Abstract
Importance
Antidepressants are one of the most commonly prescribed classes of psychotropic medications among US youths. For adults, there is emerging evidence on the increased risk of type 2 diabetes in association with antidepressant use. However, little is known about the antidepressant treatment–emergent risk of type 2 diabetes among youths.
Objective
To assess the association between antidepressant use and the risk of incident type 2 diabetes in youths by antidepressant subclass and according to duration of use, cumulative dose, and average daily dose.
Design, Setting, and Participants
A retrospective cohort study was conducted using Medicaid claims data from 4 geographically diverse, large states of youths 5 to 20 years of age who initiated antidepressant treatment from January 1, 2005, to December 31, 2009.
Exposures
Antidepressant use (selective serotonin reuptake inhibitors [SSRIs] or serotonin-norepinephrine reuptake inhibitors [SNRIs], tricyclic or other cyclic antidepressants, and other antidepressants) was assessed using the following 4 time-varying measures: current or former use, duration of use, cumulative dose, and average daily dose.
Main Outcomes and Measures
Incident type 2 diabetes was assessed using discrete-time failure models, adjusting for disease risk score estimated using more than 125 baseline and time-dependent covariates.
Results
In this cohort of 119 608 youths aged 5 to 20 years who initiated antidepressant treatment (59 087 female youths and 60 521 male youths; 54.7% between 5 and 14 years of age) with a mean follow-up of 22.8 months, 79 285 [66.3%] had SSRI or SNRI exposure. The risk of type 2 diabetes was significantly greater during current use than former use of SSRIs or SNRIs (absolute risk, 1.29 per 10 000 person-months vs 0.64 per 10 000 person-months; adjusted relative risk [RR], 1.88; 95% CI, 1.34-2.64) and tricyclic or other cyclic antidepressants (absolute risk, 0.89 per 10 000 person-months vs 0.48 per 10 000 person-months; RR, 2.15; 95% CI, 1.06-4.36), but not of other antidepressants (absolute risk, 1.15 per 10 000 person-months vs 1.12 per 10 000 person-months; RR, 0.99; 95% CI, 0.66-1.50). Furthermore, for youths currently using SSRIs or SNRIs, the risk of type 2 diabetes increased with the duration of use (RR, 2.66; 95% CI, 1.45-4.88 for >210 days and RR, 2.56; 95% CI, 1.29-5.08 for 151-210 days compared with 1-90 days) and with the cumulative dose (RR, 2.44; 95% CI, 1.35-4.43 for >4500 mg and RR, 2.17; 95% CI, 1.07-4.40 for 3001-4500 mg compared with 1-1500 mg in fluoxetine hydrochloride dose equivalents). By contrast, neither the duration nor the cumulative dose of other antidepressants was associated with an increased risk of type 2 diabetes. The risk of type 2 diabetes increased significantly with the average daily dose among youths with more than 150 days of SSRI or SNRI use (RR, 2.39; 95% CI, 1.04-5.52 for >15.0 vs ≤15.0 mg/d) but not among youths with 1 to 150 days of SSRI or SNRI use.
Conclusions and Relevance
In a large cohort of youths insured by Medicaid, the use of SSRIs or SNRIs—the most commonly used antidepressant subclass—was associated with an increased risk of type 2 diabetes that intensified with increasing duration of use, cumulative dose, and average daily dose.
This cohort study uses Medicaid claims data to assess the association between antidepressant use and the risk of incident type 2 diabetes among youths by antidepressant subclass and duration of use, cumulative dose, and average daily dose.
Key Points
Question
Does antidepressant use increase the risk of incident type 2 diabetes among youths?
Findings
In a cohort study of youths insured by Medicaid that included 119 608 youths who initiated antidepressant treatment, the current use of selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors—the most commonly used antidepressant subclass—was associated with an increased risk of incident type 2 diabetes that intensified with increasing duration of use, cumulative dose, and average daily dose.
Meaning
The study findings provide an impetus to improve monitoring for benefits vs risks of antidepressant use in pediatric care models and to shed light on the underlying biological mechanism of antidepressant treatment–emergent type 2 diabetes.
Introduction
Antidepressants are one of the most commonly prescribed psychotropic medication classes among US youths.1,2 In the past 2 decades, there has been a marked increase in the percentage of US children and adolescents who use antidepressants (from 1.5% in 1996-1998 to 2.6% in 2010-2012).1 This expansion of antidepressant use among youths was driven largely by a significantly more rapid increase in antidepressants prescribed to youths by pediatricians and other primary care physicians than by psychiatrists.3 For youths, several antidepressants have evidence-based indications approved by the US Food and Drug Administration, such as major depressive disorder, obsessive-compulsive disorder, and childhood enuresis,4 but their off-label use among youths is also not uncommon, which is a subject of concern and debate.5,6
During the past decade, there has been a growing number of published studies—all conducted for adults—that report a substantially increased risk of type 2 diabetes associated with antidepressant use.7,8,9,10,11,12,13,14 By contrast, evidence of such a risk among youths is scarce and limited to only a few studies,15,16 wherein antidepressant use concomitant with antipsychotics was associated with an additive increased risk of type 2 diabetes. To our knowledge, no population-based study has comprehensively examined the independent effect of antidepressants on the risk of incident type 2 diabetes among youths.
We conducted a large, retrospective cohort study of youths insured by Medicaid who initiated treatment with antidepressants, and we assessed the risk of incident type 2 diabetes by antidepressant subclass and according to duration of use and cumulative dose. Subsequently, in a secondary analysis, we assessed the risk of type 2 diabetes according to the average daily dose and examined the interaction between the duration of use and the average daily dose for selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs), the leading antidepressant subclass.17
Methods
Data Source
The Medicaid Analytic eXtract (MAX) database was obtained to analyze computerized administrative claim files from 4 large, geographically diverse states—California, Florida, Illinois, and New Jersey—from January 1, 2004, through December 31, 2009.18 Medicaid administrative claims data include enrollment files and claim files for inpatient and outpatient services and prescription drug dispensings. The enrollment files contain data on monthly enrollment, eligibility status, and sociodemographic characteristics. An encrypted identification number was assigned to each enrollee to link enrollment files to inpatient, outpatient, and prescription drug claim files. The inpatient and outpatient claim files contain information at the claim level, including diagnosis and procedure codes. The prescription drug claim file includes information on dispensing date, days of supply, quantity of supply, and national drug code, which was used to identify the name, strength, dosage form, and formulation of dispensed products. This study was reviewed and approved by the University of Maryland institutional review board. As this is a retrospective study with no patient contact and deidentified patient information, no patient consent was needed.
