Abstract
Objective: To determine the correlation between the degree of radiculalgia and counts of T lymphocyte subsets in the peripheral blood of patients with lumbar disc herniation.
Methods: Forty‐nine patients with lumbar disc herniation (group A) were divided into three subgroups according to Visual Analogue Scale (VAS) pain scores (group A1: n= 12, VAS 0–4.0; A2: n= 24, VAS 4.1–7.0; A3: n= 13, VAS 7.1–10.0. Twenty health blood donors who volunteered to be involved in the study comprised the control group (group B). Peripheral blood counts of various T lymphocyte subsets were measured in each group.
Results: (i) The counts of CD4+ T and CD4+/CD8+ lymphocytes were higher in group A than in group B, and the difference between the two groups was statistically significant (P < 0.05). There were also statistically significant differences between group A and group B in the counts of CD3+ and CD8+ T lymphocytes (P < 0.05); (ii) There was no correlation between the VAS scores and the counts of CD3+ T lymphocytes (r= 0.194, P > 0.05). A strong significant correlation was observed between the VAS scores and counts of CD4+ T lymphocytes (r= 0.542, P < 0.05), CD4+/CD8+ (r= 0.468, P < 0.05), which increased with increasing VAS scores in the three subgroups of group A (P < 0.05). However there was a significant negative linear correlation between CD8+ T lymphocyte counts and pain scores (r=−0.462, P < 0.05).
Conclusion: Our results suggest that changes in T lymphocyte subsets in peripheral blood take place after prolapse of lumbar intervertebral discs. The current results may provide support for involvement of immunologic mechanisms in low back pain secondary to herniation of the lumbar disc. T lymphocytes may play an important role in the development of symptoms in patients with lumbar intervertebral disc herniation.
Keywords: Intervertebral disk displacement, Low back pain, Pain measurement, T‐lymphocyte subsets
Introduction
Lumbar disc herniation (LDH) is a predominant cause of low back pain and sciatica. The classical concept is that mechanical compression or deformity of nerve roots is the primary cause of the pain or nerve dysfunction of disc herniation. Additionally, radiculalgia may be caused by an inflammatory or immune component that can occur with disc herniation 1 , 2 . The inflammatory reaction associated with LDH may play an important role in the induction of sciatica. Many investigators have studied the histology and pathology of herniated disc tissue from patients with LDH, but a correlation between cellular immune response and clinical symptoms is so far lacking. The main objective of the present study was to investigate peripheral blood counts of T lymphocyte subsets and the degree of pain in patients with LDH, so as to further elucidate the pain mechanisms in LDH.
Materials and methods
Study population
Forty‐nine patients with LDH (46.9% women, 53.1% men; mean age ± one standard deviation [SD], 44.6 ± 13.9 years; mean body mass index (BMI) ± one SD, 23.5 ± 3.1) and twenty of health blood donor volunteers (40.0% women, 60.0% men; mean age ± SD, 39.2 ± 10.9 years; mean BMI ± SD, 23.6 ± 3.5) were recruited. The mean duration of illness was 3.2 ± 2.9 years (range, 8 months to 7 years). For the diagnosis of LDH the following three criteria had to be met: (i) magnetic resonance imaging (MRI, sagittal and axial images obtained with a 1.5‐T imaging system) showing LDH; (ii) a history of unilateral leg pain radiating from the back along the femoral or sciatic nerve to the corresponding dermatome of the nerve root for at least 3 months; (iii) other lumbar diseases included lumbar stenosis, spinal tumor, spondylolisthesis, spondylosis and fracture had been excluded by physical examination and MRI or CT scans. All subjects provide informed consent after being given a detailed explanation of the aims of the study. There were no obvious differences in general characteristics between the two groups.
Pain scale
A VAS, which consists of a marked 10 cm line, was used to assess subjective pain intensity. One end of the scale was marked ‘no pain’ (0 cm) and the other end was marked ‘pain as bad as it can be’ (10 cm). All LDH patients, were asked to mark the distribution of their pain on a diagram and their pain intensity on a VAS before blood samples were collected. Forty‐nine patients with LDH were divided into three subgroups according to VAS pain scores (group A1: n= 12, VAS 0–4.0; A2: n= 24, VAS 4.1–7.0; A3: n= 13, VAS 7.1–10.0).
Sample collection
After pain assessment, a 2 ml peripheral venous blood sample was collected from every patient and volunteer, put into a test tube containing disodium ethylenediaminetetraacetic acid (EDTA‐2Na), mixed and placed into refrigerator at −80°C, preparatory to quantifying the T lymphocyte subsets.
