Abstract
Objective: To investigate the effect of Staphylococcus aureus (S. aureus) on cultured human osteoblast apoptosis and the corresponding mode of action.
Methods: Transmission electron microscopy (TEM), assessment of DNA laddering, and flow cytometry assays were used to investigate human osteoblast apoptosis following infection with S. aureus.
Results: TEM examination and DNA laddering assessment indicated that S. aureus can induce cultured human osteoblast apoptosis. Flow cytometry assays showed that human osteoblast apoptosis occurs in a dose‐dependent manner following infection with S. aureus. In addition, compared with under co‐culture conditions, inhibition of invasion by S. aureus resulted in a 64.62% reduction in the percentage of early apoptotic cells (P < 0.01); 7.09% ± 1.21% of human osteoblasts under indirect co‐culture with S. aureus at a multiplicity of infection of 250 showed an early apoptotic profile compared with uninfected controls(P < 0.01).
Conclusions: S. aureus induces cultured human osteoblast apoptosis in a dose‐dependent manner. Intracellular S. aureus is mainly responsible for cultured human osteoblast apoptosis following infection; secreted soluble factor(s) of S. aureus playing a minor role in this process.
Keywords: Apoptosis, Osteoblasts, Osteomyelitis, Staphylococcus aureus
Introduction
Osteomyelitis is an inflammation of the bone associated with abnormal bone remodeling and massive bone resorption. Staphylococcus aureus (S. aureus) is the most commonly isolated pathogen in osteomyelitis, accounting for approximately 80% of all human cases 1 . S. aureus not only colonizes the bone matrix 2 and stimulates bone resorption through secreting toxin 3 , but also invades osteoblasts, this having been demonstrated in vitro 4 , 5 , in vivo 6 and in clinical patients 7 .
Recent studies have demonstrated that S. aureus‐infected osteoblasts produce proinflammatory cytokines and chemokines to recruit immune cells that can alter the balance between bone resorption and formation 8 . S. aureus‐infected osteoblasts also produce the receptor activator of NF‐kB ligand and prostaglandin E2, which can modulate the activity or formation of osteoclasts 9 , an inducing agent involved in bone destruction. However, the pathogenesis of S. aureus induced osteomyelitis is poorly understood 9 . There is therefore a need to reevaluate the cellular pathophysiology of this disease process.
There is increasing evidence that S. aureus or other intracellular bacteria can induce apoptosis in diverse cells, such as epithelial 10 , endothelial 11 and peritoneal mesothelial cells 12 . It has also been shown that intracellular S. aureus induces apoptosis in mouse osteoblasts 13 and intracellular Salmonella induces osteoblast apoptosis 14 . In addition, S. aureus secretes a large array of soluble virulence factors 15 . However, to date, whether intracellular S. aureus and the secreted soluble factor(s) of S. aureus induce cultured human osteoblast apoptosis has not been clearly established. Therefore, in the present study, we investigated the effect of S. aureus on cultured human osteoblast apoptosis and the corresponding mode of action.
Materials and methods
Bacteria, human osteoblasts and culture conditions
S. aureus UAMS‐1 (ATCC 49230), a strain which was isolated from human osteomyelitis 9 , was used in this study. ATCC 49230 was obtained from the American Type Culture Collection (Rockville, MD, USA). S. aureus cells were grown overnight (16 h) in 5 mL of tryptic soy broth (Oxoid, Basingstoke, UK) in a shaking water bath at 37°C. The bacteria were harvested by centrifugation for 10 min at 4300 g at 4°C and washed twice in 5 mL of phosphate buffered saline (PBS). The pellets were then resuspended in 5 mL of growth medium lacking antibiotics or antimycotics. SV40 human osteoblasts (SV40 hOBs) were obtained from the American Type Culture Collection. These cells had previously been characterized as authentic osteoblasts 16 . The cells were seeded in 25‐cm2 flasks and incubated at 37°C in 5% CO2 with DMEM/F12 (Gibco, Gaithersburg, MD, USA) supplemented with 0.3 mg/mL G418 (Sigma, St Louis, MO, USA) and 10% fetal bovine serum (HyClone, Logan, UT, USA). The cells were then propagated as described by the manufacturer.
