to explore the clinical effects of ultrasound in the treatment of osteoarthritis of the knee (KOA).
Materials and methods
General data
Eighty‐seven out‐ and in‐patients with KOA were studied, including 100 knees in total. All diagnoses were made in accordance with the KOA criteria recommended by the 1995 American College of Rheumatology (ACR) 3 . There were 15 male and 72 female patients with an average of 58.3 years (range, 38–81 years). The course of disease was from 2 months to 20 years with an average of 2.8 years. All patients gave informed consent and attended for regular follow‐up. The research was approved by the Ethics Committee of our institution. Fifty knees were randomly assigned to the ultrasound group (Group A) and the other 50 to a placebo group (Group B). The ultrasound therapeutic equipment, also known as ultrasound therapeutic equipment for arthritis, was provided by the National Engineering Research Center of Ultrasound Medicine (NERCUM, Chongqing, China).
Inclusion and exclusion criteria
The inclusion criteria were as follows: (i) meeting the diagnostic criteria for KOA, the chief features being pain and movement disorder; (ii) local pain with a visual analog score (VAS) of more than three points; (ii) more than 38 years old; and (iv) stage I‐III according to the X‐ray films.
The exclusion criteria were as follows: (i) failure to meet any one of the above inclusion criteria; (ii) skin ulceration, infection, bullous lesions, and other skin conditions; (iii) major scarring as a result of previous surgery or trauma; (iv) disorders of speech or hearing or those who did not comply with the therapy; (v) “halo‐pin”; (vi) other cases that clinicians considered unsuitable for the study. Patients were excluded if they met any one of these criteria.
Treatment
Ultrasound or mock treatment was performed as follows. The patient's history was taken, a medical report form completed; the eyes of the knee (on the level of the lower border of the patella, lateral and medial to the patellar ligament), interior and lateral knee joint space marked; appropriate parameters set on the ultrasound equipment; about 2 mL of coupling agent smeared onto each head of the ultrasound treatment equipment; the heads fixed to the eyes of the knee and the knee joint space, as shown in Fig. 1; the elastic band adjusted; and ensuring that the equipment head was firmly applied to the skin and would not slip. Patients in group A were treated with the treatment model for 15 minutes and then switched over to the rehabilitation model for 20 minutes. For patients in group B only the mode was changed and they received no treatment.
Figure 1.
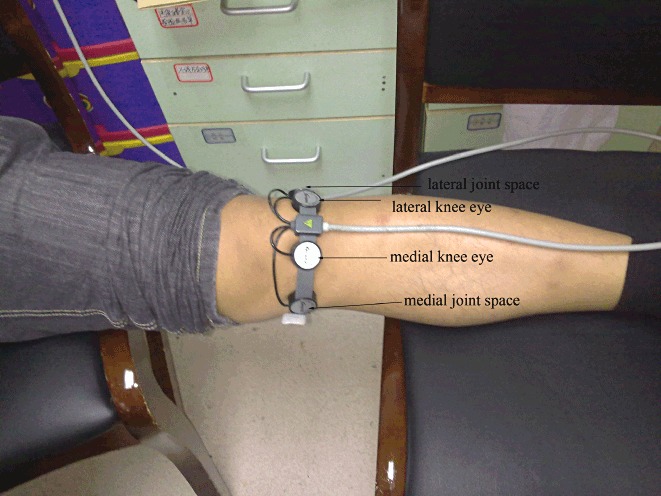
Procedure for use of therapeutic ultrasound equipment: the knee eyes, interior and lateral knee joint spaces have been marked and the heads fixed to the knee eyes and the knee joint space.
Clinical assessment
The VAS and Lequesne scores were recorded before treatment, during treatment and at four weeks after treatment to obtain the curative effect index according to the following equation.
VAS curative effect index= (VAS score before treatment−VAS score after treatment)/(VAS score before treatment) × 100%
The Lequesne curative effect index was calculated as follows.
Lequesne curative effect index= (total score before treatment−total score after treatment)/pre‐treatment total score× 100%
Both indexes were used to assess the effectiveness of the treatment. After the treatment had been completed, the patients were closely followed up to monitor the progress of the disease and evaluate the effects of the treatment.
Statistical analysis
SPSS 17.0 statistical software was used. Statistical significance was set at P < 0.05. Normal analysis of the two groups, single sample t test of the efficacy index of the control group and completely independent samples t test and rank sum test were used to compare and analyze any differences between the treatment and control groups.