Experimental materials and equipment
The immune reagents used were mouse anti‐human CD4/CD8/CD3, FITC/PE/PE‐Cy5 labeled antibody (Beijing Jingmei Ji yin,gu Technology, Beijing, China) and red blood cell lysis solution (Becton Dickinson, Sparks, MD, USA).
An FACSCalibur cytometer (Becton Dickinson) was used to quantify the lymphocyte subsets.
Detection procedure
A Facscalibur cytometer was used to detect the T lymphocyte subsets. Twenty µl of anti‐human CD4/CD8/CD3, FITC/PE/PE‐Cy5 labeled antibody was placed in the bottom of a test tube, then 100 µl of peripheral blood with EDTA anticoagulant was added to the test tube and gently shaken until the antibody was fully mixed. This was incubated in the dark at room temperature for 30 min, then 2 ml RBC lysis solution added to the bottom of the test tube and buffer blended in the dark for 10 min, to lyse the red blood cells. The test tube was then centrifuged at 1000 r/min for 5 min, the supernatant fluid discarded, 2 ml PBS solution added to suspend the cells, centrifuged at 1000 r/min for 5 min, the supernatant again discarded, and a further 0.5 ml of PBS solution added to again suspend the cells. The samples were then tested by flow cytometry, and the resultant data recorded.
Statistical analysis
Statistical analysis was implemented using SPSS 16.0 software package (SPSS, Chicago, IL, USA). Independent‐samples t‐ test and the χ2 test were used to analyze the data from patients and volunteers. Comparisons between the two groups were performed by independent samples t‐ test, and quantitative data among the A1, A2 and A3 subgroups were compared by one‐way analysis of variance (ANOVA) and group comparisons were made using Scheffe's methods. Correlation analysis between VAS scores and T lymphocyte changes was performed by Pearson correlation. The statistical significance count was set at P < 0.05 for all tests.
Results
Age and male/ female ratio between group A and B
There were no significant differences between the LDH and control groups with regard to age, BMI and the male/female ratio (Table 1). In addition, no significant differences were observed between the A1, A2 and A3 subgroups in regard to age, BMI and male/female ratio.
Table 1.
The basic characteristics of patients and volunteers
| Group A | Group B | χ2/t value | P value | |
|---|---|---|---|---|
| Male/female | 26/23 | 12/8 | χ2= 0.276 | 0.599 |
| Mean age (years) | 44.6 ± 13.9 | 39.2 ± 10.9 | t= 1.553 | 0.125 |
| BMI | 23.5 ± 3.1 | 23.6 ± 3.5 | t=−0.223 | 0.824 |
Comparison of T lymphocyte subsets between LDH and control group
Some of the T lymphocyte subset counts, such as those of CD3+ and CD4+ T lymphocytes in the 49 patients with LDH, were significantly higher than in the control group (P < 0.05, Fig. 1). Moreover, the CD4+/CD8+ ratio in the LDH group was significantly higher than in the control group (P < 0.05, Fig. 1). However, the CD8+ T lymphocyte counts in the LDH group were significantly lower than in the control group (P < 0.05, Fig. 1).
Figure 1.
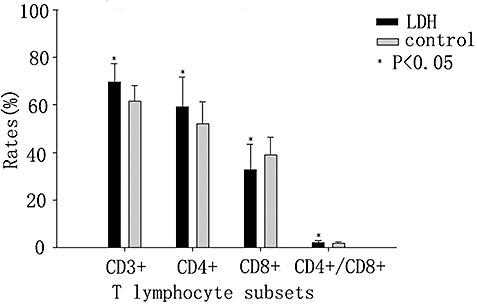
Comparison between LDH and control groups of T lymphocyte subsets.
Results of VAS and T lymphocyte subsets in the A1, A2 and A3 groups
According to their pain intensity, the patients with LDH were divided into three subgroups. There were no significant differences among the three subgroups in CD3+ T lymphocyte counts. In the high pain intensity group (A3) the CD8+ T lymphocyte counts were significantly less than in the low pain intensity group (A1). However, the CD4+ T lymphocyte counts and the CD4+/CD8+ ratio in the three subgroups increased significantly in parallel with the VAS (P < 0.05, Fig. 2).
Figure 2.
