Abstract
Importance
Antipsychotic medications for the treatment of schizophrenia have limitations, and new treatments are needed. A prior pilot investigation suggested that adjunctive sodium nitroprusside (SNP) administered intravenously had rapid efficacy in the treatment of patients with schizophrenia.
Objective
To determine the efficacy and tolerability of intravenous SNP infused at a rate of 0.5 μg/kg/min for 4 hours in patients with schizophrenia with some degree of treatment resistance.
Design, Setting, and Participants
Multicenter, randomized, double-blind acute treatment study using a sequential parallel comparison design conducted in two 2-week phases at 4 academic medical centers beginning May 20, 2015, and ending March 31, 2017. Participants were adults 18 to 65 years of age with a diagnosis of schizophrenia as confirmed by the Structured Clinical Interview for DSM-IV, taking antipsychotic medication for at least 8 weeks, and had at least 1 failed trial of an antipsychotic medication within the past year. A total of 90 participants consented, 60 participants enrolled, and 52 participants were included in the analyses. A modified intent-to-treat analysis was used.
Interventions
Participants were randomized in a 1:1:1 ratio to 1 of 3 treatment sequences: SNP and SNP, placebo and SNP, and placebo and placebo. The SNP and SNP group received SNP in phase 1 and SNP in phase 2 for the purpose of blinding, but the data from phase 2 were not included in the results. The placebo and SNP group received placebo in phase 1 and SNP in phase 2. If there was no response to placebo in phase 1, data from phase 2 were included in the analyses. The placebo and placebo group received placebo in both phases; if there was no response to placebo in phase 1, data from phase 2 were included in the analyses.
Main Outcomes and Measures
Effectiveness of SNP compared with placebo in improving Positive and Negative Syndrome Scale (PANSS) total, positive, and negative scores across each 2-week phase.
Results
Fifty-two participants (12 women and 40 men) were included in the study. In the SNP and SNP group, the mean (SD) age was 47.1 (10.5) years. In the placebo and SNP group, the mean (SD) age was 45.9 (12.3) years. In the placebo and placebo group, the mean (SD) age was 40.4 (11.0) years. There were no significant differences between the SNP and placebo groups at baseline or in change from baseline for PANSS-total (weighted β = –1.04; z = –0.59; P = .57), PANSS-positive (weighted β = –0.62; z = –0.93; P = .35), or PANSS-negative (weighted β = –0.12; z = –0.19; P = .85) scores. No significant differences in safety or tolerability measures were identified.
Conclusions and Relevance
Although intravenous SNP is well tolerated, it was not an efficacious adjunctive treatment of positive or negative symptoms of psychosis among outpatients with schizophrenia with prior history of treatment resistance.
Trial Registration
ClinicalTrials.gov identifier: NCT02164981
This randomized clinical trial examines the efficacy and tolerability of intravenous sodium nitroprusside infused at a rate of 0.5 μg/kg/min for 4 hours in patients with schizophrenia with some degree of treatment resistance.
Key Points
Question
Is adjunctive intravenous sodium nitroprusside an efficacious and safe treatment for individuals with schizophrenia who have some degree of treatment resistance?
Findings
In this multicenter, randomized double-blind clinical trial of 52 adults with chronic schizophrenia treated with antipsychotic medication, no improvement was seen in positive, negative, or cognitive symptoms after a 4-hour sodium nitroprusside infusion at 0.5 μg/kg/min; furthermore, there were no differences in response when stratified by clozapine treatment status. Overall, the treatment was well tolerated.
Meaning
Results of this well-powered clinical trial suggest that adding intravenous sodium nitroprusside infused at 0.5 μg/kg/min is not an efficacious treatment for individuals with schizophrenia with some history of treatment resistance.
Introduction
First- and second-generation antipsychotic medications have proven efficacy in the treatment of the positive symptoms of schizophrenia.1 However, current antipsychotic medications also have significant limitations, including lack of efficacy for negative and cognitive symptoms of schizophrenia and distressing adverse effects, and reduction in psychotic symptoms is often quite delayed.2,3 Furthermore, there is a subset of patients who do not fully respond to multiple trials of antipsychotic medications, including clozapine.4,5
In an effort to identify more efficacious treatments, a proof-of-concept clinical trial investigated intravenous sodium nitroprusside (SNP).6 Sodium nitroprusside releases nitric oxide (NO), and N-methyl-d-aspartate (NMDA) receptor activation also produces intracellular NO release through activation of neuronal NO synthase (nNOS).7 N-methyl-d-aspartate hypofunction may contribute to the underlying neurobiology of schizophrenia. Among 20 individuals, Hallak and colleagues6 showed that a single 4-hour infusion of SNP at 0.5 μg/kg/min resulted in a significant decrease in psychotic symptoms, as measured by the Brief Psychiatric Rating Scale. A follow-up placebo-controlled study demonstrated improvement in impairments in cognitive function with SNP treatment in 20 patients with schizophrenia.8 However, a subsequent study from a different group failed to show any benefit of SNP either in reducing psychotic symptoms or in improving spatial working memory performance among 20 patients with schizophrenia.9 Clarifying the potential therapeutic effect of SNP has both clinical and mechanistic importance: if efficacy could be confirmed, it might point the way to a new class of interventions, as with ketamine hydrochloride in major depressive disorder.