Study Design and Population
In this retrospective cohort study, the study population comprised youths insured by Medicaid who were 5 to 20 years of age. To attenuate prevalent user bias,19,20 a new-user design was applied by restricting the study cohort to those who initiated treatment with antidepressant medications (SSRIs or SNRIs, tricyclic or other cyclic antidepressants [TCAs], and other antidepressants) from January 1, 2005, through December 31, 2009 (eTable 1 in the Supplement).
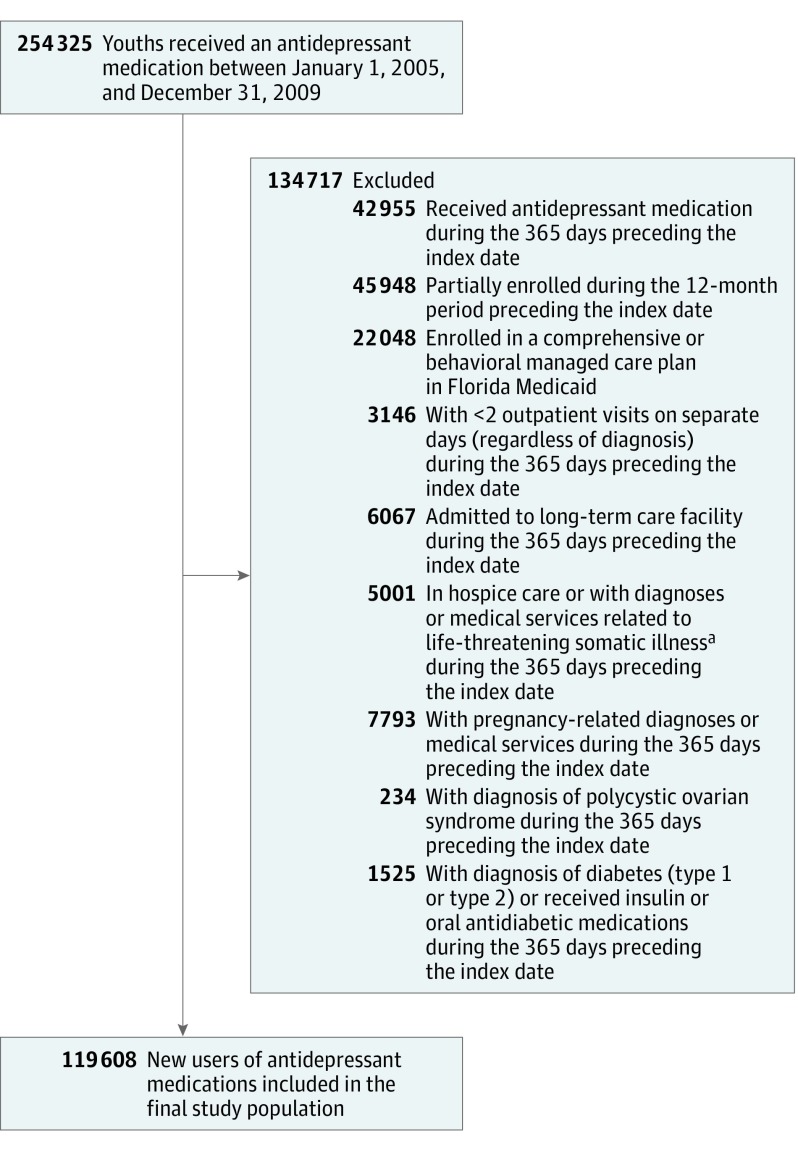
The flowchart in Figure 1 illustrates the inclusion and exclusion criteria that were applied to define the study cohort of 119 608 youths who initiated antidepressant treatment. The antidepressant initiation date served as the index date for cohort entry. New users of antidepressants were selected by excluding youths who received antidepressant medications during the 365 days preceding the index date. In addition, we excluded youths who were partially enrolled during the 365 days preceding the index date. Youths were also excluded if they had an enrollment in a comprehensive or behavioral managed care plan in Florida because of concerns about the quality of managed care data from Florida. To ensure that there would be an active contact with the health care system, youths were required to have at least 2 outpatient visits (on separate days) in the 365 days prior to the index date. Other criteria (an admission to a long-term care facility or hospice care and a diagnosis of a life-threatening or serious somatic condition [eTable 2 in the Supplement]) were applied to exclude youths who might have received frequent care in noncommunity settings. Because gestational diabetes is common during pregnancy and oral antidiabetic medications (eg, metformin) are frequently used to treat women with polycystic ovarian syndrome,21 these cases were excluded. Finally, because the main objective of the study was to assess incident type 2 diabetes, youths were excluded if they had a diabetes-related inpatient or outpatient claim or an antidiabetic medication dispensed during the 365-day period prior to the index date.
Figure 1. Flowchart for the Study Cohort of Youths Insured by Medicaid (5-20 Years of Age) Who Were New Users of Antidepressant Medications, 2005-2009.
aLife-threatening or serious somatic conditions included sickle cell disease, cystic fibrosis, cerebral palsy, cancer, HIV infection, organ transplant, dialysis or end-stage renal disease, respiratory failure, aplastic anemia, congenital immune deficiencies, Down syndrome, other lethal chromosomal anomalies, fatal metabolic diseases, and serious neuromuscular disease.
Incident Type 2 Diabetes
The main study outcome was diagnosis of incident type 2 diabetes, which was identified by the use of a validated, computerized database algorithm. This algorithm—validated in a cohort of youths insured by Medicaid—had a positive predictive value of 83.9%.22 In brief, new cases of diabetes were identified from inpatient stays or outpatient visits with a diabetes diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification codes 250, 250.0, 250.1, 250.2, 250.3, and 250.9), as well as the dispensing of antidiabetic medications (insulin, insulin adjuncts, or oral hypoglycemic agents). To meet the case definition of diabetes, we required an inpatient stay with a primary diagnosis of diabetes or a combination of 2 or more different diabetes-related medical care encounters (ie, an outpatient visit with a primary diagnosis of diabetes, an inpatient stay with a diagnosis [any] of diabetes, or the dispensing of an antidiabetic medication) within a 120-day period. The initial diagnosis date of diabetes was recorded as the date for the initial diabetes-related medical care encounter. Youths were excluded from meeting the case definition if they had a polycystic ovarian syndrome–related medical encounter within 120 days of the diagnosis date of diabetes. Cases were considered type 1 diabetes if insulin was the sole treatment within 120 days of the diagnosis date of diabetes. Otherwise, the cases were considered type 2 diabetes.