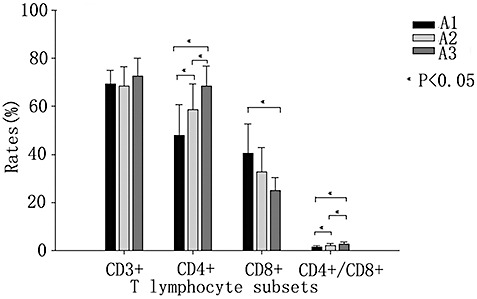
Comparisons of T lymphocyte subsets in the A1, A2 and A3 groups.
Correlation between VAS and T lymphocyte subsets
There was no significant linear correlation between counts of CD3+ T lymphocytes and VAS scores (r= 0.194, P > 0.05). However there was a strong linear correlation between CD4+ T lymphocyte counts and VAS scores (r= 0.542, P < 0.05), a strong negative linear correlation between CD8+ T lymphocyte counts and VAS scores (r=−0.462, P < 0.05), and a strong linear correlation between CD4+/CD8+ ratio and VAS scores (r= 0.468, P < 0.05).
Discussion
Lumbosacral radiculopathy secondary to LDH is one of the leading cause of occupational disability in the world and the most common cause of lost workdays, affecting approximately 1%–10% of the population 3 , 4 . Although many risk factors have been reported for LDH, its etiology and pathogenesis remains uncertain. Two pathological mechanisms have been proposed for low back and sciatic pain owing to LDH, these are nerve root compression and an inflammatory reaction induced by the herniated disc material 5 , 6 , 7 , 8 . It is known that the degree of prolapse of the lumbar intervertebral disc and nerve root compression do not necessarily match the clinical symptoms and signs. During surgery the nerve root is often found to be hyperemic, edematous and to show other evidence of inflammatory changes, which could indicate that mechanical compression caused by disc herniation is not the only factor in nerve root dysfunction. Whether the inflammation is induced by chemical irritation by the nucleus pulposus (NP) itself, or is secondary to an autoimmune response to the NP remains uncertain; several authors have demonstrated the infiltration of inflammatory cells into the extruded herniated mass 7 , 8 . However, both the role of immune factors in pathogenesis and how they cause nerve root symptoms is not entirely clear, which limits the assessment of pain and of the effects of treatment. According to the clonal selection theory of the immunologist Burnet, exposure of autologous NP to the immune system can lead to an autoimmune response, producing chronic inflammation and subsequent low back pain and sciatica 9 .
This study shows that the CD4+ T lymphocyte count and the CD4+/CD8+ ratio in the peripheral blood are higher in patients with LDH than in an age‐ and sex‐matched control group. This indicates that some changes take place in the immune system after disc herniation, supporting the immune theory of LDH and confirming the results of other scholars 2 , 6 , 13 , 14 . CD4+ T lymphocytes are central regulators of both humoral and cellular immune responses. CD4+ and CD8+ T lymphocytes are functional T lymphocytes from two different subgroups, and exert a positive and negative regulatory role in the immune response respectively. The relative immune balance is maintained mainly by interaction between CD4+ and CD8+ T lymphocytes, and imbalance in the proportion of these two T lymphocyte subsets results in immune dysfunction, therefore the CD4+/CD8+ ratio represents the overall immune balance 10 . According to Geha et al., degeneration or trauma leads to exposure of antigenic disc components to the circulating blood, generating an autoimmune response, which manifests as abnormalities in cell‐mediated and humoral immune responses 11 . MA et al. collected specimens of lumbar intervertebral discs from nineteen patients to study their histopathologic features by immunohistochemical staining, and showed that there were more T lymphocytes and macrophages in herniated lumbar disc specimens than in protruded type specimens 12 . Immunoglobulin counts of IgM and IgG in peripheral blood were higher in patients with the herniated type than in patients with the protruding type 12 . Arai et al. observed inflammatory‐cell infiltration, mainly by T cells and macrophages, in herniated disc tissue 13 . In a study of 205 disc herniations, Johanna et al. observed abundant T cells in 17% of the disc herniations, activated T cells in 17%, B cells in 16% and macrophages in 37% 14 . All these cell types were 2−3 times higher in sequestrated discs than in extrusions. In protrusions macrophages were abundant in 25% (4 of 16), while no other inflammatory cells were seen in these patients. Tian et al. found that IgM and IgG are deposited around the new blood vessels and NP cells in herniated lumbar intervertebral discs and concluded that IgM and IgG‐mediated immune responses play an important role in lumbar disc herniation 15 . In an experimental study on pigs, Geiss et al. discovered that, after autologous NP was placed subcutaneously in a perforated titanium chamber, the proportion of activated T cells (CD4 and CD8) was significantly higher in the exudate from the NP filled chambers than in that from the empty chambers 2 . The proportion of activated B cells expressing Ig κ was also significantly greater in the exudate of the NP filled chambers. Windsor et al. observed that lymphocytic pleocytosis is common in dogs with chronic progression or acute‐on‐chronic intervertebral disc herniation and suggested that lymphocytic inflammation in the CSF of some dogs might suggest an immune‐mediated response to chronically herniated disc material 16 .