Results
Treatment
Over the course of the treatment, no patients had adverse reactions such as skin irritation, blisters and so on. Three patients complained of mental stress, dizziness, palpitation, or fatigue. Their treatment was stopped immediately, they were asked to lie down, given oxygen, oral glucose water and so on and their symptoms resolved quickly.
Normal analysis of the treatment group (group A)
After the fifth treatment session the VAS and Lesquesne effect indexes for group A were analyzed for their Wishart (W) distributions. According to this method of checking distribution, the VAS curative effect index yielded W = 0.958, P= 0.071, and the Lequesne curative effect index W = 0.959, P= 0.080, verifying that both of them had a normal distribution.
Normal analysis of the placebo group (group B)
After the fifth treatment session the curative and Lequesne effect indexes for group B were analyzed for their W distributions. According to this method of checking distribution, the VAS curative effect index yielded W = 0.955, P= 0.053, and the Lequesne curative effect index W = 0.954, P= 0.051, verifying that both of them had a normal distribution.
Single sample t test of the efficacy index in the treatment group
Single sample t test of the efficacy index of the treatment group (VAS efficacy index, mean = 0.3640, SD = 0.28062, P= 0.000; Lequesne efficacy index, mean = 0.3080, SD = 0.42076, P= 0.000) showed that both the VAS and Lequesne efficacy indexes of group A after treatment were statistically significantly different from the pre‐treatment scores, indicating that the treatment was effective.
Single sample t test of the efficacy index in the control group
Single sample t test of the efficacy index of group B (VAS efficacy index, M = 0.1000, SD = 0.18729, P= 0.000; Lequesne efficacy index, M = 0.0364, SD = 0.11082, P= 0.024) showed that both the VAS and Lequesne efficacy indexes of the control group after the mock treatment were statistically significantly different from the pre‐treatment scores, indicating that the treatment was effective.
Completely independent samples t test and rank sum test comparing effectiveness between the treatment and placebo groups
The VAS efficacy indexes of the treatment and control groups after treatment were compared. As is shown in Table 1, Levene's test showed heterogeneity of variance (P= 0.003) and t test showed there was a statistically significant difference between the two groups (P= 0.000). The rank sum test also showed a statistically significant difference (P= 0.000). This is also evident from the mean of the treatment group being 0.364 whereas that of the control group was 0.1000. Accordingly it was concluded that the efficacy of treatment was better than that of placebo (mock treatment) when the VAS efficacy indexes weres used as the evaluation criterion.
Table 1.
Analysis of differences in the VAS curative effect index between the two groups after treatment
| Items | Levene's test | t test | Mean | Rank sum test | |
|---|---|---|---|---|---|
| P | P | Group A | Group B | P | |
| Equal variance | 0.003 | 0.000 | 0.3640 | 0.1000 | 0.000 |
| Unequal variance | — | 0.000 | |||
The Lequesne efficacy indexes of the treatment and placebo groups after treatment were also compared. As is shown in Table 2, Levene's test showed heterogeneity of variance (P= 0.000) and t test showed a statistically significant difference between the means of the two groups (P= 0.000). The rank sum test also showed a statistically significant difference (P= 0.001). This was also evident from the mean of the treatment group being 0.3080 while that of the control group was 0.0364. Accordingly it was concluded that the efficacy of treatment was better than that of the mock treatment when the Lequesne efficacy indexes were used as the evaluation criteria.
Table 2.
Analysis of differences in the Lequesne curative effect index between the two groups after treatment
| Items | Levene's test | t test | Mean | Rank sum test | |
|---|---|---|---|---|---|
| P | P | Group A | Group B | P | |
| Equal variance | 0.000 | 0.000 | 0.3080 | 0.0364 | 0.001 |
| Unequal variance | — | 0.000 | |||
Change in means of the efficacy index in the treatment group
The means of the VAS efficacy index of the treatment group during (1–5 days) and after the treatment (3, 7, 14, and 28 days) were calculated and plotted on a bar graph. As shown in Fig. 2, the symptom of pain was significantly relieved during the treatment. The efficacy was still evident 28 days after treatment.
Figure 2.

Average VAS curative effect index during and after treatment. The symptom of pain was significantly relieved during treatment and the therapeutic effect continued to gradually improve up until 28 days after treatment.