The incidence of type 2 diabetes was expressed as the number of new cases of type 2 diabetes per 10 000 person-months of follow-up. Youths in the study were followed up until the incident type 2 diabetes, their 21st birthday, the end of continuous enrollment in the state Medicaid programs, or the end of the study (December 31, 2009), whichever came first. During follow-up, youths were also censored if they had a diabetes-related medical care encounter that did not meet the case definition of type 2 diabetes (ie, type 1 diabetes, having a dispensing of an antidiabetic medication in the absence of a diabetes diagnosis, or having a polycystic ovarian syndrome–related medical encounter within 120 days of the diagnosis date of diabetes).
Antidepressant Medication Use
In this cohort of youths who initiated antidepressant treatment, medication use was operationalized using 4 time-dependent measures, which accounted for status of use (current vs former use), duration of use (in days), cumulative dose exposure, and average daily dose. Medication use was considered current if medications were not discontinued for more than 90 days. The rationale for the 90-day time period was to account for carryover effects23 and to allow a reasonable time window for the latency or detection of antidepressant treatment–emergent type 2 diabetes. Medication use was considered former if medications were discontinued for more than 90 days. Of note, we also conducted a sensitivity analysis in which we did not require the 90-day time window to account for the carryover effects.
The duration of use was calculated in each month of follow-up as the sum of the total days of supply that were available starting from the cohort entry date (index date). Although the 90-day time period was applied to account for carryover effects in categorizing months of current use, the duration-of-use measure was calculated solely using the days of supply information. Similarly, the cumulative dose was calculated as the sum of total dosage that was available starting from the index date. To set comparable doses across antidepressant medications, the cumulative dosage was calculated in fluoxetine dosage equivalents (in milligrams) (eTable 3 in the Supplement). The dose conversion factors were adapted from previously published clinical research.24,25 Finally, the average daily dose (milligrams per day) for current users was calculated (in a time-dependent manner) as the cumulative dose (in fluoxetine equivalents) divided by the duration of use.
Statistical Analysis
All analyses were conducted using SAS, version 9.3 (SAS Institute Inc). Discrete-time failure models were used to assess the adjusted incidence of type 2 diabetes. The unit of analysis in these models was person-months. Discrete-time analyses provide computational efficiency and practical advantages over Cox proportional hazards regression models when there are multiple time-dependent medication exposure groups.26,27
To adjust for confounding, we identified more than 125 baseline and time-dependent covariates that encompassed a wide range of sociodemographic, administrative, and regional characteristics; psychiatric and nonpsychiatric clinical characteristics; and health care use characteristics (eTable 4 in the Supplement). Because of the high volume of study covariates, we used the disease risk score method to generate a confounder summary score. The disease risk score method—like the propensity score method—allows for parsimonious models but provides advantages over the propensity score when multiple time-dependent main exposure groups are present.28,29,30 Because the study cohort was nested in a cohort of youths who initiated antidepressant treatment, we used the Miettinen full cohort approach31,32 to calculate the disease risk score, which was estimated as the probability of incident type 2 diabetes conditional on study covariates. The disease risk score was estimated in a time-dependent manner for each person in each month of follow-up.
The final regression models that assessed the risk of type 2 diabetes according to antidepressant exposure groups were adjusted for time-dependent disease risk score, expressed as percentile rank groups (eFigure in the Supplement), and time from the index date. First, we assessed the risk of type 2 diabetes during current vs former use of antidepressants, as well as each antidepressant subclass. Subsequent analyses were restricted to current users to evaluate the risk of type 2 diabetes according to duration of use and dose.
The duration-response and dose-response analyses were not conducted for TCAs owing to low frequency of use. For current users of SSRIs or SNRIs, we assessed the risk of type 2 diabetes according to the duration of SSRI or SNRI use and the cumulative SSRI or SNRI dose. Similarly, for current users of other antidepressants, we assessed the risk of type 2 diabetes according to the duration of use and the cumulative dose of other antidepressants.
Furthermore, we conducted secondary analyses to explore whether the average daily dose would be associated with the risk of type 2 diabetes for SSRIs or SNRIs, the leading antidepressant subclass. Also, we assessed whether the risk of type 2 diabetes associated with the average daily dose differs according to the duration of use (ie, the interaction between the average daily dose and the duration of use).
We conducted several subgroup and sensitivity analyses. We conducted an analysis of older youths (10-20 years of age) wherein antidepressant use was most prevalent.1,17 In another analysis, we did not require the 90-day time window to account for the carryover effects. Moreover, we excluded youths who used any other psychotropic medication prior to the index date. The latter analysis (in psychotropic medication–naive youth) attempted to attenuate confounding by disease severity because prior use of other classes of psychotropic medications could suggest greater severity of a psychiatric condition. Finally, to attenuate confounding by indication, we conducted an analysis by restricting the cohort to youths with a diagnosis of depressive disorders.
Results
Characteristics of the Study Cohort
The study cohort comprised 119 608 youths aged 5 to 20 years who initiated treatment with antidepressants from 2005 through 2009 (Table 1). The study cohort largely represented youths who were between 10 and 17 years of age (83 122 [69.5%]), nonwhite (64 170 [53.7%]), and eligible for Medicaid based on low family income (81 219 [67.9%]). Male and female youths were equally represented.