In a healthy lumbar intervertebral disc, cells within the NP produce extracellular matrix (ECM) containing a high percentage of collagens and proteoglycans. These NP cells may also secrete small amounts of cytokines as well as matrix metalloproteinases (MMP). These cytokines and MMP help to regulate the metabolism of the NP cells. When the NP is exposed to the immune system, it may cause an autoimmune response. In a pig model, Geiss et al. performed a study in which they created exposure of the NP to the immune system 17 . They reported that this resulted predominantly in production of IL‐4 together with little production of IFN‐γ in T cells, indicating that exposure of the NP to the immune system, as can occur in association with disc herniation, may prime T helper (TH) cells to develop into interleukin‐4 (IL‐4) ‐producing TH2 cells. Shamji et al., studied the immunohistochemistry of sectioned dorsal root ganglions which had been exposed to autologous NP and demonstrated local expression of IL‐23 and IL‐17, which could provide evidence of a role for both local inflammatory and immune activation 18 . However, Kawaguchi et al. observed abundant infiltration of CD68‐positive cells which lacked CD33 but had a variable amount of CD11b, CD11c and CD40 and thought that this likely represented a process of differentiation from monocytes to macrophages. They concluded that their findings were consistent with an immunophenotype of inflammatory responses to tissue injury or chemical irritation rather than antigen‐specific immune responses 19 .
According to their VAS scores 20 , the LDH patients were divided into three subgroups. In this study, among the three subgroups of group A the counts of CD4+ T and CD4+/CD8+ lymphocytes increased in parallel with increasing VAS score. It is usually considered that low back pain and sciatica are induced by mechanical stimulation of herniated lumbar intervertebral discs, but it has also been proposed that pain due to nerve damage involves inflammatory mediators. In a study of human lumbar intervertebral discs, Tian et al. found deposits of IgM and IgG and vascular formation in the herniated discs 15 . They observed that the presence of deposits of IgM and IgG was correlated with the degree of pain, which could indicate that IgM and IgG‐mediated immune responses play an important role in the occurrence and development of symptoms in such patient. Katsuno et al. showed an age‐related cytokine change, as represented by counts of IFN‐γ and IL‐10. Stress and aging are thought to affect the extracellular matrix synthesis and influence the type of immunologic response 21 . Younger rat NP cells have greater cell‐mediated immunity activity, while older rats have greater humoral immunity activity 21 . These results demonstrate that age affects the immunologic response attributable to NP cells' activities.
The current study shows that significant changes in peripheral T lymphocyte subsets take place in patients with LDH. Our results indicate that an autoimmune response plays a role in the pathogenic mechanism of LDH, and suggest that CD4+ T lymphocytes and the CD4+/CD8+ ratio may be involved in low back pain in patients with LDH. This may provide a new indicator for evaluation of symptoms in, and diagnosis of, LDH. A drawback of this study is that immune changes and inflammatory mediators in herniated discs of LDH patients were not assessed at the same time. Further studies are needed to elucidate the mechanism of symptom formation in LDH.
Disclosure
This is an original study and the manuscript was completed by all authors.
Acknowledgments
This work was supported by the Scientific Research Fund of Tianjin Medical University (Grant No.2008ky22) and the Tianjin Public Health Bureau Technology Fund (Grant No.06kz48).