We conducted an adequately powered randomized, double-blind, placebo-controlled, multicenter clinical trial to characterize the efficacy and safety of a single dose of intravenous SNP in treating the positive, negative, and cognitive symptoms of patients with schizophrenia. We used a sequential parallel comparison design (SPCD) to increase statistical power and diminish placebo response.10
Methods
Participants
This study enrolled outpatients at 4 academic medical centers (Massachusetts General Hospital, University of Massachusetts Medical School, New York University, and the Zucker Hillside Hospital) beginning May 20, 2015, with the final study visit completed March 31, 2017. The study was approved by the Institutional Review Boards at each site. Written informed consent was obtained from all participants (the trial protocol is available in Supplement 1).
We intended to recruit a total of 60 individuals between the ages of 18 and 65 years with a primary diagnosis of schizophrenia assessed using the Structured Clinical Interview for DSM-IV. Participants were included if they had a total score of 70 or more on the Positive and Negative Syndrome Scale (PANSS), with a score of 4 or more on 2 or more of the following PANSS items: delusions, conceptual disorganization, hallucinatory behavior, suspiciousness, and unusual thought content.11 Participants were also required to have a score of 4 or more on the Clinical Global Impression–Severity. A confirmation of both schizophrenia diagnosis and symptom severity was carried out by an independent, expert clinician remote rater from Massachusetts General Hospital (H.E.B.). Participants must have had ongoing antipsychotic medication treatment for at least 8 weeks, with stable dosing for at least 4 weeks. In addition, in the past year, they must have failed to achieve a clinically significant reduction in symptoms after treatment for at least 8 weeks with at least 1 antipsychotic medication at a therapeutic dose. Antipsychotic medication treatment history was confirmed using the Massachusetts General Hospital Fast Additive Summary of Treatment. This questionnaire is an efficient, structured way to capture individuals’ historical treatments across mood and psychotic disorders, and includes treatment duration, response, and reason for discontinuation.
Participants were excluded if they had any major medical illness, symptomatic orthostatic hypotension, treatment with medications that may interfere with the metabolism or excretion of SNP, medications associated with drug interactions with SNP, medications that could pose a significant risk to the participants’ health, current alcohol or substance use disorders (except nicotine), were pregnant or breastfeeding, or were at imminent risk for suicide or injury to self or others. All participants underwent a physical examination, routine laboratory tests, urine toxicology test, and 12-lead electrocardiogram to ensure medical stability. A board-certified psychiatrist (H.E.B.) was the medical monitor for this study and reviewed all adverse events and issues related to participant eligibility.
Procedure
Study Design
The study was conducted in 2 phases. A SPCD design was used for the 4-week randomized, double-blind phases (phase 1 and phase 2, both lasting 2 weeks). eFigure 1 in Supplement 2 provides further details of the study design. The SPCD reduces placebo response rate and sample size requirement.10 Participants who met eligibility criteria were randomized in a 1:1:1 ratio to 1 of 3 treatment sequences as follows: SNP and SNP, placebo and SNP, and placebo and placebo. Both participants and clinicians were blinded to treatment. Participants in the SNP and SNP group received SNP in phase 1 and SNP in phase 2 for the purpose of blinding, but the data in phase 2 were not included in the study results. Patients in the placebo and SNP group received placebo in phase 1 and received SNP in phase 2. If they did not respond to placebo (ie, >20% reduction on the PANSS-total) in phase 1, their data in phase 2 were included in the analyses. Patients in the placebo and placebo group received placebo in both phases; if they did not respond to placebo in phase 1, their data from phase 2 were included in the analyses. Participants were also stratified by antipsychotic treatment status: those who were taking clozapine and those who were taking an antipsychotic medication other than clozapine. The number of patients taking clozapine was restricted to 20. Finally, we used a median split to stratify participants by negative symptom severity, as measured by the PANSS-negative symptom subscale.