Table 1. Baseline Characteristics of Youths Insured by Medicaid Who Initiated Treatment With Antidepressant Medications, 2005-2009.
| Characteristic | Youths, No. (%) (N = 119 608) |
|---|---|
| Age, y | |
| 5-9 | 21 660 (18.1) |
| 10-14 | 43 724 (36.6) |
| 15-17 | 39 398 (32.9) |
| 18-20 | 14 826 (12.4) |
| Sex | |
| Male | 60 521 (50.6) |
| Female | 59 087 (49.4) |
| Race/ethnicity | |
| White | 55 438 (46.3) |
| African American | 20 720 (17.3) |
| Hispanic | 33 793 (28.3) |
| Othera | 9657 (8.1) |
| Medicaid eligibility group | |
| Foster care | 22 548 (18.9) |
| SSI | 15 841 (13.2) |
| TANF or CHIP | 81 219 (67.9) |
| State Medicaid program | |
| California | 66 524 (55.6) |
| Florida | 9341 (7.8) |
| Illinois | 34 330 (28.7) |
| New Jersey | 9413 (7.9) |
| Payment system | |
| Fee-for-service | 66 350 (55.5) |
| Managed care | 53 258 (44.5) |
| Psychiatric diagnostic group | |
| Schizophrenia or other psychoses | 4351 (3.6) |
| Tic disorders | 439 (0.4) |
| Bipolar disorder | 8815 (7.4) |
| Disruptive behavior disorders | 20 357 (17.0) |
| ADHD | 30 975 (25.9) |
| Depressive disorders | 44 753 (37.4) |
| Anxiety disorders | 21 189 (17.7) |
| Adjustment disorder | 14 144 (11.8) |
| Communication and learning disorder | 6850 (5.7) |
| Pervasive developmental disorder or intellectual disability | 4910 (4.1) |
| Personality disorder | 822 (0.7) |
| Somatoform spectrum disorders | 2049 (1.7) |
| Sleep disorders of nonorganic origin | 443 (0.4) |
| Alcohol and other substance abuse | 4269 (3.6) |
| Other psychiatric disorders | 18 701 (15.6) |
| Psychiatric medications | |
| Any psychiatric medication use | 42 398 (35.4) |
| ADHD drugs | 29 516 (24.7) |
| Atomoxetine | 5345 (4.5) |
| Central α-agonists | 8057 (6.7) |
| Stimulants | 25 235 (21.1) |
| Anticonvulsant-mood stabilizers | 9083 (7.6) |
| Antipsychotics | 18 554 (15.5) |
| Atypical antipsychotics | 18 433 (15.4) |
| Conventional antipsychotics | 463 (0.4) |
| Anxiolytic or hypnotics | 4186 (3.5) |
| Lithium | 1047 (0.9) |
| Metabolic screening procedures | 33 642 (28.1) |
| Diabetes screening | 5738 (4.8) |
| Hyperlipidemia screening | 11 424 (9.6) |
| Metabolic panelb | 29 929 (25.0) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CHIP, Children’s Health Insurance Program (youths eligible for Medicaid based on low family income); SSI, Supplemental Security Income (youths with disabilities); TANF, Temporary Assistance for Needy Families (youths eligible for Medicaid based on low family income).
Includes youths of Asian, Native Hawaiian, or other Pacific Islander race/ethnicity and youths with more than 1 race or unknown race/ethnicity.
Includes testing for blood glucose level.
In rank order, the most common clinician-reported psychiatric diagnoses were depressive disorders (44 753 [37.4%]), attention-deficit/hyperactivity disorder (30 975 [25.9%]), and anxiety disorders (21 189 [17.7%]). Most of the study cohort (77 210 [64.6%]) did not have a dispensing of any other psychiatric medication class in the year prior to antidepressant initiation.
The study cohort had a mean follow-up of 22.8 months (median, 19.0 months; interquartile range, 9.0-35.0 months). During follow-up, SSRIs and SNRIs (79 285 [66.3%]) were the most commonly used antidepressant subclass with a mean duration of 179.7 days (median, 90.0 days; interquartile range, 30.0-213.0 days) (eTable 5 in the Supplement). The use of antidepressants that were not SSRIs or SNRIs, particularly TCAs (22 143 [18.5%]), was less common.
Risk of Incident Type 2 Diabetes
During follow-up, there were 233 incident cases of type 2 diabetes, of which 156 occurred during current use (absolute risk, 1.16 per 10 000 person-months) and 77 occurred during former use of antidepressants (absolute risk, 0.56 per 10 000 person-months) (Table 2). Overall, when compared with former use, current use of antidepressants was associated with a 1.92-fold increased risk of type 2 diabetes (95% CI, 1.43-fold to 2.57-fold increased risk). The risk of type 2 diabetes according to current vs former use differed by antidepressant medication subclass (in terms of both absolute risk and relative risk [RR] estimates). Compared with former use, current use of SSRIs or SNRIs (absolute risk, 1.29 per 10 000 person-months vs 0.64 per 10 000 person-months; RR, 1.88; 95% CI, 1.34-2.64) or TCAs (absolute risk, 0.89 per 10 000 person-months vs 0.48 per 10 000 person-months; RR, 2.15; 95% CI, 1.06-4.36) was associated with an increased risk of type 2 diabetes. By contrast, current use of other antidepressants was not significantly associated with an increased risk of type 2 diabetes compared with former use (absolute risk, 1.15 per 10 000 person-months vs 1.12 per 10 000 person-months; RR, 0.99; 95% CI, 0.66-1.50).
Table 2. Risk of Incident Type 2 Diabetes According to Exposure to Antidepressant Subclasses Among Youths Insured by Medicaid (5-20 Years of Age) Who Initiated Treatment With Antidepressant Medications, 2005-2009.
| Exposure Status | Person-Months | Cases, No. | Absolute Risk per 10 000 Person-Months |
Adjusted Relative Risk (95% CI)a |
|---|---|---|---|---|
| Antidepressants | ||||
| Former use | 1 378 212 | 77 | 0.56 | 1 [Reference] |
| Current use | 1 342 896 | 156 | 1.16 | 1.92 (1.43-2.57) |
| SSRIs or SNRIs | ||||
| Former use | 873 299 | 56 | 0.64 | 1 [Reference] |
| Current use | 863 290 | 111 | 1.29 | 1.88 (1.34-2.64) |
| TCAs | ||||
| Former use | 331 094 | 16 | 0.48 | 1 [Reference] |
| Current use | 178 883 | 16 | 0.89 | 2.15 (1.06-4.36) |
| Other antidepressants | ||||
| Former use | 464 172 | 52 | 1.12 | 1 [Reference] |
| Current use | 399 251 | 46 | 1.15 | 0.99 (0.66-1.50) |
Abbreviations: SNRIs, serotonin-norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic and other related cyclic antidepressants.
Adjusted for disease risk score (expressed as percentile ranks) and time from cohort entry (ie, follow-up month).