Infection procedure
Once the osteoblasts had reached approximately 80% confluency, the cells were trypsinized (0.025% trypsin‐0.01% ethylenediaminetetraacetic acid), washed in medium and seeded into 6 or 12‐well plates (Corning, Cambridge, MA, USA). Two to three hours before the addition of bacteria, the cells were washed twice with PBS and then incubated with an assay medium (growth medium without antibiotics and antimycotics). S. aureus was added at the designated multiplicity of infection (MOI) at designated times. Next, the supernatants were removed and the osteoblasts washed twice with PBS. Finally, a growth medium containing gentamicin (25 µg/mL) (Sigma) was added to kill the remaining extracellular bacteria as described previously 13 .
Transmission electron microscopy for apoptosis
One × 106 cells/well in 6‐well plates were infected with S. aureus at an MOI of 250 for 2 h. After infection, they were incubated for 0, 16 or 20 h, then floating and adherent cells were collected by trypsinization, washed three times with PBS and collected by centrifugation at 1000 g for 10 min. The cell pellets were fixed for 2 h at 4°C in a solution containing 2.5% glutaraldehyde (Sigma) and washed three times with PBS. The pellets were then post‐fixed with osmium tetroxide (Sigma), dehydrated and embedded in epoxy resin (Sigma). Ultrathin sections were collected on 100‐ to 150‐mesh nickel grids (Plano, Wetzlar, Germany), stained with lead citrate and uranyl acetate and then examined by transmission electron microscopy (TEM) (Tecnai‐10; Philips, the Netherlands). Uninfected SV40 hOBs were used as controls with the same wash and addition of gentamicin.
DNA laddering assessment of apoptosis
Two × 106 cells/well in 6‐well plates were infected with S. aureus at an MOI of 250 for 2 h. After infection, they were incubated for 20 h. Detached human osteoblasts were centrifuged at 1000 g for 5 min at 4°C, the pellets washed with ice‐cold PBS and resuspended in a lysis buffer. Adherent cells were lysed directly in the dish and these two samples combined into a single sample. DNA was isolated from the lysates with an Enhanced Apoptotic DNA Ladder Detection Kit (BioVision, San Diego, CA, USA) according to the manufacturer's guidelines and analyzed on an ethidium bromide‐stained 1.5% TreviGel (Genzyme, Cambridge, MA, USA). Uninfected human osteoblasts were used as controls, the same steps being followed.
Flow cytometry assays to detect apoptosis
To further confirm apoptosis in human osteoblasts and determine whether that apoptosis occurred in a dose‐dependent manner, 5 × 104 cells/well in 12‐well plates were infected with S. aureus at MOIs from 25 to 500 for 2 h. Uninfected SV40 hOBs were used as controls, the same steps being followed.
To determine the role of intracellular S. aureus in human osteoblast apoptosis, 5 × 104 cells/well in 12‐well plates were exposed to S. aureus at an MOI of 250 for 2 h in the presence of cytochalasin D (5 µg/mL) (Sigma) added 1 h prior to infection. Cells in the presence of 5 µg/mL cytochalasin D alone were used as controls, the same steps being followed. Cytochalasin D at 5 µg/mL inhibits invasion of S. aureus into human osteoblasts by 99.85% compared to controls 4 .
To determine the role in human osteoblast apoptosis of the soluble factor(s) secreted by S. aureus, 5 × 104 cells/well were grown in the lower compartment of a 12‐well Transwell bicameral chamber (Corning). This chamber contains a 0.4‐µm pore size polycarbonate filter that separated the cells from S. aureus, which was added to the upper chamber at an MOI of 250 for 2 h.