To monitor safety and tolerability, participants were also administered the Abnormal Involuntary Movement Scale and the Systematic Assessment for Treatment Emergent Effects (SAFTEE) prior to each infusion, after each infusion, and at follow-up.12,13 Participants also completed the Columbia-Suicide Severity Rating Scale14 as well as the Measurement and Treatment Research to Improve Cognition in Schizophrenia15 Consensus Cognitive Battery, and the University of California San Diego Performance-based Skills Assessment Brief at 3 different time points.16
Treatment
Prior to starting the initial infusion (phase 1), participants underwent 2 screening visits that included assessment of eligibility. Patients were then observed for 28 days, which included 2 treatment phases (phase 1 and phase 2) and a final follow-up visit. In both phases, participants underwent a baseline visit, including safety assessments, review of concomitant medications, electrocardiogram, and vital sign monitoring. Participants then returned for a second study visit during which they received an infusion (either SNP or placebo) and then a follow-up visit 1 week later. All participants completed a final follow-up study visit at day 28, including safety assessments, PANSS, electrocardiogram, and vital sign monitoring (eFigure 2 in Supplement 2).
The clinical trials management software generated a randomization identifier for each participant; the identifier was accessible to the site pharmacy and was used to prepare the corresponding infusion treatment. Participants received either SNP diluted with dextrose, 5%, infused at a rate of 0.5 μg/kg/min for 4 hours or a placebo solution of dextrose, 5%, infused at a rate of 0.5 μg/kg/min for 4 hours. Participants were recumbent during the infusions and blood pressure (BP), heart rate, blood oxygen saturation, and electrocardiogram results were continuously monitored during the course of the infusion.
Outcomes
The primary outcome measures examined were the PANSS total, positive, and negative scores with SNP compared with placebo across each 2-week phase. The secondary outcome evaluated the safety and tolerability of SNP compared with placebo as measured by BP and heart rate, as well as the SAFTEE and Abnormal Involuntary Movement Scale. Other outcomes examined included cognitive changes as measured by the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery and life skills as measured by the University of California San Diego Performance-based Skills Assessment Brief.
Statistical Analysis
The primary outcome regarding improvement in symptoms as measured by the PANSS total, positive, and negative scales was tested using the Tamura and Huang17 approach to SPCD for continuous data, where effect estimates from the 2 phases were weighted (using a weighted z test) to compare differences between the SNP and placebo groups. The primary outcome analysis was performed for all participants in the SNP and placebo groups, and then in distinct treatment subgroups (clozapine vs no clozapine treatment, and more severe vs less severe negative symptoms). The secondary outcome regarding safety and tolerability as measured by the SAFTEE, BP, and heart rate was examined at baseline, 7 hours after each infusion, and after the final infusion. A modified intent-to-treat analysis was used (ie, only including participants who at least started the infusion), and per the SPCD design, only placebo nonresponders were included in the phase 2 analyses, whereas all participants from phase 1 were included.
We planned to randomize a sample size of 60 participants to ensure that at least 48 participants completed the study, based on power calculations indicating that 60 participants needed to be randomized to obtain at least 81% power to detect a weighted mean difference of 10 points in PANSS total scores (9 points in phase 1 and 11 points in phase 2, with SD of 14) between the SNP group and placebo group with a type I error rate of 0.05. This calculation was based on a 20% attrition rate by the fourth week and 30% placebo response at the end of phase 1. The RCT logic calculator18 was used for this sample size calculation.