Grant Sources: This work was supported by the Scientific Research Fund of Tianjin Medical University (2008ky22) and the Tianjin Public Health Bureau Technology Fund (06kz48)
References
- 1. Mclain RF, Kapural L, Mekhail NA. Epidural steroids for back and leg pain: mechanism of action and efficacy. Cleve Clin J Med, 2004, 71: 961–970. [DOI] [PubMed] [Google Scholar]
- 2. Geiss A, Larsson K, Rydevik B, et al. Autoimmune properties of NP: an experimental study in pigs. Spine, 2007, 32: 168–173. [DOI] [PubMed] [Google Scholar]
- 3. Bucquet D, Colvez A. Sciatica and other low‐back disorders in private practice: extent of the problem and therapeutic approaches. Rev Epidemiol Sante Publique, 1985, 33: 1–8. [PubMed] [Google Scholar]
- 4. Younes M, Béjia I, Aguir Z, et al. Prevalence and risk factors of disk‐related sciatica in an urban population in Tunisia. Joint Bone Spine, 2006, 5: 538–42. Epub 19 April 2006. [DOI] [PubMed] [Google Scholar]
- 5. Nygaard OP, Mellgren SI, Osterud B. The inflammatory properties of contained and noncontained lumbar disc herniation. Spine, 1997, 22: 2484–2488. [DOI] [PubMed] [Google Scholar]
- 6. Olmarker K, Blomquist J, Strömberg J, et al. Inflammatogenic properties of NP. Spine, 1995, 20: 665–669. [DOI] [PubMed] [Google Scholar]
- 7. Rothoerl RD, Woertgen C, Brawanski A. Pain resolution after lumbar disc surgery is influenced by macrophage tissue infiltration. A prospective consecutive study on 177 patients. J Clin Neurosci, 2002, 9: 633–666. [DOI] [PubMed] [Google Scholar]
- 8. Woertgen C, Rothoerl RD, Brawanski A. Influence of macrophage infiltration of herniated lumbar disc tissue on outcome after lumbar disc surgery. Spine, 2000, 25: 871–875. [DOI] [PubMed] [Google Scholar]
- 9. Burnet FM. A modification of Jerne's theory of antibody production using the concept of clonal selection. CA Cancer J Clin, 1976, 26: 119–121. [DOI] [PubMed] [Google Scholar]
- 10. Stockinger B, Bourgeois C, Kassiotis G. CD4+ memory T cells: functional differentiation and homeostasis. Immunol Rev, 2006, 211: 39–48. [DOI] [PubMed] [Google Scholar]
- 11. Geha RS, Leung DY. Cellular abnormalities in patients with elevated serum IgE counts. J Allergy Clin Immunol, 1986, 78: 995–999. [DOI] [PubMed] [Google Scholar]
- 12. Ma XL, Xu YQ, Zhang YX, et al. The study on autoimmune factors of lumbar disc herniation (Chin). Zhongguo Xian Dai Shen Jing Ji Bing Za Zhi, 2004, 4: 291–296. [Google Scholar]
- 13. Arai Y, Yasuma T, Shitoto K, et al. Immunohistological study of intervertebral disc herniation of lumbar spine. J Orthop Sci, 2000, 5: 229–231. [DOI] [PubMed] [Google Scholar]
- 14. Virri J, Grönblad M, Seitsalo S, et al. Comparison of the prevalence of inflammatory cells in subtypes of disc herniations and associations with straight leg raising. Spine, 2001, 26: 2311–2315. 11679814 [Google Scholar]
- 15. Tian W, Cui GY, Zhao D‐h, et al. Clinical signs and symtoms relate to IgM and IgG in herniated lumbar intervertebral disc (Chin). Zhonghua Gu Ke Za Zhi, 2008, 28: 288–291. [Google Scholar]
- 16. Windsor RC, Vernau KM, Sturges BK, et al. Lumbar cerebrospinal fluid in dogs with type I intervertebral disc herniation. J Vet Intern Med, 2008, 22: 954–960. [DOI] [PubMed] [Google Scholar]
- 17. Geiss A, Larsson K, Junevik K, et al. Autologous NP primes T cells to develop into interleukin‐4‐producing effector cells: an experimental study on the autoimmune properties of NP. J Orthop Res, 2009, 27: 97–103. [DOI] [PubMed] [Google Scholar]
- 18. Shamji MF, Allen KD, So S, et al. Gait abnormalities and inflammatory cytokines in an autologous NP model of radiculopathy. Spine, 2009, 34: 648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kawaguchi S, Yamashita T, Yokogushi K, et al. Immunophenotypic analysis of the inflammatory infiltrates in herniated intervertebral discs. Spine, 2001, 26: 1209–1214. [DOI] [PubMed] [Google Scholar]
- 20. Hirayama J, Yamagata M, Ogata S, et al. Relationship between low‐back pain, muscle spasm and pressure pain thresholds in patients with lumbar disc herniation. Eur Spine J, 2006, 15: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katsuno R, Hasegawa T, Iwashina T, et al. Age‐related effects of cocultured rat NP cells and macrophages on nitric oxide production and cytokine imbalance. Spine, 2008, 33: 845–849. [DOI] [PubMed] [Google Scholar]


