Abstract
Objective: The purpose of this prospective clinical trial, with a minimum two‐year follow‐up, was to evaluate the clinical effects of a titanium‐coated lumbar interbody fusion system in the treatment of lumbar instability.
Methods: The study cohort consisted of 94 patients with lumbar instability who accepted posterior lumbar interbody fusion with a titanium‐coated fusion system. The patients were examined at the sixth, 12th and 24th month postoperatively. The clinical outcomes of all patients were evaluated according to the Japanese Orthopaedic Association (JOA) score and Oswestry disability index (ODI). Radiological studies, which included assessment of loss of disc space height, intervertebral angle and isodense bone bridging, were used to evaluate the fusion.
Results: The overall fusion rate was 95.75% at the 24th month after surgery. Ninety‐two (97.87%) patients were able to work while 53 patients (56.38%) were capable of performing heavy manual labor. Neurological assessment showed 77 patients (81.92%) had no sensory or motor deficit. The mean JOA score had increased from 15.34 to 28.92 and ODI had decreased from 45 to 15 at the 24th month after surgery. No implant fracture or displacement was found.
Conclusion: The titanium‐coated intervertebral fusion cage is effective and safe for treatment of lumbar instability.
Keywords: Lumbar vertebrae, Spinal fusion, Spinal stenosis
Introduction
Spinal instability is one of the important factors in low back pain and lumbar fusion is a well‐accepted procedure in the correction of spinal stability. Lumbar interbody fusion been widely applied in the treatment of lumbar instability because of its high fusion rate and ability to immediately restore spinal stability. Recent developments in interbody fixation, such as use of a cage, have enriched the possibilities for lumbar interbody fusion. However, subsidence of cages into the vertebral body is a common complication after interbody fusion, especially in older patients with low bone mineral density. In an attempt to avoid these complications, the use of newly designed titanium‐coated fusion cage for the treatment of lumbar instability was studied. In this study, we present the two‐year follow‐up clinical data on 94 patients with lumbar instability treated surgically by posterior lumbar interbody fusion (PLIF) using the titanium‐coated fusion cage.
Materials and methods
The titanium‐coated fusion system
The titanium‐coated implant used for PLIF is a solid titanium block coated with pure titanium powder. The parallel and lordotic shaping of these implants guarantee maximum contact between implant and endplate for minimum implant dimensions. Primary stability is ensured by optimal bone contact and additional posterior stabilization.
Patient selection
The cohort for this study consisted of 94 patients with lumbar instability. There were 36 males and 58 females, with an average age of 54 years (range, 21–71 years). The primary diagnosis was degenerative spinal stenosis in 14 patients, degenerative spondylolisthesis in 42, isthmic spondylolisthesis in 22, degenerative disc disease in 13, and post nucleotomy syndrome in 3.
Inclusion criteria were: (i) clinical manifestations including chronic back pain with repeated acute episodes, sciatica and intermittent claudication and forward bending restricted by ‘unstable locking’ characterized by swaying and jerking movements, all of which symptoms could be relieved by rest; (ii) X‐ray diagnosis: the amount of sagittal rotation between the extremes of movements or the amount of vertebral translation on flexion‐extension (F‐E) radiographs were recorded. Values of 10° for rotation and 3 mm for translation in the lumbar or 20° for rotation and 4 mm for translation in the lumbar‐sacral joint were taken to indicate instability 1 ; (iii) informed consent was obtained from all patients before surgery; (iv) the unstable segments were between L3 and S1, and had proved unresponsive to conservative treatment for at least 6 months.
Exclusion criteria: instability caused by trauma, tumor, tuberculosis, active infection, osteopenia, symptomatic vascular disease, active malignancy, gross obesity and pregnancy.
Surgical technique
All surgeries were performed under general anesthesia. Satisfactory exposure was obtained by proper laminectomy and facetectomy. The pedicle screw instrument was used according to the three‐column spine concept. For patients with spinal stenosis or spondylolisthesis, adequate decompression or reduction was achieved. During preparation of the disc space, the bony endplates were reserved. After distraction of the disc space, two titanium‐coated fusion cages were inserted and the bone removed at laminectomy was grafted post‐laterally. It was ensured that the distance between the cages and posterior wall of the vertebrae was at least 3 mm.