Results
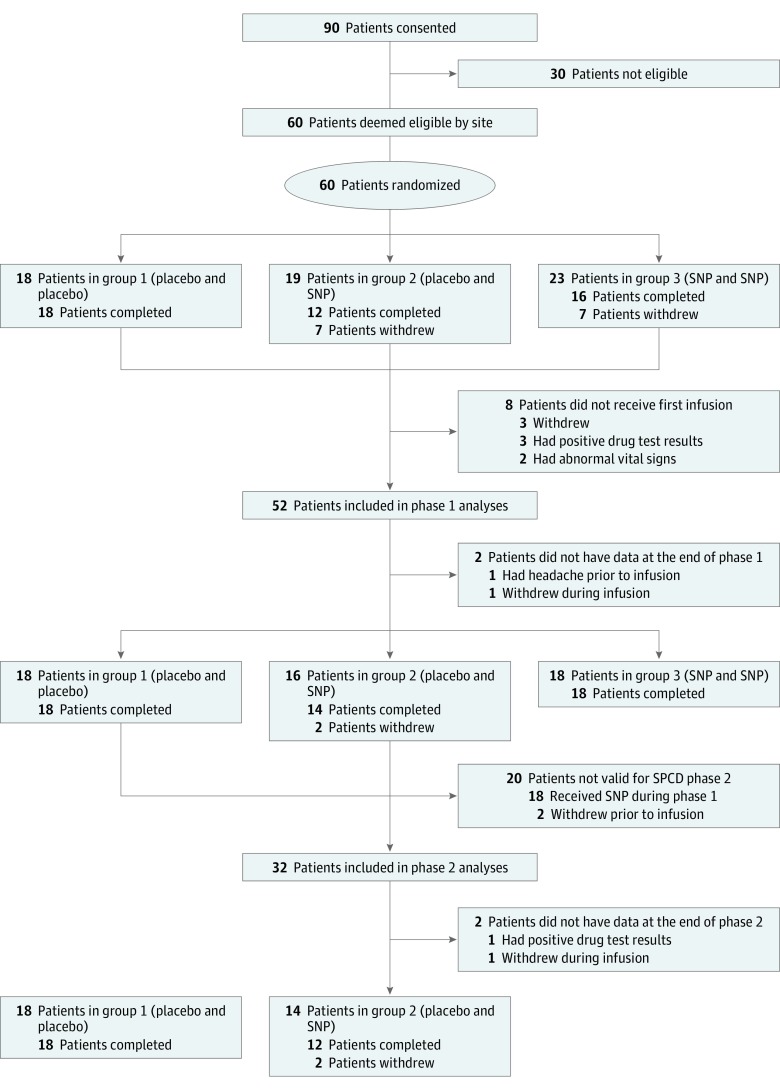
A total of 60 participants were randomized, and 52 participants (12 women and 40 men) received the first infusion and were included in the phase 1 analyses. Baseline clinical and demographic characteristics are summarized in Table 1. Briefly, in the SNP and SNP group, the mean (SD) age was 47.1 (10.5) years, 10 of 18 (55.5%) were white, 6 (33.3%) were black, 1 (5.6%) was Asian, and 1 participant’s race was identified as “other.” In the placebo and SNP group, the mean (SD) age was 45.9 (12.3) years, 9 of 16 (56.3%) were white, 5 (31.3%) were black, and 2 (12.5%) were Asian. In the placebo and placebo group, the mean (SD) age was 40.4 (11.0) years, 6 of 18 (33.3%) were white, 11 (61.1%) were black, and 1 (5.6%) was Asian. Participants had a baseline mean PANSS-total score of 81.0 (SNP and SNP group, 83.6; placebo and SNP group, 77.6; and placebo and placebo group, 81.7), which clinically corresponds to between moderately and markedly ill.19 Fifty participants (96%) completed phase 1; 2 participants terminated the study early. Of these 50 participants, 32 were included in the phase 2 outcome analyses as placebo nonresponders. Per the SPCD design, the 18 participants randomized to the SNP and SNP group were excluded from phase 2 analyses, and there were no placebo responders (ie, >20% reduction on the PANSS-total) to exclude from phase 2. However, 2 participants withdrew prior to receiving the second infusion, and thus were excluded from phase 2 analyses. Of the 32 participants who entered phase 2, 30 (94%) completed phase 2. Participant information is detailed in Figure 1.
Table 1. Baseline Clinical and Demographic Characteristics.
| Characteristic | SNP and SNP (n = 18) | Placebo and SNP (n = 16) | Placebo and Placebo (n = 18) |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD), y | 47.1 (10.5) | 45.9 (12.3) | 40.4 (11.0) |
| Female sex, No. (%) | 4 (22.2) | 4 (25.0) | 4 (22.2) |
| Hispanic ethnicity, No. (%) | 1 (5.6) | 1 (6.3) | 2 (11.1) |
| Race, No. (%) | |||
| White | 10 (55.6) | 9 (56.3) | 6 (33.3) |
| Black | 6 (33.3) | 5 (31.3) | 11 (61.1) |
| Asian | 1 (5.6) | 2 (12.5) | 1 (5.6) |
| Other | 1 (5.6) | 0 | 0 |
| Clinical Severity at Phase 1 Baseline (Visit 3) | |||
| PANSS score, mean (SD) | |||
| Total | 83.6 (10.0) | 77.6 (8.8) | 81.7 (8.8) |
| Positive | 24.9 (3.5) | 22.3 (3.4) | 22.6 (3.1) |
| Negative | 20.9 (5.5) | 20.3 (5.0) | 21.4 (4.6) |
Abbreviations: PANSS, Positive and Negative Syndrome scale; SNP, sodium nitroprusside.