After infection, the cells were incubated for 10 h. Adherent and floating cells were collected by trypsinization and centrifuged at 1000 g for 5 min, washed in cold PBS and incubated for 15 min with Annexin V‐FITC and propidium iodide (PI) according to the manufacturer's instructions (Caltag Laboratories, Burlingame, CA, USA). The cells were then analyzed by FACS Calibur flow cytometry (Beckman Coulter, Fullerton, CA, USA). Cells in the early phases of apoptosis bind with Annexin V but exclude PI, while necrotic cells or cells in the later stages of apoptosis stain simultaneously with Annexin V and PI.
Statistical analysis
All assays were repeated in three independent experiments. Data are presented as mean ± SD. Statistical analysis among groups was performed by analysis of variance and the Student‐Newman‐Keuls test using the SPSS17.0 software package (SPSS, Chicago, IL, USA). Statistically significant values were defined as P < 0.05.
Results
Results of transmission electron microscopy
Morphological changes in human osteoblasts after infection with S. aureus were identified by TEM. Figure 1A shows the normal morphology of human osteoblasts that have not been infected with S. aureus, and Fig. 1B demonstrates that S. aureus invades human osteoblasts. Moreover, as shown in Fig. 1C and D, following infection with S. aureus human osteoblasts manifested features of apoptosis.
Figure 1.
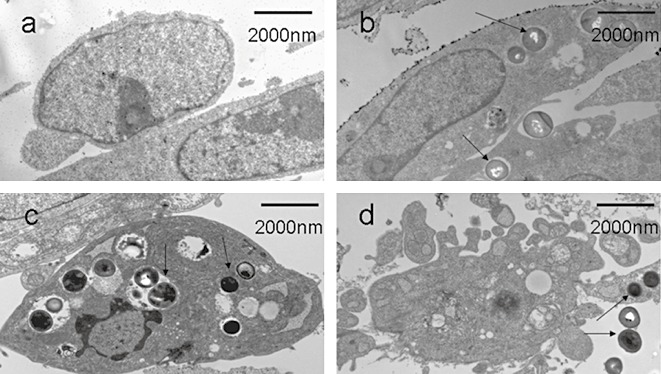
Investigation of apoptosis by TEM. (A) Uninfected human osteoblasts showing normal cellular morphology. (B) S. aureus has invaded human osteoblasts (black arrows). (C, D) Infected human osteoblasts manifesting features of apoptosis: shrunken cells, cytoplasmic concentration and apoptotic bodies (black arrows).
Results of DNA laddering assessment
Internucleosomal DNA fragmentation, a hallmark of apoptotic cells, was examined for the presence of a characteristic 180‐bp laddering pattern on agarose gels (Fig. 2, lane a). As shown in Fig. 2, lane b, DNA extracted from uninfected controls failed to display this laddering. However, DNA from human osteoblasts infected with S. aureus did display the characteristic fragmentation (Fig. 2, lane c).
Figure 2.
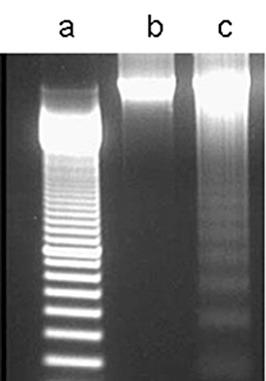
Investigation of apoptosis by DNA laddering electrophoresis. Lane a, 100‐bp standard DNA ladder; lane b, DNA from uninfected human osteoblasts; lane c, DNA from S. aureus‐infected human osteoblasts at an MOI of 250, showing the characteristic ladder of fragmented DNA bands in multiples of 180 bp.
Apoptosis detection by flow cytometry assays
As shown in Fig. 3, human osteoblasts infected with S. aureus demonstrated an apoptotic profile in a dose‐dependent manner at MOIs from 25 to 250. The maximum percentage of early apoptotic cells (annexin V +/PI −) was reached at an MOI of 250 (29.65% ± 1.05%). There were no further increases in the early apoptotic profile (18.58% ± 0.63%), but there were increases in later stages of apoptosis (annexin V +/PI +) (22.86% ± 2.67%) and necrosis (annexin V −/PI +) (data not shown) at an MOI of 500. However, control cells generally exhibited a low percentage of apoptosis (1.32% ± 0.54%).
