Abstract
Objective
To review and analyze cage migration and related risk factors in patients who have undergone transforaminal lumbar interbody fusion (TLIF).
Methods
A retrospective study was conducted to review the complications of cage migration in 512 patients who had undergone a TLIF procedure from January 2010 to June 2011 in five spinal centers. In all, 263 men and 249 women with a mean age of 54.7 years were included. All patients were followed up at 3, 6 and 12 months after the procedure. The clinical outcomes were evaluated by visual analogue scores, the Oswestry disability index, plain radiography and three‐dimensional CT scanning to analyze the incidence of, and risk factors related to, cage migration.
Results
Cage migration was found in 6 of the 512 patients (1.17%). Significant differences were found between all pairs of centers. Different shapes and sizes of cages had different incidences of migration. Analysis showed that rectangular‐shaped cages had a significantly greater incidence of cage migration (3.11%, 5/161) than did kidney‐shaped cages (0.28%, 1/351; P < 0.05). Small cages had a tendency to more frequent post‐operative cage migration (5.13%, 4/78) than did large cages (0.46%, 2/434; P < 0.05). Double segment TLIF cages migrated more frequently (5.75%, 5/87) than did mono‐segment cages (0.24%, 1/425; P < 0.05)). Furthermore, when the adjacent endplates were of linear type, the cages migrated much more frequently (3.50%) than when they were of concave‐concave type (0.27%; P < 0.05).
Conclusion
Cage size, shape, number of fused segments and adjacent endplate shape might be risk factors for cage migration in addition to surgical technique, disc height and bone mineral density.
Keywords: Cage migration, Risk factors, Transforaminal lumbar interbody fusion
Introduction
The procedure of transforaminal lumbar interbody fusion (TLIF) has become increasingly popular for treatment of degenerative disc disease, spondylolisthesis and lumbar stenosis with instability. Many clinical studies have demonstrated its safety and effectiveness1, 2, 3, 4, 5. Compared with other fusion procedures, TLIF has many advantages, including minimal invasiveness, less blood loss and shorter hospitalization time6. In addition, complications such as nerve root injury and spinal dura mater avulsion are less frequent and severe than with the PLIF procedure7, 8, 9. Depending on specific patient characteristics, open, mini‐open or mini‐invasive approaches can be used to perform TLIF.
Increasing numbers of surgeons have encountered cage migration, one complication of spinal fusion. After migrating forwards into the retroperitoneum or backwards into the vertebral canal, malpositioned cages can cause serious clinical consequences. Of these, posterior migration is more serious because it can cause compression of nerve roots or dura mater, intensify neurological symptoms, and make the fusion unsuccessful. To date, several cases of cage migration have been reported. However, a systematic analysis is necessary.
Materials and Methods
Clinical Data
In all, 512 patients (263 men and 249 women with a mean age of 54.7 years) who underwent a TLIF procedure from January 2010 to June 2011 in five spinal centers were included in this study. All patients had polyether ether ketone (PEEK) cages implanted with bilateral pedicle screw fixation and were followed up 3, 6 and 12 months after the procedure. General factors such as age, sex, body mass index and preoperative diagnosis were similar between the five centers (Table 1). The adjacent endplates of each intervertebral disc were classified into linear and concave‐concave types according to sagittal MRI scan images. The clinical outcomes were evaluated using visual analogue scores (VAS), the Oswestry disability index (ODI)6, conventional radiography and three‐dimensional (3D)‐CT scanning to analyze the incidence of, and risk factors related to, cage migration. Additional thin‐section CT scanning was performed in patients whose cages were found by plain radiography to have migrated.
Table 1.
Clinical data on patients from the five participating spinal centers
| Index | Center 1 | Center 2 | Center 3 | Center 4 | Center 5 |
|---|---|---|---|---|---|
| Cases (number) | 132 | 185 | 81 | 65 | 49 |
| Age(mean age in years ± s.d) | 52.3 ± 10.6 | 54.8 ± 11.7 | 53.5 ± 9.9 | 56.6 ± 8.6 | 55.8 ± 8.9 |
| Sex (male/female) | 62/70 | 103/82 | 37/44 | 31/34 | 22/27 |
| Pre‐operative diagnosis (cases [%]) | |||||
| Spondylolisthesis | 64 (48.4) | 94 (50.8) | 39 (48.2) | 32 (49.2) | 24 (49.0) |
| Intervertebral disc herniation | 53 (40.2) | 71 (38.4) | 32 (39.5) | 25 (38.5) | 19 (38.8) |
| Spinal stenosis | 15 (11.4) | 20 (10.8) | 10 (12.3) | 8 (12.3) | 6 (12.2) |
| Body mass index (mean ± s.d, kg/m2) | 24.3 ± 2.3 | 26.4 ± 3.1 | 23.8 ± 1.9 | 24.3 ± 3.0 | 22.9 ± 2.6 |
Center 1: Department of Orthopaedics, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University; Center 2: Department of Orthopaedics, Tongde Hospital of Zhejiang Province; Center 3: Department of Orthopaedics, Taizhou Hospital; Center 4: Department of Orthopaedics, Shaoxing People's Hospital; Center 5: Department of Orthopaedics, The Central Hospital of Lishui City.