Furthermore, for youths currently using SSRIs or SNRIs, the risk of type 2 diabetes intensified with increasing duration of SSRI or SNRI use, with an RR of 2.66 (95% CI, 1.45-4.88) for more than 210 days of use and an RR of 2.56 (95% CI, 1.29-5.08) for 151 to 210 days of use compared with 1 to 90 days of use (Table 3). By contrast, for current users of other antidepressants, the duration of use was not significantly associated with an increased risk of type 2 diabetes.
Table 3. Risk of Incident Type 2 Diabetes According to Cumulative Duration and Cumulative Dose Among Youths Insured by Medicaid (5-20 Years of Age) Who Initiated Treatment With Antidepressant Medications, 2005-2009.
| Exposure Status | Person-Months | Cases, No. | Absolute Risk per 10 000 Person-Months |
Adjusted Relative Risk (95% CI)a |
|---|---|---|---|---|
| Among Current Users of SSRIs or SNRIs | ||||
| Cumulative duration, d | ||||
| 1-90 | 380 416 | 20 | 0.53 | 1 [Reference] |
| 91-150 | 122 031 | 13 | 1.07 | 1.68 (0.83-3.40) |
| 151-210 | 83 246 | 15 | 1.80 | 2.56 (1.29-5.08) |
| >210 | 277 597 | 63 | 2.27 | 2.66 (1.45-4.88) |
| Cumulative dose, mgb | ||||
| 1-1500 | 358 385 | 19 | 0.53 | 1 [Reference] |
| 1501-3000 | 148 939 | 12 | 0.81 | 1.22 (0.59-2.52) |
| 3001-4500 | 85 822 | 14 | 1.63 | 2.17 (1.07-4.40) |
| >4500 | 270 144 | 66 | 2.44 | 2.44 (1.35-4.43) |
| Among Current Users of Other Antidepressants | ||||
| Cumulative duration, d | ||||
| 1-90 | 182 812 | 20 | 1.09 | 1 [Reference] |
| 91-210 | 90 342 | 15 | 1.66 | 1.36 (0.68-2.73) |
| >210 | 126 097 | 11 | 0.87 | 0.74 (0.30-1.82) |
| Cumulative dose, mgb | ||||
| 1-1500 | 202 735 | 20 | 0.99 | 1 [Reference] |
| 1501-4500 | 102 674 | 14 | 1.36 | 1.33 (0.65-2.72) |
| >4500 | 93 842 | 12 | 1.28 | 1.37 (0.58-3.24) |
Abbreviations: SNRIs, serotonin-norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors.
All regression models were adjusted for disease risk score (expressed as percentile ranks), time from cohort entry (ie, follow-up month), and exposure to tricyclic and other related cyclic antidepressants. In youths currently using SSRIs or SNRIs, the use of other antidepressants was also adjusted. Likewise, in youths currently using other antidepressants, the use of SSRIs or SNRIs was also adjusted.
The cumulative dose was calculated in fluoxetine hydrochloride equivalents.
Similar significant findings were observed when the risk of type 2 diabetes was assessed according to the cumulative dose. For youths currently using SSRIs or SNRIs, the risk of type 2 diabetes intensified with increasing cumulative dose (in fluoxetine hydrochloride dose equivalents) (Table 3). Compared with youths with a 1- to 1500-mg cumulative SSRI or SNRI dose exposure, there was a 2.44-fold increased risk (95% CI, 1.35- to 4.43-fold increased risk) of type 2 diabetes among youths with more than a 4500-mg SSRI or SNRI cumulative dose and a 2.17-fold increased risk (95% CI, 1.07- to 4.40-fold increased risk) of type 2 diabetes among youths with a 3001- to 4500-mg cumulative SSRI or SNRI dose. By contrast, for youths who were currently using other antidepressants, the cumulative dose was not significantly associated with an increased risk of type 2 diabetes.
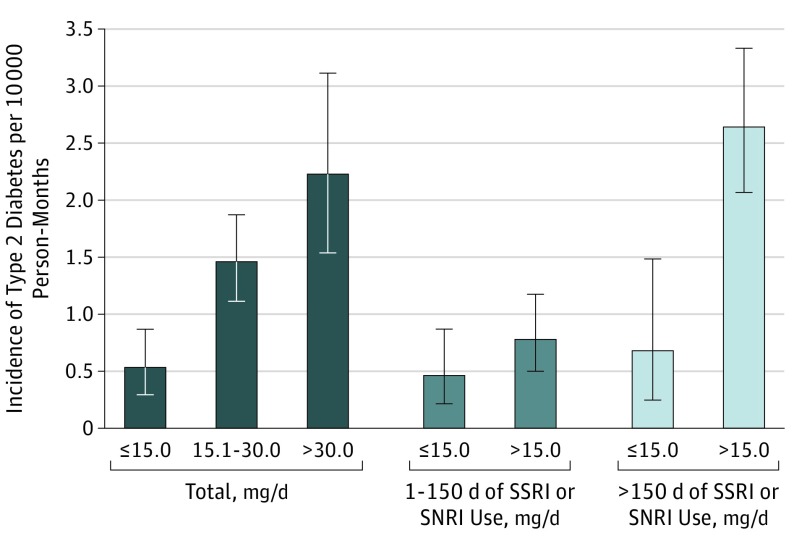
Finally, because both the duration and the cumulative dose were associated with an increased risk of type 2 diabetes in youths currently using SSRIs or SNRIs, we explored the interaction between the duration of use and the average daily dose on the risk of type 2 diabetes (Figure 2). Overall, for youths currently using SSRIs or SNRIs, the risk of type 2 diabetes increased significantly with the average daily dose (in fluoxetine hydrochloride dose equivalents), with an RR of 1.96 (95% CI, 1.05-3.64) for more than 30.0 mg/d and an RR of 1.83 (95% CI, 1.04-3.24) for 15.1 to 30.0 mg/d compared with 15.0 mg/d or less. However, the risk of type 2 diabetes associated with the average daily dose differed according to the duration of SSRI or SNRI use. There was a significant interaction between the duration of SSRI or SNRI use and the average daily SSRI or SNRI dose on the risk of type 2 diabetes. For youths with more than 150 days of SSRI or SNRI use, more than 15.0 mg/d was associated with a 2.39-fold increased risk (95% CI, 1.04- to 5.52-fold increased risk) of type 2 diabetes compared with 15.0 mg/d or less. By contrast, for youths with 1 to 150 days of SSRI or SNRI use, the average daily dose was not significantly associated with an increased risk of type 2 diabetes (RR, 1.22; 95% CI, 0.57-2.63).