Outcome measures
Data collection consisted of clinical assessment, questionnaires and radiographs.
Fusion
Adequacy of fusion was determined with antero‐posterior and F‐E lateral radiographs. Fusion was considered solid when the range of vertebral movement was less than 3° in the sagittal plane. Lucency >2 mm on more than 50% of the fusion cages surfaces or movement >3° on the F‐E films were considered to constitute fusion failure 2 .
Imaging evaluation
The loss of height of disc space was measured by Mochida's index (Fig. 1). The stability of the fusion segments was assessed by sagittal motion in F‐E lateral radiographs (intervertebral angle). The gap around the cages and isodense bony trabecular bridging in the fusion area was also assessed (2, 3).
Figure 1.
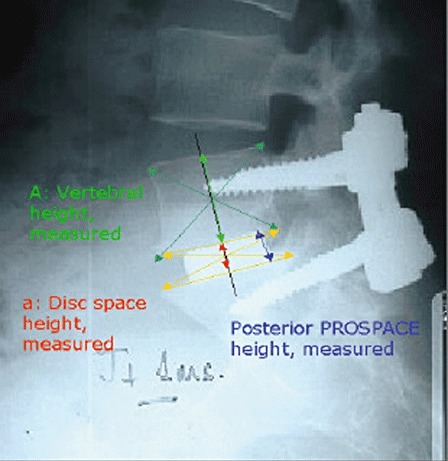
Showing where the values required to calculate Mochida's index are measured.
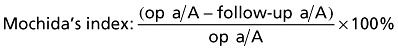 A, vertebral height measured; a, disc space height measured; op, immediately post‐operation.
PROSPACE, name of the fusion cage.
A, vertebral height measured; a, disc space height measured; op, immediately post‐operation.
PROSPACE, name of the fusion cage.
Figure 2.
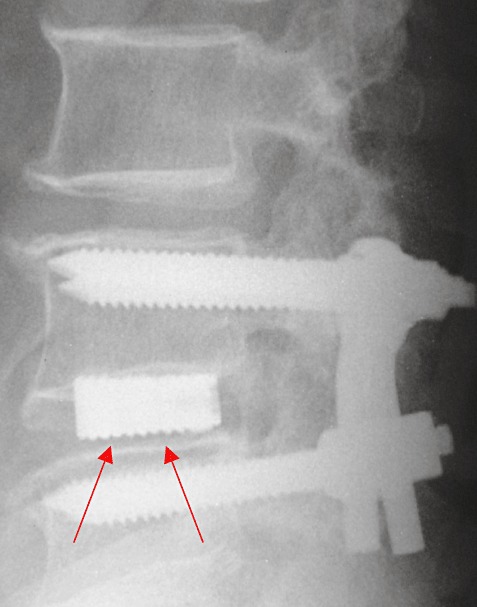
Follow‐up radiograph. The arrows indicate absence of anterior fusion.
Figure 3.
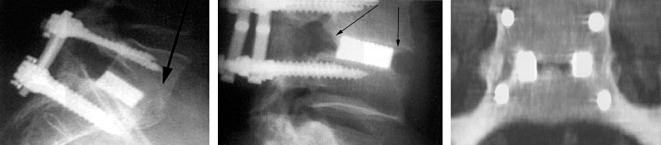
Radiographs in which arrows indicate trabecular bridges anterior, around and between the fusion cages.
Function
The Oswestry disability index (ODI) 3 and JOA score were used to evaluate the patients' clinical symptoms. Return‐to work status was also assessed.
Statistics analysis
The data were analyzed with SPSS 10.0 (SPSS, Chicago, IL, USA) and P < 0.05 was considered to be significant.
Results
All patients underwent single level fusion (7 at L3‐L4, 66 at L4‐L5 and 21 at L5‐S1). The average operation time and estimated blood loss were 159 min and 448 ml, respectively. The average follow‐up time was 744 days (Fig. 4).