The means of the Lequesne efficacy index of the treatment group after the treatment (1, 3, 7, 14, and 28 days) were calculated and plotted on a bar graph. As shown in Fig. 3, the efficacy did not appear to decline within 28 days after treatment.
Figure 3.

Average Lequesne curative effect index after treatment. There was no obvious decline in efficacy over the 28 day follow‐up period.
Follow‐up
All patients were followed up for about one month. We went to their homes or they came to our hospital after the treatment (1, 3, 7, 14, and 28 days) to record their VAS and Lequesne scores. The VAS (visual analogue scale) score represents the degree of pain, zero being no pain and ten the most severe pain. The patients indicated their scores on the VAS themselves according to the pain they were experiencing. The Lequesne score criterion includes six indicators, namely rest pain, pain on movement, tenderness, swelling, morning stiffness, and walking ability. We assessed the scores (0–3) for each indicator, zero representing normal and three the worst. One patient from the treatment group and two from the control group took oral analgesics during follow‐up, but the amount of medication they took was small, and this was not statistically significant.
Discussion
Osteoarthritis of the knee
Osteoarthritis of the knee is a disorder in which there is bone and cartilage degeneration, articular cartilage degeneration including fibrosis, cracks, ulcers and even loss of the full thickness of the articular cartilage 1 . KOA not only affects the articular cartilage, but also involves the entire joint including the subchondral bone, ligaments, joint capsule, synovial membrane and periarticular muscles. The major clinical symptoms are knee joint pain and instability 2 . Most people of 65 years or older have varying symptoms of OA, such as pain, joint dysfunction, loss of working ability or total disability. At present, the main treatment is non‐surgical and the goal of rehabilitation is to relieve pain, and reduce further strain and the degree of the disability.
The mechanism of ultrasound treatment
Ultrasound is a newly‐emerging rehabilitation treatment. Its effects include the following: mechanical effects, cavitation, thermal effects and chemical effects. Ultrasound can promote or accelerate certain chemical reactions. When applied to articular cartilage, joint capsules and synovial membranes, it can change the membrane potential, enhancing the permeability of ions and colloids; promote blood circulation; soften tissue; stimulate cells; accelerate chemical reactions; enhance metabolism; affect enzyme function and content of biologically active substances; change the pH; decrease the excitability of sensory nerves; increase the pain threshold, and thus achieve a therapeutic effect. It has been proved that ultrasound can promote the repair of defects in full‐thickness articular cartilage and result in formation of hyaline cartilage‐like tissue at the the sites of defects 3 , 4 . In elderly patients, ultrasound applied during the consolidation phase of distraction osteogenesis accelerates callus maturation after opening‐wedge high tibial osteotomy by hemicallotasis 5 .
Research has suggested that ultrasound is likely to increase type II collagen synthesis in articular cartilage via activation of chondrocytes and induction of type II collagen mRNA expression 6 . Ultrasound stimulates chondrocyte proliferation and matrix production in chondrocytes of human articular cartilage in vitro. Therefore, ultrasound might provide a feasible tool for cartilage tissue repair in osteoarthritic patients 7 .
Matrix metalloproteinase‐9 (MMP‐9), a glycosylated protease secreted by stromal cells 8 , has the specific capabilities of degrading and causing degeneration in type I, II and III collagen proteins. MMP‐9, the amount of which increases with worsening of osteoarthritis, is an important biological indicator of osteoarthritis. Ultrasound can enhance cell metabolism 9 , promote cartilage matrix components synthesis such as proteoglycan, chondroitin sulfate 10 , inhibit the production of cartilage degrading enzymes, and reduce cartilage damage and degradation of the cartilage matrix. It can therefore promote cartilage repair, improve joint mobility, relieve joint pain and slow the pathological changes of osteoarthritis. Studies have shown that ultrasound may increase intra‐articular type II collagen synthesis 6 and stimulate cartilage cell proliferation 7 .