Figure 2. Risk of Incident Type 2 Diabetes By Average Daily Dose According to Duration of Use Among Youths Insured by Medicaid (5-20 Years of Age) Who Were Current Users of SSRIs or SNRIs, 2005-2009.
The risk of type 2 diabetes increased significantly with the average daily dose (in fluoxetine hydrochloride dose equivalents), with a relative risk [RR] of 1.96 (95% CI, 1.05-3.64) for more than 30.0 mg/d and an RR of 1.83 (95% CI, 1.04-3.24) for 15.1 to 30.0 mg/d compared with 15.0 mg/d or less. For youths with 1 to 150 days of selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) use, the average daily dose was not significantly associated with an increased risk of type 2 diabetes (RR, 1.22; 95% CI, 0.57-2.63). For youths with more than 150 days of SSRI or SNRI use, more than 15.0 mg/d was associated with a 2.39-fold (95% CI, 1.04- to 5.52-fold) increased risk of type 2 diabetes compared with 15.0 mg/d or less. Relative risk is adjusted for disease risk score (expressed as percentile ranks), time from cohort entry (ie, follow-up month), and exposures to tricyclic and other related cyclic antidepressants and other antidepressants. Error bars indicate 95% CIs.
Sensitivity and Subgroup Analyses
The risk of incident type 2 diabetes remained elevated during current use of SSRIs or SNRIs compared with former SSRI or SNRI use across all the subgroup and sensitivity analyses (eTable 6 in the Supplement): (1) for youths 10 to 20 years of age (RR, 1.85; 95% CI, 1.32-2.60), (2) when the 90-day time window was not allowed to account for carryover effects (RR, 1.78; 95% CI, 1.30-2.43), (3) when youths with any psychotropic medication use prior to the index date were excluded (RR, 1.88; 95% CI, 1.18-2.99), and (4) for youths with a diagnosis of depressive disorders (RR, 2.73; 95% CI, 1.64-4.56). By contrast, there was no significant difference in the risk of type 2 diabetes between current and former use of other antidepressants in all the listed subgroup and sensitivity analyses, consistent with the main study findings.
Discussion
In this large cohort of youths insured by Medicaid who initiated treatment with antidepressant medications, our main finding indicates that there was a substantially greater risk of incident type 2 diabetes for those currently using SSRIs or SNRIs than for those who formerly used these medications. Furthermore, for youths currently using SSRIs or SNRIs, the risk of type 2 diabetes intensified with increasing duration of use, cumulative dose, and average daily dose. The increased risk was most apparent for youths with long-term SSRI or SNRI use at higher average daily doses. Current use of TCAs was also associated with an increased risk of type 2 diabetes. By contrast, current use of other antidepressants was not associated with an increased risk of type 2 diabetes. Moreover, for patients currently using other antidepressants, neither duration nor cumulative dose was associated with an increased risk of type 2 diabetes.
There is a dearth of published reports examining the risk of type 2 diabetes associated with pediatric use of antidepressants. To our knowledge, this is the first population-based study of youths that comprehensively examines the risk of incident type 2 diabetes following treatment initiation with an antidepressant. Previous population-based studies reported that, among youths insured by Medicaid who were treated with antipsychotics, concomitant antidepressant use was associated with an increased risk of incident type 2 diabetes.15,16 In another study also focused on antipsychotics,33 there was a statistically increased risk of incident type 2 diabetes among youths treated with antipsychotics when compared with those who did not use psychotropic medications but not when compared with youths treated with antidepressants.
There is a growing number of studies7,8,9,10,11,12,13,14 of adults that corroborate the increased risk observed in our study. For example, long-term use of SSRIs in moderate to high daily doses by adults was associated with an increased risk of type 2 diabetes.7 Adult studies also suggest that long-term use of antidepressants, particularly those with a high affinity for serotonin receptors, may be associated with increased weight gain, albeit not substantially.8,34,35 Mechanisms other than weight gain may play a greater role in serotonin reuptake inhibitor treatment-emergent type 2 diabetes, including disturbances in glucose homeostasis,36 decreased pancreatic insulin secretion,12,37 and increased cellular insulin resistance.38 Nevertheless, much remains to be elucidated about the biological pathway for incident type 2 diabetes following antidepressant use among youths.
Strengths and Limitations
In this large cohort of youths insured by Medicaid, we used a new-user design to mitigate prevalent user bias,20 and we used a previously validated algorithm to assess incident type 2 diabetes.22 Also, we nested our study solely in a cohort of youths who initiated antidepressant treatment wherein the incidence of type 2 diabetes was assessed according to current vs former use (rather than comparing users vs nonusers). This approach aimed to attenuate bias due to unmeasured confounding since current users should be more similar to former users than to nonusers regarding factors associated with antidepressant use.39,40,41 In addition, for current users, we conducted duration-response and dose-response analyses, all of which provided consistent estimates for SSRIs and SNRIs (significantly increased risk) and other antidepressants (no significantly increased risk). Several limitations should also be noted. First, causality cannot be inferred from observational studies; thus, the study findings should be interpreted with caution. Nevertheless, the study used a rigorous design and rigorous statistical approaches to account for confounding, and it provides new information on the risk of a rare, but serious, adverse outcome that is often difficult to assess in randomized clinical trials owing to limited sample size and inadequate follow-up.42 Second, drug dispensings are not commensurate with actual use, but the emphasis on duration and cumulative dose increases the likelihood of actual use. Third, duration-response and dose-response analyses could not be conducted for TCAs or for individual drugs owing to limited exposure, but the study provides compelling safety information for SSRIs and SNRIs, the major antidepressant subclass. Finally, previous research on adults suggests that depression, in itself, may be associated with weight gain, which may, in turn, increase the likelihood of developing type 2 diabetes.8,43 However, in the present study, most youths who initiated antidepressant treatment (74 855 [62.6%]) did not have a clinician-reported diagnosis of depressive disorders. A large majority of youths who initiated antidepressant treatment had a clinician-reported psychiatric diagnosis other than depression. Also, compared with antipsychotics (a drug class known to induce weight gain), treatment-emergent weight gain with SSRIs or SNRIs appears to be more limited.8,34 Furthermore, to attenuate confounding by indication, when the study cohort was limited to youth diagnosed with depressive disorders, the findings were consistent with the main study results (eTable 6 in the Supplement).