Figure 3.
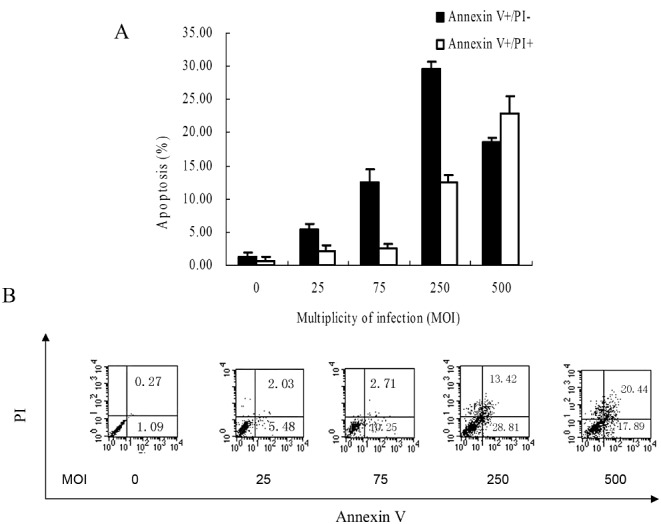
Investigation of apoptosis by flow cytometry with annexin V and PI assay. (A) Human osteoblasts infected with S. aureus demonstrate an apoptotic profile in a dose‐dependent manner at MOIs from 25 to 250. (B) Representative scatter plots are shown.
Comparing the percentage of apoptotic human osteoblasts infected with S. aureus at an MOI of 250 in the presence or absence of 5 µg/mL cytochalasin D by flow cytometry assays, as shown in Fig. 4, the percentage of early apoptotic cells at an MOI of 250 was dramatically reduced by 64.62% (P < 0.01) in the presence of cytochalasin D (10.49% ± 1.32%) compared with absence of cytochalasin D (29.65% ± 1.05%). These results indicate that intracellular S. aureus is mainly responsible for cultured human osteoblasts apoptosis following infection.
Figure 4.
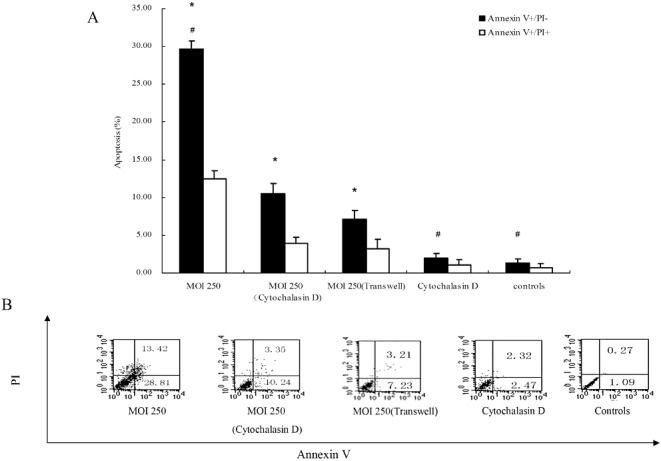
The roles of intracellular S. aureus and the secreted soluble factor(s) of S. aureus on cultured human osteoblasts apoptosis following infection with these bacteria. (A)The percentage of early apoptotic cells at an MOI of 250 is dramatically reduced in the presence of cytochalasin D compared with absence of cytochalasin D. (B)Representatives scatter plots are shown. *P < 0.01, versus controls; #P < 0 01, MOI 250 (cytochalasin D) versus MOI 250, cytochalasin D alone and controls.
In addition, Fig. 4 also clearly demonstrates that, under indirect co‐cultured conditions with the Transwell bicameral chamber, 7.09% ± 1.21% of cells showed an early apoptotic profile, this being significantly different (P < 0.01) than the findings with uninfected controls. This result indicates that soluble bacterial factors secreted by S. aureus may also, to some extent, play a role in apoptosis of cultured human osteoblasts.