Surgical Procedures
L4,5 mini‐open TLIF with decompression on the left side was selected as an example of the procedures and was performed as follows:
The patients were placed in a prone position after anaesthesia and bent forward to open the posterior structures of the relevant motion segment. The skin was prepared with povidone iodine and draped in a sterile manner.
The location of the L4–5 intervertebral space was identified by C‐arm X‐ray and marked on the body surface, as were the pedicle roots of L4 and L5.
Incisions were made on both sides about 2 cm from the spinous processes, the left sided thoracolumbar fascia opened, the vertebral lamina reached by blunt dissection, and the soft tissue separated from the vertebral lamina. The right side was approached via the interspace between the multifidus and longissimus.
Pedicle screws were inserted into L4 and L5 with C‐arm X‐ray vision and a connecting rod installed on the right side to distract the intervertebral space.
Next, left hemilaminectomy and medial facetectomy were performed. Adequate decompression was achieved and the lateral recess or nerve root canal was also decompressed if necessary. The ligamentum flavum was resected and the nerve roots retracted medially. A complete discectomy was performed, after which the disc space was sequentially distracted. The endplates were then prepared for fusion. The anterior disc space was packed with autologous bone graft, after which an interbody cage packed with autograft was put in place. Once interbody fusion had been completed, pedicle rod instrumentation was put in place.
Data Analysis
The patients were classified into cage migration and non‐migration groups. The cages' shapes, sizes and adjacent endplate styles were analyzed to evaluate possible risk factors for cage migration. T‐tests and X2 tests were used for analysis. A two‐sided test with a significance level of 0.05 was used and P < 0.05 was considered significant without multiple test adjustment. All statistical calculations were performed by SPSS 16.0 statistics software (IBM, Chicago, IL, USA).
Typical Case
A 55‐year‐old man complained of pain in the low back and both lower limbs for 17 months. Systematic conservative treatments had had little effect and his symptoms were intensifying. MRI showed disc herniation of L4–5 and L5S1, the endplate of L4–5 being of concave‐concave type and of L5S1 was linear type. TILF was performed and rectangular‐shaped PEEK cages implanted in both affected segments. The distance between the posterior edge of the cage and posterior edge of the vertebral body was greater than 3 mm postoperatively. In this operation, the compression procedure was not performed on the side that required decompression before it was performed on the other side. Post‐operative X‐ray films showed that the size and location of both cages was appropriate and his symptoms were relieved. At his 2‐month follow‐up X‐ray films showed that the L5S1 cage had migrated. 3D‐CT‐reconstruction showed that the cage had migrated backwards into the vertebral canal (Fig. 1). Luckily, the patient did not have obvious nerve root symptoms in subsequent monthly follow‐ups. The migration may have been related to the linear shape of the endplate.
Figure 1.
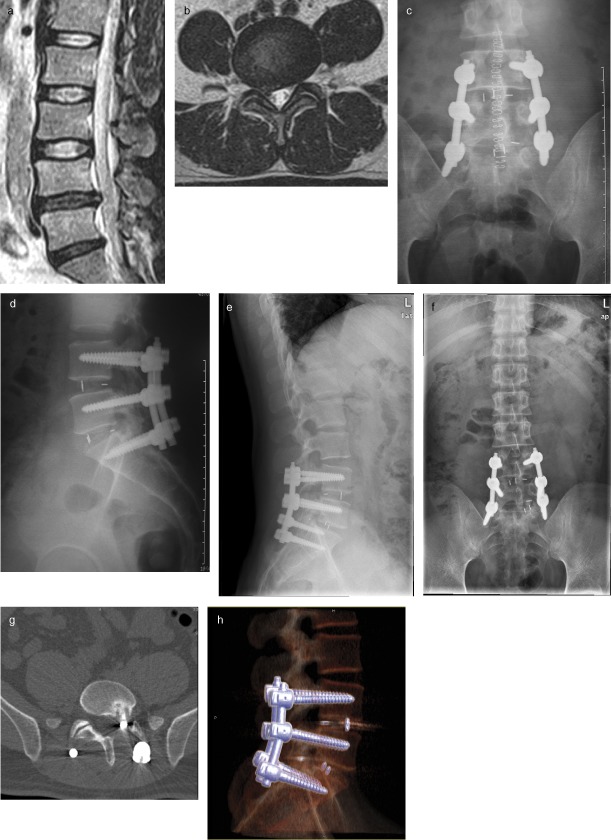
Radiological findings in a 55‐year‐old man who underwent TLIF for back and leg pain.(a, b) Pre‐operative MR imaging showing intervertebral disc herniation of L 4–5 and L 5 S 1. The dura mater and nerve root are compressed. (c, d) Post‐operative X‐ray film showing the size and location of both cages is appropriate. (e, f) Two‐month follow‐up showing migration of the L 5 S 1 cage on X‐ray films. (g, h) 3D‐CT‐reconstruction showed the cage has migrated backwards into the vertebral canal.