Conclusions
In a large cohort of youths insured by Medicaid, the use of SSRIs and SNRIs was associated with an increased risk of type 2 diabetes, which intensified with increasing duration of use, cumulative dose, and average daily dose. The increased risk was particularly prominent for long-term use of SSRIs or SNRIs that occurred in greater daily doses. In the face of recent growth in the pediatric use of antidepressants in the United States, as well as other Western countries,17,44 the findings from this study support the need for further research to shed light on the underlying biological mechanism of treatment-emergent type 2 diabetes associated with antidepressants. Also, given that more than half of antidepressant prescriptions to US youths occur in outpatient visits to pediatricians and other primary care physicians,3 the study findings provide an impetus for policy development to improve monitoring for the benefits vs risks of antidepressant use in pediatric care models, specifically for serotonin reuptake inhibitors, the most commonly used antidepressant subclass.
eTable 1. Antidepressant Subclasses
eTable 2. Operational Definitions of Life-Threatening or Serious Somatic Conditions Used to Exclude Youth
eTable 3. Antidepressant Dose Equivalence Conversion Factors
eTable 4. Operational Definition of Study Covariates That Included a Range of Sociodemographic, Administrative, Clinical, and Other Health Care Utilization Characteristics
eTable 5. Exposure to Antidepressant Subclasses During Follow-up Among Medicaid-Insured Youth (5-20 Years Old) Who Initiated Treatment With Antidepressant Medications, 2005-2009
eTable 6. Subgroup and Sensitivity Analyses Assessing the Risk of Incident Type 2 Diabetes According to Exposure to Antidepressant Subclasses Among Medicaid-Insured Youth (5-20 Years Old) Who Initiated Treatment With Antidepressant Medications, 2005-2009
eFigure. Incidence of Type 2 Diabetes Per 10 000 Person-Months According to Disease Risk Score, Expressed as Percentile Ranks
eReferences.
References
- 1.Olfson M, Druss BG, Marcus SC. Trends in mental health care among children and adolescents. N Engl J Med. 2015;372(21):2029-2038. [DOI] [PubMed] [Google Scholar]
- 2.Jonas BS, Gu Q, Albertorio-Diaz JR. Psychotropic medication use among adolescents: United States, 2005-2010. NCHS Data Brief. 2013;(135):1-8. [PubMed] [Google Scholar]
- 3.Olfson M, Blanco C, Wang S, Laje G, Correll CU. National trends in the mental health care of children, adolescents, and adults by office-based physicians. JAMA Psychiatry. 2014;71(1):81-90. [DOI] [PubMed] [Google Scholar]
- 4.United States Department of Health and Human Services, Centers for Medicare & Medicaid Services Antidepressant medications: use in pediatric patients. https://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Pharmacy-Education-Materials/Downloads/ad-pediatric-factsheet11-14.pdf. Published October 2015. Accessed January 9, 2017.
- 5.Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848-856. [DOI] [PubMed] [Google Scholar]
- 6.Zito JM, Safer DJ, dosReis S, et al. . Rising prevalence of antidepressants among US youths. Pediatrics. 2002;109(5):721-727. [DOI] [PubMed] [Google Scholar]
- 7.Andersohn F, Schade R, Suissa S, Garbe E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 2009;166(5):591-598. [DOI] [PubMed] [Google Scholar]
- 8.Kivimäki M, Hamer M, Batty GD, et al. . Antidepressant medication use, weight gain, and risk of type 2 diabetes: a population-based study. Diabetes Care. 2010;33(12):2611-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin RR, Ma Y, Marrero DG, et al. ; Diabetes Prevention Program Research Group . Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the diabetes prevention program. Diabetes Care. 2008;31(3):420-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin RR, Ma Y, Peyrot M, et al. ; Diabetes Prevention Program Research Group . Antidepressant medicine use and risk of developing diabetes during the diabetes prevention program and diabetes prevention program outcomes study. Diabetes Care. 2010;33(12):2549-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu CS, Gau SS, Lai MS. Long-term antidepressant use and the risk of type 2 diabetes mellitus: a population-based, nested case-control study in Taiwan. J Clin Psychiatry. 2014;75(1):31-38. [DOI] [PubMed] [Google Scholar]
- 12.Noordam R, Aarts N, Peeters RP, Hofman A, Stricker BH, Visser LE. Selective serotonin reuptake inhibitors decrease pancreatic insulin secretion in older adults and increase the risk of insulin dependence in type 2 diabetes patients. J Clin Psychiatry. 2016;77(9):e1124-e1129. [DOI] [PubMed] [Google Scholar]
- 13.Raeder MB, Bjelland I, Emil Vollset S, Steen VM. Obesity, dyslipidemia, and diabetes with selective serotonin reuptake inhibitors: the Hordaland Health Study. J Clin Psychiatry. 2006;67(12):1974-1982. [DOI] [PubMed] [Google Scholar]
- 14.Pan A, Sun Q, Okereke OI, et al. . Use of antidepressant medication and risk of type 2 diabetes: results from three cohorts of US adults. Diabetologia. 2012;55(1):63-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin DM, Kreider AR, Matone M, et al. . Risk for incident diabetes mellitus following initiation of second-generation antipsychotics among Medicaid-enrolled youths. JAMA Pediatr. 2015;169(4):e150285. [DOI] [PubMed] [Google Scholar]
- 16.Burcu M, Zito JM, Safer DJ, et al. . Concomitant use of atypical antipsychotics with other psychotropic medication classes and the risk of type 2 diabetes mellitus. J Am Acad Child Adolesc Psychiatry. 2017;56(8):642-651. [DOI] [PubMed] [Google Scholar]
- 17.Bachmann CJ, Aagaard L, Burcu M, et al. . Trends and patterns of antidepressant use in children and adolescents from five western countries, 2005-2012. Eur Neuropsychopharmacol. 2016;26(3):411-419. [DOI] [PubMed] [Google Scholar]
- 18.Medicaid.gov. Medicaid Analytic eXtract (MAX) general information. https://www.medicaid.gov/medicaid/data-and-systems/macbis/max/index.html. Accessed September 3, 2017.