Figure 4.
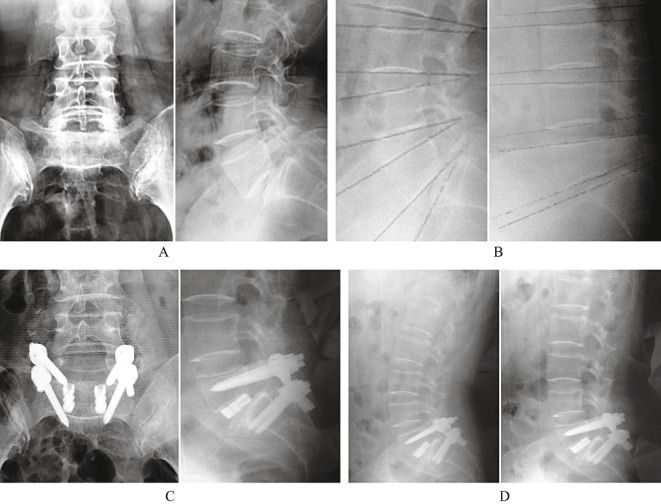
(A) Preoperative radiographs of a 40‐year‐old woman with symptomatic grade II isthmic spondylolisthesis. (B) Preoperative F‐E lateral radiographs show segmental instability in L5S1. (C) Post‐operative radiographs after treatment by PLIF using the titanium‐coated fusion system. (D) F‐E lateral radiograph at 2‐year follow‐up show the fused segment is stable.
Fusion
One‐level procedures were performed in all of the patients and the rate of total fusion was 95.75% (90/94) at the 24th month.
Functional outcomes
Return to work
Table 1 shows a summary of return‐to‐work statistics for all patients at different stages. Before surgery, only 3.19% of the patients were capable of performing heavy manual labor. The percentage had increased to 56.38% at the 24th month after surgery.
Table 1.
Patients' return‐to work status (%)
| Pre‐op | 6 months | 12 months | 24 months | |
|---|---|---|---|---|
| Unable | 15 (15.96%) | 2 (2.13%) | 2 (2.13%) | 2 (2.13%) |
| Able‐sedentary | 36 (38.30%) | 12 (12.77%) | 2 (2.13%) | 2 (2.13%) |
| Able‐light manual | 40 (42.55%) | 60 (63.83%) | 50 (53.19%) | 37 (39.36%) |
| Able‐heavy manual | 3 (3.19%) | 20 (21.27%) | 40 (42.55%) | 53 (56.38%) |
| Total | 94 | 94 | 94 | 94 |
Neurological assessment
Table 2 showed the neurological status of patients at the sixth, 12th and 24th month after surgery.
Table 2.
Patients' clinical symptoms (%)
| Pre‐op | 6 months | 12 months | 24 months | |
|---|---|---|---|---|
| No deficit | 23 (24.47%) | 63 (67.02%) | 69 (73.41%) | 77 (81.92%) |
| Sensory deficit only | 31 (32.98%) | 23 (24.47%) | 19 (20.21%) | 9 (9.57%) |
| Motor deficit only | 8 (8.51%) | 3 (3.19%) | 1 (1.06%) | 1 (1.06%) |
| Sensory and motor deficit | 32 (34.04%) | 5 (5.32%) | 5 (5.32%) | 7 (7.45%) |
| Total | 94 | 94 | 94 | 94 |
JOA Score and ODI
The JOA Score had increased from 15.34 to 28.92 and ODI had decreased from 45 to 15 at the 24th month after surgery (3, 4).
Table 3.
Neurological assessment (JOA score)
| Pre‐op | 6 months | 12 months | 24 months | |
|---|---|---|---|---|
| Mean | 15.34 | 27.23 | 28.52 | 28.92 |
| Standard Deviation | 5.31 | 5.41 | 4.55 | 4.08 |
| Minimum | 1.00 | 5.00 | 11.00 | 18.00 |
| Maximum | 31.00 | 33.00 | 33.00 | 33.00 |
| Total | 94 | 94 | 94 | 94 |
Table 4.