The knee joint fluid of patients with OA contains large amounts of cytokines, among which interleukin‐1 (IL‐1) is representative. IL‐1 is mainly involved in increasing the expression of endothelial cell adhesion molecule receptor and promoting synthesis of collagenase and other neutral proteases. It thereby enhances the inflammatory response within the synovium and synovial fluid, and promotes the proliferation of synovial cells. In the normal state, the amount of IL‐1 maintains a balance. When that balance is disturbed, it plays an important role in causing destruction of bone and cartilage and inflammation of synovial fluid. In KOA patients, the amount of IL‐1 is greater than the normal, the amount being positively correlated with the severity of KOA. Increased amounts of IL‐1 are closely correlated with synovitis and articular cartilage matrix degradation and interfere with the function of chondrocytes 11 . Ultrasound promotes the diffusion of small molecules in the blood into the synovial fluid, and can probably increase the exudation of synovial fluid 12 , degrade or eliminate inflammatory mediators and other transmitters, and reduce the production and release of inflammatory mediators 13 .
Clinical research on ultrasound
Ultrasound is a safe and effective treatment modality for achieving pain relief and improvement of function in patients with KOA 14 , 15 . Further research is required to investigate its long‐term efficacy 16 . Köybaşi et al. recommended using therapeutic ultrasound in the management of hip osteoarthritis, and believed that large clinical trials on ultrasound were necessary in order to standardize the treatment modality in this patient group 17 . Because the extent to which ultrasound produces pain relief and functional improvement in KOA patients is still uncertain, further study is needed 18 .
Ultrasound is not effective in the absence of sufficient functional chondrocytes. In the later stages of OA, chondrocytes gradually degenerate and undergo apoptosis, resulting in a reduction in the number of number of functional cartilage cells. Two studies of osteoarthritis induced in animals have suggested that ultrasound can enhance cartilage repair in early osteoarthritis 19 , 20 . Therefore the effect of ultrasound would be significantly greater in patients with early osteoarthritis than in those with severe osteoarthritis 21 , 22 .
Conclusion
This study suggests that ultrasound is beneficial for KOA, as assessed by evaluation indexes, such as those derived from VAS and Lequesne scores. The effect of mock treatment on the placebo group was statistically significant, suggesting that psychological factors may play a role. The treatment group had better results than the control group and this difference was statistically different, showing that ultrasound has a clear therapeutic effect on KOA. However, the target and mechanism of the beneficial effects of ultrasound on OA are still not entirely clear, and require further study. An important direction for future research is elucidation of the biologic impact of various ultrasound waveform variables, such as the peak pulse intensity 23 , 24 .
Disclosure
No benefits in any form have been, or will be, received from a commercial party related directly or indirectly to the subject of this manuscript.
Acknowledgments
Support from the Second Affiliated Hospital of Chongqing Medical University and of the National Engineering Research Center of Ultrasound Medicine is gratefully acknowledged.
References
- 1. Zhang HB. Gene treatment of osteoarthritis. Zhongguo Lin Chuang Kang Fu, 2003, 7: 4390–4391. [Google Scholar]
- 2. Cook SD, Salkeld SL, Popich‐Patron LS, et al Improved cartilage repair after treatment with low‐intensity pulsed ultrasound. Clin Orthop Relat Res, 2001, 391 (Suppl. 1): S231–S243. [DOI] [PubMed] [Google Scholar]
- 3. Altman RD. The classification of osteoarthritis. J Rheumatol Suppl, 1995, 43: 42–43. [PubMed] [Google Scholar]
- 4. Jia XL, Chen WZ, Zhou K, et al Effects of low‐intensity pulsed ultrasound in repairing injured articular cartilage. Chin J Traumatol, 2005, 8: 175–178. [PubMed] [Google Scholar]