Discussion
S. aureus induces apoptosis of diverse cells in a variety of ways. Kahl et al. have reported that S. aureus RN 6390 can induce human epithelial cell apoptosis 10 . They thought that this apoptosis was associated with bacterial invasion and their intracellular replication. It has been shown that S. aureus a‐toxin contributes to the triggering of apoptosis from the intracellular environment, and that cell apoptosis induced by S. aureus appears to be dependent on invasiveness and agr‐ and sigB‐controlled gene products 11 . Furthermore, that intracellular S. aureus induces mouse osteoblast apoptosis 13 and intracellular Salmonella induces osteoblast apoptosis 14 have also been demonstrated.
In the current study, we first demonstrated that S. aureus induces apoptosis of cultured human osteoblasts in a dose‐dependent manner (1, 2, 3). In addition, we observed that intracellular S. aureus not only induces human osteoblast apoptosis, but is also mainly responsible for cultured human osteoblast apoptosis following infection. The above conclusions are based on two findings. First, Fig. 1C and D show that apoptotic human osteoblasts contain S. aureus, suggesting that S. aureus induced human osteoblast apoptosis may occur through invasion of cells. This morphological confirmation that apoptotic cells contain intracellular bacteria was not reported by Tucker et al. 13 Second, as shown in Fig. 4, compared to controls in which cytochalasin D was absent, the percentage of early apoptotic cells at an MOI of 250 was dramatically reduced by 64.62% (P < 0.01) in the presence of cytochalasin D (5 µg/mL), which means that intracellular S. aureus is mainly responsible for apoptosis of cultured human osteoblasts following infection.
Furthermore, we demonstrated for the first time that the soluble bacterial factors secreted by S. aureus can also induce cultured human osteoblast apoptosis (Fig. 4), although these factor(s) are not the main reason for human osteoblast apoptosis following infection with S. aureus. It has been reported that S. aureus secretes a large array of soluble factors, such as a‐toxin, exotoxins, toxic shock syndrome toxin‐1 (TSST‐1) and Panton‐Valentine leukocidin (PVL) 17 . PVL can trigger phagocyte apoptosis 18 ; a‐toxin can induce mesothelial cell apoptosis12 and is required for induction of endothelial cell apoptosis 11 ; whereas TSST‐1 can inhibit monocyte apoptosis 19 and exotoxins have the ability to inhibit eosinophil apoptosis 20 . Thus, the mechanisms by which the secreted soluble factor(s) of S. aureus induced cultured human osteoblast apoptosis in the current experiments may be complex and intricate. Indeed, although they only play a minor role in cultured human osteoblast apoptosis following infection, further work is needed to explore how the secreted soluble factor(s) of S. aureus triggers human osteoblast apoptosis.
In summary, in the present study, we demonstrated that S. aureus induces cultured human osteoblast apoptosis in a dose‐dependent manner. In addition, we revealed that intracellular S. aureus is mainly responsible for cultured human osteoblast apoptosis following infection, while the secreted soluble factor(s) of S. aureus play a minor role in such apoptosis. These finding suggest that the destruction of osteoblasts through apoptosis induced by intracellular S. aureus and its secreted soluble factor(s) might be one reason for the bone loss caused by osteomyelitis.
Disclosure
All authors declared that there have no conflicts of interest.
Acknowledgments
We thank Xiao‐li Lou for her technical assistance. The research was financially supported by the National Natural Science Foundation of China (No. 30470455).