Neurological assessment (ODI score)
| Pre‐op | 6 months | 12 months | 24 months | |
|---|---|---|---|---|
| Mean | 45 | 20 | 16 | 15 |
| Standard deviation | 17 | 18 | 15 | 14 |
| Minimum | 0 | 0 | 0 | 0 |
| Maximum | 98 | 78 | 66 | 70 |
| Total | 94 | 94 | 94 | 94 |
Radiological evaluation
Loss of disc height
We found increasing loss of disc height during follow‐up. The Mochida's Index (loss of height in %) increased from 1.60 at the sixth month to 7.56 at the 24th month postoperatively (Table 5).
Table 5.
Loss of disc height
| Loss of disc height | 6 months | 12 months | 24 months |
|---|---|---|---|
| mm | 0.30 | 0.60 | 1.00 |
| Mochida index | 1.60 | 3.89 | 7.56 |
Segmental stability
Segmental stability was assessed by the difference in the intervertebral angle in F‐E lateral radiographs (Table 6).
Table 6.
Segmental stability according to degree of intervertebral angle
| Stability (Cobb's ) | 6 months | 12 months | 24 months |
|---|---|---|---|
| Mean | 1.10 | 0.90 | 0.80 |
| Standard deviation | 2.00 | 1.50 | 1.50 |
| Total n | 94 | 94 | 94 |
Isodense bone bridging
Isodense bony trabecular bridging in the fusion area was assessed (Table 7).
Table 7.
Incidence of complete isodense bone bridge from endplate to endplate
| Bone bridge | Count | % |
|---|---|---|
| Around the fusion cage | 20 | 21.28% |
| Lateral to the fusion cage | 21 | 22.34% |
| Between two fusion cages | 4 | 4.26% |
Complications
Regarding complications, eight patients experienced temporary postoperative motor and sensory deficits, which differed from their preoperative symptoms. All of them were managed nonsurgically. No implant fracture or migration was found and no revision surgery was needed.
Discussion
Low back pain remains a major public health problem 4 and spinal instability is one of the important factors in low back pain. Although White and Panjabi 5 have provided a working definition of clinical instability as ‘loss of the ability of the spine under physiologic loads to maintain its pattern of displacement so that there is no initial or additional neurological deficit, no major deformity and no incapacitation pain’, how this definition might be useful clinically remains controversial. The clinical manifestations of instability might better be understood in terms of specific pain patterns: pain typically increases throughout the day and is relieved by rest and recumbency 6 . In addition, forward bending is restricted by pain and characterized by swaying or jerking movements. Radiologically, instability is interpreted in terms of gross movements, with the common criteria being either the amount of sagittal rotation observed between the extremes of movement (on F‐E radiographs) or the amount of vertebral translation. In this study, we used both clinical and radiological manifestations as diagnostic criteria for patient selection.
Fusion of the lumbar spine is a well‐accepted surgical procedure for the correction of spinal stability. Lumbar interbody fusion has gained wide application in treatment of lumbar instability because of its high fusion rate and ability to immediately restore spinal stability. Recent developments in interbody fixation devices, known as cages, have renewed interest in lumbar interbody fusion. However, some post‐operative complications have occurred with the increasing use of interbody fusion cages. Subsidence of the cages into the vertebral body is a well‐known complication after interbody fusion,especially in the old patients with low bone mineral density 2 .
Threaded fusion cages such as the Bagby and Kuslich (BAK) and Ray threaded fusion cage (Ray TFC) have been widely used in PLIF and have shown satisfactory early clinical outcomes. In a multicenter study, Ray reported a fusion rate of 96% for the Ray TFC (Surgical Dynamics, Norwalk, CT, USA) at 24‐month follow‐up in a group of 226 patients who underwent PLIF 7 . However, long‐term follow‐up indicated that they had a high incidence of subsidence which resulted in loss of disc and foraminal height, and symptoms caused by compression of the nerve roots 8 . The main cause of the subsidence is destruction of the bony endplate, which plays an important role in interbody fusion during the threading of the cages 9 . Firstly, the cylindrical shape of the device does not match the anatomy of the interbody space, so the contact area between the fusion cage and the endplate is limited, which results in poor fusion. Secondly, the bony endplate; especially in the middle column, which bears most of the axial compression load; is seriously damaged during insertion of the threading fusion cages because of the trapeziform shape of the interbody space, and this damage is the main cause of postoperative subsidence.