- 19.McMahon AD, MacDonald TM. Design issues for drug epidemiology. Br J Clin Pharmacol. 2000;50(5):419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915-920. [DOI] [PubMed] [Google Scholar]
- 21.Legro RS, Arslanian SA, Ehrmann DA, et al. ; Endocrine Society . Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobo WV, Cooper WO, Stein CM, et al. . Positive predictive value of a case definition for diabetes mellitus using automated administrative health data in children and youth exposed to antipsychotic drugs or control medications: a Tennessee Medicaid study. BMC Med Res Methodol. 2012;12:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleophas TJ. Carryover bias in clinical investigations. J Clin Pharmacol. 1993;33(9):799-804. [DOI] [PubMed] [Google Scholar]
- 24.Hayasaka Y, Purgato M, Magni LR, et al. . Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord. 2015;180:179-184. [DOI] [PubMed] [Google Scholar]
- 25.Safer DJ. Raising the minimum effective dose of serotonin reuptake inhibitor antidepressants: adverse drug events. J Clin Psychopharmacol. 2016;36(5):483-491. [DOI] [PubMed] [Google Scholar]
- 26.Allison PD. Analysis of tied or discrete data with proc logistic In: Allison PD, ed. Survival Analysis Using SAS: A Practical Guide. 2nd ed Cary, NC: SAS Institute, Inc; 2010:235-256. [Google Scholar]
- 27.D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med. 1990;9(12):1501-1515. [DOI] [PubMed] [Google Scholar]
- 28.Arbogast PG, Ray WA. Use of disease risk scores in pharmacoepidemiologic studies. Stat Methods Med Res. 2009;18(1):67-80. [DOI] [PubMed] [Google Scholar]
- 29.Hansen BB. The prognostic analogue of the propensity score. Biometrika. 2008;95(2):481-488. doi: 10.1093/biomet/asn004 [DOI] [Google Scholar]
- 30.Arbogast PG, Ray WA. Performance of disease risk scores, propensity scores, and traditional multivariable outcome regression in the presence of multiple confounders. Am J Epidemiol. 2011;174(5):613-620. [DOI] [PubMed] [Google Scholar]
- 31.Glynn RJ, Gagne JJ, Schneeweiss S. Role of disease risk scores in comparative effectiveness research with emerging therapies. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):138-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miettinen OS. Stratification by a multivariate confounder score. Am J Epidemiol. 1976;104(6):609-620. [DOI] [PubMed] [Google Scholar]
- 33.Andrade SE, Lo JC, Roblin D, et al. . Antipsychotic medication use among children and risk of diabetes mellitus. Pediatrics. 2011;128(6):1135-1141. [DOI] [PubMed] [Google Scholar]
- 34.Blumenthal SR, Castro VM, Clements CC, et al. . An electronic health records study of long-term weight gain following antidepressant use. JAMA Psychiatry. 2014;71(8):889-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noordam R, Aarts N, Tiemeier H, Hofman A, Stricker BH, Visser LE. Sex-specific association between antidepressant use and body weight in a population-based study in older adults. J Clin Psychiatry. 2015;76(6):e745-e751. [DOI] [PubMed] [Google Scholar]
- 36.Derijks HJ, Meyboom RH, Heerdink ER, et al. . The association between antidepressant use and disturbances in glucose homeostasis: evidence from spontaneous reports. Eur J Clin Pharmacol. 2008;64(5):531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isaac R, Boura-Halfon S, Gurevitch D, Shainskaya A, Levkovitz Y, Zick Y. Selective serotonin reuptake inhibitors (SSRIs) inhibit insulin secretion and action in pancreatic β cells. J Biol Chem. 2013;288(8):5682-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levkovitz Y, Ben-Shushan G, Hershkovitz A, et al. . Antidepressants induce cellular insulin resistance by activation of IRS-1 kinases. Mol Cell Neurosci. 2007;36(3):305-312. [DOI] [PubMed] [Google Scholar]
- 39.Ray WA, Meredith S, Thapa PB, Hall K, Murray KT. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther. 2004;75(3):234-241. [DOI] [PubMed] [Google Scholar]
- 40.Schneeweiss S, Suissa S. Advanced approaches to controlling confounding in pharmacoepidemiologic studies In: Strom BL, Kimmel SE, Hennessy S, eds. Textbook of Pharmacoepidemiology. 2nd ed West Sussex, UK: Wiley Blackwell; 2013:324-336. [Google Scholar]
- 41.Schneeweiss S, Patrick AR, Stürmer T, et al. . Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45(10)(suppl 2):S131-S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strom BL. When should one perform pharmacoepidemiologic studies? In: Strom BL, Kimmel SE, Hennessy S, eds. Textbook of Pharmacoepidemiology. 2nd ed West Sussex, UK: Wiley Blackwell; 2013:54-62. [Google Scholar]
- 43.Rotella F, Mannucci E. Depression as a risk factor for diabetes: a meta-analysis of longitudinal studies. J Clin Psychiatry. 2013;74(1):31-37. [DOI] [PubMed] [Google Scholar]
- 44.Mittal M, Harrison DL, Miller MJ, Brahm NC. National antidepressant prescribing in children and adolescents with mental health disorders after an FDA boxed warning. Res Social Adm Pharm. 2014;10(5):781-790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Antidepressant Subclasses
eTable 2. Operational Definitions of Life-Threatening or Serious Somatic Conditions Used to Exclude Youth
eTable 3. Antidepressant Dose Equivalence Conversion Factors
eTable 4. Operational Definition of Study Covariates That Included a Range of Sociodemographic, Administrative, Clinical, and Other Health Care Utilization Characteristics
eTable 5. Exposure to Antidepressant Subclasses During Follow-up Among Medicaid-Insured Youth (5-20 Years Old) Who Initiated Treatment With Antidepressant Medications, 2005-2009
eTable 6. Subgroup and Sensitivity Analyses Assessing the Risk of Incident Type 2 Diabetes According to Exposure to Antidepressant Subclasses Among Medicaid-Insured Youth (5-20 Years Old) Who Initiated Treatment With Antidepressant Medications, 2005-2009
eFigure. Incidence of Type 2 Diabetes Per 10 000 Person-Months According to Disease Risk Score, Expressed as Percentile Ranks
eReferences.