References
- 1. Lew DP, Waldvogel FA. Osteomyelitis. Lancet, 2004, 364: 369–379. [DOI] [PubMed] [Google Scholar]
- 2. Foster TJ, Höök M. Surface protein adhesins of Staphylococcus aureus . Trends Microbiol, 1998, 6: 484–488. [DOI] [PubMed] [Google Scholar]
- 3. Nair S, Song Y, Meghji S, et al Surface‐associated proteins from Staphylococcus aureus demonstrate potent bone resorbing activity. J Bone Miner Res, 1995, 10: 726–734. [DOI] [PubMed] [Google Scholar]
- 4. Ellington JK, Reilly SS, Ramp WK, et al Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb Pathog, 1999, 26: 317–323. [DOI] [PubMed] [Google Scholar]
- 5. Jevon M, Guo C, Ma B, et al Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect Immun, 1999, 67: 2677–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reilly SS, Hudson MC, Kellam JF, et al In vivo internalization of Staphylococcus aureus by embryonic chick osteoblasts. Bone, 2000, 26: 63–70. [DOI] [PubMed] [Google Scholar]
- 7. Bosse MJ, Gruber HE, Ramp WK. Internalization of bacteria by osteoblasts in a patient with recurrent, long‐term osteomyelitis. A case report. J Bone Joint Surg Am, 2005, 87: 1343–1347. [DOI] [PubMed] [Google Scholar]
- 8. Marriott I, Gray DL, Rati DM, et al Osteoblasts produce monocyte chemoattractant protein‐1 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Bone, 2005, 37: 504–512. [DOI] [PubMed] [Google Scholar]
- 9. Somayaji SN, Ritchie S, Sahraei M, et al Staphylococcus aureus induces expression of receptor activator of NF‐kappaB ligand and prostaglandin E2 in infected murine osteoblasts. Infect Immun, 2008, 76: 5120–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kahl BC, Goulian M, van Wamel W, et al Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect Immun, 2000, 68: 5385–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haslinger‐Löffler B, Kahl BC, Grundmeier M, et al Multiple virulence factors are required for Staphylococcus aureus‐induced apoptosis in endothelial cells. Cell Microbiol, 2005, 7: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 12. Haslinger‐Löffler B, Wagner B, Brück M, et al Staphylococcus aureus induces caspase‐independent cell death in human peritoneal mesothelial cells. Kidney Int, 2006, 70: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 13. Tucker KA, Reilly SS, Leslie CS, et al Intracellular Staphylococcus aureus induces apoptosis in mouse osteoblasts. FEMS Microbiol Lett, 2000, 186: 151–156. [DOI] [PubMed] [Google Scholar]
- 14. McCall SH, Sahraei M, Young AB, et al Osteoblasts express NLRP3, a nucleotide‐binding domain and leucine‐rich repeat region containing receptor implicated in bacterially induced cell death. J Bone Miner Res, 2008, 23: 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karahan M, Açik MN, Cetinkaya B. Investigation of toxin genes by polymerase chain reaction in Staphylococcus aureus strains isolated from bovine mastitis in Turkey. Foodborne Pathog Dis, 2009, 6: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 16. Zhu J, Zhang X, Wang C, et al Periprosthetic strain magnitude‐dependent upregulation of type I collagen synthesis in human osteoblasts through an ERK1/2 pathway. Int Orthop, 2009, 33: 1455–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tseng CW, Kyme P, Low J, et al Staphylococcus aureus Panton‐Valentine leukocidin contributes to inflammation and muscle tissue injury. PLoS ONE, 2009, 4: e6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Genestier AL, Michallet MC, Prévost G, et al Staphylococcus aureus Panton‐Valentine leukocidin directly targets mitochondria and induces Bax‐independent apoptosis of human neutrophils. J Clin Invest, 2005, 115: 3117–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bratton DL, May KR, Kailey JM, et al Staphylococcal toxic shock syndrome toxin‐1 inhibits monocyte apoptosis. J Allergy Clin Immunol, 1999, 103 (5 Pt 1):895–900. [DOI] [PubMed] [Google Scholar]
- 20. Wedi B, Wieczorek D, Stünkel T, et al Staphylococcal exotoxins exert proinflammatory effects through inhibition of eosinophil apoptosis, increased surface antigen expression (CD11b, CD45, CD54, and CD69), and enhanced cytokine‐activated oxidative burst, thereby triggering allergic inflammatory reactions. J Allergy Clin Immunol, 2002, 109: 477–484. [DOI] [PubMed] [Google Scholar]