Mechanical stability of an instrumented spinal segment relies mainly on a distraction–compression mechanism, and the achievement of optimal sagittal alignment is one of the major goals in fusion surgery 10 , 11 . A decrease in the sagittal spinal curvature indexes after fusion surgery increases the probability of segmental breakdown above and below the fusion level. Extended sagittal malalignment can even cause iatrogenic flatback syndrome 12 . An increased incidence of low back pain is highly associated with loss of segmental lumbar lordosis 13 , 14 . Therefore, it is necessary to provide geometrically optimized implants whose shape and size achieve sufficient distraction and promote segmental realignment. Because the disc space of the lower lumbar spine is wedge‐shaped, wedge‐shaped interbody implants theoretically offer many potential advantages. So, in instrumented PLIF procedures, wedge‐shaped cages are obviously superior to rectangular cages in restoring sagittal alignment and avoiding flatback deformity. Burkus et al. have found that wedge‐shaped fusion cages are much better in regard to restoration of interbody height than threaded cages, and can achieve better long‐term clinical results 15 . The titanium‐coated cage we have used for PLIF has been anatomically designed and incorporates two kinds of wedge angles (5° and 10°) to maintain the physiological curvature of the lumbar spine. This anatomically accurate design not only maintains the physiological curvature of the lumbar spine but also protects the integrity of the bony endplate. The greatest force of the vertebral body is on the bony endplate, which withstands 40%–75% of the force and maintains the height of disc space, and thus can prevent subsidence of the fusion cage.
Surface coating, initially used in joint replacement, is now also used in spinal surgery. The titanium‐coated implant we used is solid‐titanium metal coated with pure titanium powder. With the titanium coating, the fusion cage‐endplate contact area is 16 times as large as that of the surface of the cage. Therefore, fusion efficiency is significantly increased compared to threaded fusion cages, and in addition the increased contact area greatly reduces the axial compression load on the bony endplate. This minimizes the risk of microfracture of the endplate. In addition, the implant is not likely to be forced into the vertebral endplates because the coralloid microstructure of the coating facilitates creeping bone substitution which greatly increases fusion efficiency. Tropiano et al. implanted titanium‐coated and uncoated fusion cages into sheep to provide interbody fusion, and found that both the extraction force and bone formation adhering to the implant were better in the coated group than that in the uncoated group 16 . They also found that abundant bone formation occurred between the fusion cage and the endplate as well as on the side (non‐contact area), which indicates that the microstructure provides a good environment for fusion 16 . In contrast to threaded cages, initial fixation of the wedged fusion cage is obtained from ‘distraction‐compression’ by expanding the interbody space, so the pedicle screw system provides stability of the fusion segment and prevents migration of the fusion cages.
In this study, radiological evaluation showed satisfactory reconstruction of segmental stability according to the intervertebral angles and the complete isodense bone bridges between endplates, which indicated successful fusion of the unstable segments. Meanwhile, we also found continuing loss of disc height during follow‐up, Mochida's index increasing from 1.60 at the sixth month to 7.56 at the 24th month. In regard to measures of functional outcome, the ability of patients to return to work was assessed in this study. Before surgery, only 3.19% of the patients were capable of performing heavy manual labor and this percentage increased to 56.38% at the 24th month after surgery. The percentage of patients unable to perform manual labor decreased from 15.96% to 2.13%. At the same time, neurological assessment showed that sensory and motor function improved significantly. We used the JOA and ODI scoring systems for evaluation of function, and positive results were observed. Significant improvement in neurological function was determined by the ODI, a patient‐based evaluation system which is widely accepted, reliable and validated, and avoids interviewer bias by employing a self‐administered questionnaire 3 .