Results
Clinical outcome analysis showed that the ODI and VAS scores were significantly lower after the procedure (P < 0.05); there was no statistically significant difference between final follow‐up and 3 or 6 month follow‐ups (Table 2). We found postoperative cage migration in six patients at or before 3 months. The five spinal centers had different incidences of cage migration (Table 3). Cages of different shapes and sizes (Fig. 2) also had different incidences of migration. The cages migrated in 5 of the 161 patients (3.11%) with rectangular‐shaped cages and 1 of the 351 patients (0.28%) with kidney‐shaped cages. This difference was statistically significant (χ2 = 7.58, P = 0.006). The cages migrated postoperatively in 4 of the 78 patients (5.13%) with small cages (smaller than 28 mm × 14 mm × 9 mm) and 2 of the 434 patients (0.46%) with large cages (larger than 31 mm × 18 mm × 11 mm). This difference was statistically significant (χ2 = 8.73, P = 0.0004). Bilateral screw fixations were performed in all patients during surgery. The double segment TLIF cages migrated more frequently (5.75%, 5/87) than did mono‐segment ones (0.24%, 1/425, χ2 = 14.48, P = 0.00001). Furthermore, when the adjacent endplates were of linear type, migration occurred much more frequently (3.50%, 5/143) than when they were of concave‐concave type (0.27%, 1/369, χ2 = 6.68, P = 0.0012).
Table 2.
Comparison of pre‐ and post‐operative ODI and VAS scores of 512 patients (mean ± s.d)
| Index | Pre‐operative | 3 months post‐operative | 6 months post‐operative | Last follow‐up |
|---|---|---|---|---|
| ODI score | 68.5 ± 15.6 | 14.6 ± 3.2* | 13.4 ± 3.2*† | 13.5 ± 3.1*†‡ |
| VAS score | 8.0 ± 2.1 | 2.2 ± 0.6* | 2.0 ± 0.3*† | 1.9 ± 0.6*†‡ |
*Compared with pre‐operative, the difference is significant (P < 0.05); †Compared with 3 months post‐operative, the difference is not significant (P > 0.05); ‡Compared with 6 months post‐operative, the difference is not significant.
Table 3.
The incidence of cage migration at the different spinal centers
| Center | Cage migration | Total number of patients | Incidence (%) |
|---|---|---|---|
| 1 | 2 | 132 | 1.52 |
| 2 | 1 | 185 | 0.54 |
| 3 | 1 | 81 | 1.23 |
| 4 | 1 | 65 | 1.54 |
| 5 | 1 | 49 | 2.04 |
Figure 2.

Rectangular‐shaped cage (left) and kidney‐shaped cage (right).
Discussion
With the aging population, increasingly more health problems related to degeneration and aging have emerged. Many aged patients suffer from diseases such as spondylolisthesis, scoliosis and spinal stenosis. Lumbar interbody fusion can help to relieve these patients symptoms and improve their quality of life. TLIF is widely accepted because it is easy to perform, is very safe and has little effect on spinal stability. However, implantation of a single cage from one side can result in certain side effects. In addition to nerve root injury, dura mater injury and pedicle screw mal‐positioning, cage migration is an important complication. Cage migration can be classified as posterior, anterior or sagittal migration according to the direction in which the cage migrates. Of these, posterior migration is the most serious because it can compress the nerve root or dura mater and intensify neurological symptoms.