- 5. Tsumaki N, Kakiuchi M, Sasaki J, et al Low‐intensity pulsed ultrasound accelerates maturation of callus in patients treated with opening‐wedge high tibial osteotomy by hemicallotasis. J Bone Joint Surg Am, 2004, 86: 2399–2405. [DOI] [PubMed] [Google Scholar]
- 6. Naito K, Watari T, Muta T, et al Low‐intensity pulsed ultrasound (LIPUS) increases the articular cartilage type II collagen in a rat osteoarthritis model. J Orthop Res, 2010, 28: 361–369. [DOI] [PubMed] [Google Scholar]
- 7. Korstjens CM, van der Rijt RH, Albers GH, et al Low‐intensity pulsed ultrasound affects human articular chondrocytes in vitro . Med Biol Eng Comput, 2008, 46: 1263–1270. [DOI] [PubMed] [Google Scholar]
- 8. Fujiki M, Shineha J, Yamanokuchi K, et al Effects of treatment with polysulfated glycosaminoglycan on serum cartilage oligomeric matrix protein and C‐reactive protein concentrations, serum matrix metalloproteinase‐2 and ‐9 activities, and lameness in dogs with osteoarthritis. Am J Vet Res, 2007, 68: 827–833. [DOI] [PubMed] [Google Scholar]
- 9. Warden SJ, Favaloro JM, Bennell KL, et al Low‐intensity pulsed ultrasound stimulates a bone‐forming response in UMR‐106 cells. Biochem Biophys Res Commun, 2001, 286: 443–450. [DOI] [PubMed] [Google Scholar]
- 10. Nishikori T, Ochi M, Uchio Y, et al Effects of low‐intensity pulsed ultrasound on proliferation and chondroitin sulfate synthesis of cultured chondrocytes embedded in Atelocollagen gel. J Biomed Mater Res, 2002, 59: 201–206. [DOI] [PubMed] [Google Scholar]
- 11. Kaneko S, Satoh T, Chiba J, et al Interleukin‐6 and interleukin‐8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther, 2000, 6: 71–79. [DOI] [PubMed] [Google Scholar]
- 12. Weishaupt D, Schweitzer ME, Rawool NM, et al Indirect MR arthrography of the knee: effects of low‐intensity ultrasound on the diffusion rate of intravenously administered Gd‐DTPA in healthy volunteers. Invest Radiol, 2001, 36: 493–499. [DOI] [PubMed] [Google Scholar]
- 13. Tang J, Huang LK, Li D, et al Efficacy of low‐intensity pulsed ultrasound in the treatment of knee osteoarthritis. J Third Mil Med Univ, 2010, 32: 2562–2564. [Google Scholar]
- 14. Tascioglu F, Kuzgun S, Armagan O, et al Short‐term effectiveness of ultrasound therapy in knee osteoarthritis. J Int Med Res, 2010, 38: 1233–1242. [DOI] [PubMed] [Google Scholar]
- 15. Ozgönenel L, Aytekin E, Durmuşoglu G. A double‐blind trial of clinical effects of therapeutic ultrasound in knee osteoarthritis. Ultrasound Med Biol, 2009, 35: 44–49. [DOI] [PubMed] [Google Scholar]
- 16. Loyola‐Sánchez A, Richardson J, MacIntyre NJ. Efficacy of ultrasound therapy for the management of knee osteoarthritis: a systematic review with meta‐analysis. Osteoarthritis Cartilage, 2010, 18: 1117–1126. [DOI] [PubMed] [Google Scholar]
- 17. Köybaşi M, Borman P, Kocaoğlu S, et al The effect of additional therapeutic ultrasound in patients with primary hip osteoarthritis: a randomized placebo‐controlled study. Clin Rheumatol, 2010, 29: 1387–1394. [DOI] [PubMed] [Google Scholar]
- 18. Rutjes AW, Nüesch E, Sterchi R, et al Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database Syst Rev, 2010, (1): CD003132. [DOI] [PubMed] [Google Scholar]
- 19. Choi BH, Woo JI, Min BH, et al Low‐intensity ultrasound stimulates the viability and matrix gene expression of human articular chondrocytes in alginate bead culture. J Biomed Mater Res A, 2006, 15: 858–864. [DOI] [PubMed] [Google Scholar]
- 20. Min BH, Woo JI, Cho HS, et al Effects of low‐intensity ultrasound (LIUS) stimulation on human cartilage explants. Scand J Rheumatol, 2006, 35: 305–311. [DOI] [PubMed] [Google Scholar]
- 21. Wang XH, Wang H, Yang SH, et al Effect of low intensity pulsed ultrasound in repairing rabbits cartilage injury caused by experimental osteoarthritis. Int J Orthop, 2009, 30: 191–193. [Google Scholar]
- 22. Gurkan I, Ranganathan A, Yang X, et al Modification of osteoarthritis in the guinea pig with pulsed low‐intensity ultrasound treatment. Osteoarthritis Cartilage, 2010, 18: 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Srbely JZ. Ultrasound in the management of osteoarthritis: part I: a review of the current literature. J Can Chiropr Assoc, 2008, 52: 30–37. [PMC free article] [PubMed] [Google Scholar]
- 24. Nyborg WL. Optimization of exposure conditions for medical ultrasound. Ultrasound Med Biol, 1985, 11: 245–260. [DOI] [PubMed] [Google Scholar]