According to our observations, restoration of spinal stability and improvement in neurological function were satisfactory. No complications such as migration of the fusion cages or hardware failure were found. The results of this study of PLIF, using titanium‐coated interbody fusion cages for treatment of lumbar instability, are positive and include satisfactory clinical outcomes. Long‐term pain relief and functional improvement are yet to be confirmed.
References
- 1. Hanley EN Jr. The indications for lumbar spinal fusion with and without instrumentation. Spine, 1995, 20 (24 Suppl.): 143–153. [PubMed] [Google Scholar]
- 2. Kuslich SD, Danielson G, Dowdle JD, et al. Four‐year follow‐up results of lumbar spine arthrodesis using the Bagby and Kuslich lumbar fusion cage. Spine, 2000, 25: 2656–2662. [DOI] [PubMed] [Google Scholar]
- 3. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine, 2000, 25: 2940–2952. [DOI] [PubMed] [Google Scholar]
- 4. Andersson GBJ. The epidemiology of spinal disorders In: Frymoyer JW, ed. The Adult Spine. Philadelphia: Lippincott–Raven, 1997; 107–146. [Google Scholar]
- 5. White AAIII Panjabi MM. Clinical Biomechanics of the Spine, 2nd edn. Philadelphia: JB Lippincott, 1990. [Google Scholar]
- 6. Eisenstein SM, Parry CR. The lumbar facet arthrosis syndrome. Clinical presentation and articular surface changes. J Bone Joint Surg Br, 1987, 69: 3–7. [DOI] [PubMed] [Google Scholar]
- 7. Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine, 1997, 22: 667–679. [DOI] [PubMed] [Google Scholar]
- 8. Beutler WJ, Peppelman WC Jr. Anterior lumbar fusion with paired BAK standard and paired BAK Proximity cages: subsidence incidence, subsidence factors, and clinical outcome. Spine J, 2003, 3: 289–293. [DOI] [PubMed] [Google Scholar]
- 9. Steffen T, Tsantrizos A, Aebi M. Effect of implant design and endplate preparation on the compressive strength of interbody fusion constructs. Spine, 2000, 25: l077–l084. [DOI] [PubMed] [Google Scholar]
- 10. Goh JC, Wong HK, Thambyah A, et al. Influence of PLIF cage size on lumbar spine stability. Spine, 2000, 25: 35–39. [DOI] [PubMed] [Google Scholar]
- 11. Langrana NA. Point of view: influence of PLIF cage size on lumbar spine stability. Spine, 2000, 25: 40. [DOI] [PubMed] [Google Scholar]
- 12. Booth KC, Bridwell KH, Lenke LG, et al. Complications and predictive factors for the successful treatment of flatback deformity (fixed sagittal imbalance). Spine, 1999, 24: 1712–1720. [DOI] [PubMed] [Google Scholar]
- 13. Kuslich SD, Ulstrom CL, Griffith SL, et al. The Bagby and Kuslich method of lumbar interbody fusion. History, techniques, and 2‐year follow‐up results of a United States prospective, multicenter trial. Spine, 1998, 23: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 14. Tribus CB, Belanger TA, Zdeblick TA. The effect of operative position and short‐segment fusion on maintenance of sagittal alignment of the lumbar spine. Spine, 1999, 24: 58–61. [DOI] [PubMed] [Google Scholar]
- 15. Burkus JK, Schuler TC, Gornet MF, et al. Anterior lumbar interbody fusion for the management of chronic lower back pain: current strategies and concepts. Orthop Clin North Am, 2004, 35: 25–32. [DOI] [PubMed] [Google Scholar]
- 16. Tropiano P, Diop A, Dejou J, et al. Interbody arthrodesis using a plasmapore titanium block. Mechanical and histological experimental study in sheep. Chirurgie, 1999, 124: 58–65. [DOI] [PubMed] [Google Scholar]