We conducted a retrospective study to review the complications of cage migration in 512 patients who underwent TLIF procedures in five spinal centers in our province. The incidence of migration differed in each center. Although all patients underwent TLIF procedures, there were differences, including the cage size, shape, surgical skills, indications and shape of the endplates of adjacent segments. We found that rectangular‐shaped and small cages migrated more frequently than did kidney‐shaped and large cages, which we concluded are more stable. The incidence of mono‐segment cage migration was lower than that of double‐segment and adjacent endplates of linear type were associated with a higher rate of migration than were concave‐concave type. In addition, treatment of the cartilaginous endplate is a very important factor; too little abrasion can prejudice fusion of the interface, whereas too much abrasion can damage the bony endplate and lead to cage subsidence. Also, when compressing the connecting rod, compressing the side that requires decompression before the other side can make the combination stronger and more stable.
Many researchers have analyzed factors influencing case migration. Most of these concern the number, shape, size and implantation site of the cages. A spinal biomechanical study showed that the interface between endplate and fusion cage is under huge pressure. Failure load and flexibility vary significantly across the endplate surfaces, posterolateral regions being stronger and less flexible than the anterior and central regions of lumbar vertebral bodies10, 11. These findings were supported by a study of 40 patients with 80 implanted cages that showed that cage migration rate occurred more frequent in central regions than in posterolateral regions and that cages without grafts in them had a significantly higher migration rate than did those with grafts12. Aoki et al. followed up 125 patients with TLIF and reported that the incidence of cage migration was 3.20% and use of bullet‐shaped cages, higher posterior disc height, presence of scoliotic curvature, and undersized fusion cages were possible risk factors for cage migration, all of which confirms the present authors' findings13. Smith et al. reviewed 81 patients with TLIF using implants made of carbon fiber (37 patients) or biodegradable poly‐L/DL‐lactide (44 patients) and drew the conclusion that poly‐L/DL‐lactide implants migrate significantly more frequently than do carbon fiber implants (P < 0.01)14.
Recombinant human bone morphogenetic protein‐2 (rhBMP‐2) is widely used in spinal fusion surgery to promote osteogenesis and enhance the fusion rate. However, recent research has shown that rhBMP‐2 used in TLIF is associated with a significantly higher risk of postoperative osteolysis and may increase the incidence of cage migration15, 16, 17, 18.
Traditional TLIF is performed with open approaches following bilateral pedicle screw fixation. Alternatively, minimally invasive TLIF is safe and effective and has become increasingly popular recently. Research has shown that patients with unilateral pedicle screw fixation have a significantly shorter operative time, less blood loss and lower costs than do those with bilateral pedicle screw fixation, whereas the clinical outcomes are not significantly different19. Chen et al. reported that the rate of cage migration in patients without posterior instrumentation is significantly higher than in those with posterior instrumentation20. Aoki et al. considered that cage migration occurs more commonly in patients treated by unilateral fixation than in those treated by bilateral fixation. However, the difference was not significant13. More recent research has shown that the TLIF procedure without pedicle screw support achieves good clinical outcomes and that the operative time, blood loss, implant costs and complications are less than with pedicle screwing21.
Although this multicenter retrospective research has provided important data, it did have some limitations. The first is that, because of the low incidence, the total number of case migration cases is insufficient to draw many conclusions. In addition, not all patients had bone mineral density measurements and the follow‐up period was not long enough to investigate all aspects. Further studies should pay more attention to the above aspects to clarify which factors influence cage migration.
Conclusion
This study analyzes the radiographic findings and clinical outcomes of patients who underwent TLIF procedures with bilateral screw fixation in five spinal centers. The results suggest that use of rectangular‐shaped cages, small cages, conducting double segment fusion and adjacent endplates of linear type might be risk factors for cage migration.
Disclosure: No funds were received in support of this work. No benefits in any form have been, or will be, received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1. Rosenberg WS, Mummaneni PV. Transforaminal lumbar interbody fusion: technique, complications, and early results. Neurosurgery, 2001, 48: 569–575. [DOI] [PubMed] [Google Scholar]
- 2. Salehi SA, Tawk R, Ganju A, et al Transforaminal lumbar interbody fusion: surgical technique and results in 24 patients. Neurosurgery, 2004, 54: 368–374. [DOI] [PubMed] [Google Scholar]
- 3. Lowe TG, Tahernia AD, O'Brien MF, et al Unilateral transforaminal posterior lumbar interbody fusion (TLIF): indications, technique, and 2‐year results. J Spinal Disord Tech, 2002, 15: 31–38. [DOI] [PubMed] [Google Scholar]
- 4. Potter BK, Freedman BA, Verwiebe EG, et al Transforaminal lumbar interbody fusion: clinical and radiographic results and complications in 100 consecutive patients. J Spinal Disord Tech, 2005, 18: 337–346. [DOI] [PubMed] [Google Scholar]
- 5. Holly LT, Schwender JD, Rouben DP, et al Minimally invasive transforaminal lumbar interbody fusion: indications, technique, and complications. Neurosurg Focus, 2006, 20: E6. [DOI] [PubMed] [Google Scholar]
- 6. Hee HT, Castro FP Jr, Majd ME, et al Anterior/posterior lumbar fusion versus transforaminal lumbar interbody fusion: analysis of complications and predictive factors. J Spinal Disord, 2001, 14: 533–540. [DOI] [PubMed] [Google Scholar]
- 7. Humphreys SC, Hodges SD, Patwardhan AG, et al Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine, 2001, 26: 567–571. [DOI] [PubMed] [Google Scholar]
- 8. Xiao YX, Chen QX, Li FC. Unilateral transforaminal lumbar interbody fusion: a review of the technique, indications and graft materials. J Int Med Res, 2009, 37: 908–917. [DOI] [PubMed] [Google Scholar]
- 9. DiPaola CP, Molinari RW. Posterior lumbar interbody fusion. J Am Acad Orthop Surg, 2008, 16: 130–139. [DOI] [PubMed] [Google Scholar]
- 10. Fairbank JC, Couper J, Davies JB, O'Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy, 1980, 66: 271–273. [PubMed] [Google Scholar]
- 11. Grant JP, Oxland TR, Dvorak MF. Mapping the structural properties of the lumbosacral vertebral endplates. Spine, 2001, 26: 889–896. [DOI] [PubMed] [Google Scholar]
- 12. Labrom RD, Tan JS, Reilly CW, et al The effect of interbody cage positioning on lumbosacral vertebral endplate failure in compression. Spine, 2005, 30: E556–E561. [DOI] [PubMed] [Google Scholar]
- 13. Abbushi A, Cabraja M, Thomale UW, et al The influence of cage positioning and cage type on cage migration and fusion rates in patients with monosegmental posterior lumbar interbody fusion and posterior fixation. Eur Spine J, 2009, 18: 1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aoki Y, Yamagata M, Nakajima F, et al Examining risk factors for posterior migration of fusion cages following transforaminal lumbar interbody fusion: a possible limitation of unilateral pedicle screw fixation. J Neurosurg Spine, 2010, 13: 381–387. [DOI] [PubMed] [Google Scholar]
- 15. Smith AJ, Arginteanu M, Moore F, et al Increased incidence of cage migration and nonunion in instrumented transforaminal lumbar interbody fusion with bioabsorbable cages. J Neurosurg Spine, 2010, 13: 388–393. [DOI] [PubMed] [Google Scholar]
- 16. Sethi A, Craig J, Bartol S, et al Radiographic and CT evaluation of recombinant human bone morphogenetic protein‐2‐assisted spinal interbody fusion. AJR Am J Roentgenol, 2011, 197: W128–W133. [DOI] [PubMed] [Google Scholar]
- 17. Knox JB, Dai JM 3rd, Orchowski J. Osteolysis in transforaminal lumbar interbody fusion with bone morphogenetic protein‐2. Spine, 2011, 36: 672–676. [DOI] [PubMed] [Google Scholar]
- 18. Vaidya R, Sethi A, Bartol S, et al Complications in the use of rhBMP‐2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech, 2008, 21: 557–562. [DOI] [PubMed] [Google Scholar]
- 19. Mroz TE, Wang JC, Hashimoto R, et al Complications related to osteobiologics use in spine surgery: a systematic review. Spine, 2010, 35 (Suppl. 9): S86–S104. [DOI] [PubMed] [Google Scholar]
- 20. Xue H, Tu Y, Cai M. Comparison of unilateral versus bilateral instrumented transforaminal lumbar interbody fusion in degenerative lumbar diseases. Spine J, 2012, 12: 209–215. [DOI] [PubMed] [Google Scholar]
- 21. Chen L, Yang H, Tang T. Cage migration in spondylolisthesis treated with posterior lumbar interbody fusion using BAK cages. Spine, 2005, 30: 2171–2175. [DOI] [PubMed] [Google Scholar]
- 22. Kotil K, Ali Akçetin M, Savaş Y. Clinical and radiologic outcomes of TLIF applications with or without pedicle screw: a double center prospective pilot comparative study. J Spinal Disord Tech, 2012. Feb 8; doi: 10.1097/BSD.0b013e318249599f. [DOI] [PubMed] [Google Scholar]