Figure 1. CONSORT Flow Diagram.
SNP indicates sodium nitroprusside; and SPCD, sequential parallel comparison design.
Primary Outcome: PANSS Scores
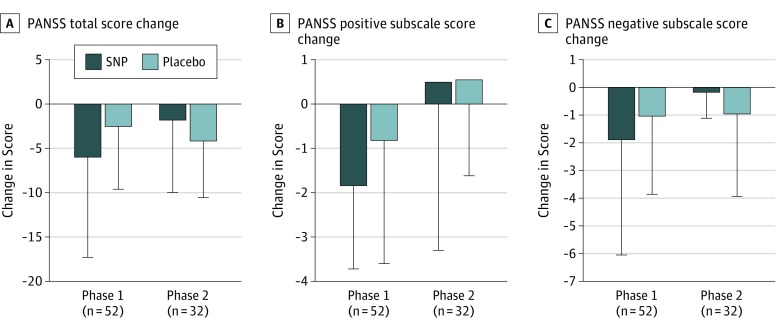
There were no significant differences between the SNP and placebo groups for change in PANSS-total (weighted β = −1.04; z = −0.59; P = .57), PANSS-positive (weighted β = −0.62; z = −0.93; P = .35), or PANSS-negative (weighted β = −0.12; z = −0.19; P = .85) scores (Figure 2 and Table 2). When stratified by treatment status at study entry, there were no significant differences between the SNP and placebo groups for PANSS-total, PANSS-positive, PANSS-negative, or PANSS-general scores in either the clozapine-treated or the non–clozapine-treated groups (eTable 1 in Supplement 2). When stratified by severity of PANSS-negative scores, there were no significant differences between the SNP and placebo groups for PANSS-total, PANSS-positive, PANSS-negative, or PANSS-general scores (eTable 2 in Supplement 2). The baseline mean (SD) PANSS-negative score was 25 (3).
Figure 2. Change in Positive and Negative Syndrome Scale (PANSS) Scores for Phases 1 and 2.
A, Mean change scores for the PANSS total score. B, Mean change scores for the PANSS positive subscale. C, Mean change scores for the PANSS negative subscale. For phase 1, change scores are scores at day 14 minus scores at day 0; for phase 2, day 28 minus day 14. Error bars represent 1 SD. SNP indicates sodium nitroprusside.
Table 2. PANSS Scores (Total, Positive, and Negative) for Phases 1 and 2.
| SPCD Phase | Total Score, Mean (SD) | Positive Subscale Score, Mean (SD) | Negative Subscale Score, Mean (SD) | |||
|---|---|---|---|---|---|---|
| SNP | Placebo | SNP | Placebo | SNP | Placebo | |
| Phase 1 (n = 52) | ||||||
| Baseline | 83.6 (10.0) | 79.8 (8.9) | 24.9 (3.5) | 22.5 (3.2) | 20.9 (5.5) | 20.9 (4.8) |
| End | 77.6 (12.2) | 76.7 (11.0) | 23.1 (4.6) | 21.3 (3.6) | 19.1 (4.7) | 19.8 (5.1) |
| Change | −6.0 (11.3) | −2.5 (7.1) | −1.8 (1.9) | −0.8 (2.8) | −1.9 (4.2) | −1.0 (2.8) |
| Phase 2 (n = 32) | ||||||
| Baseline | 74.0 (12.0) | 78.8 (10.1) | 20.7 (3.6) | 21.8 (3.6) | 18.5 (5.8) | 20.8 (4.4) |
| End | 71.8 (10.6) | 74.7 (9.0) | 21.3 (4.6) | 22.4 (3.5) | 18.1 (6.2) | 19.8 (4.7) |
| Change | −1.8 (8.2) | −4.2 (6.4) | 0.5 (3.8) | 0.6 (2.2) | −0.2 (0.9) | −0.9 (3.0) |
Abbreviations: PANSS, Positive and Negative Syndrome scale; SNP, sodium nitroprusside; SPCD, sequential parallel comparison design.
Secondary Outcome: Safety and Tolerability
There were no statistically significant differences in mean SAFTEE scores between groups at baseline, 7 hours after the first infusion, 7 hours after the second infusion, and at the final follow-up visit, either as a main effect (F2,47 = 0.68; P = .51) or an interaction effect over time (F8,47 = 1.64; P = .14). There were significant differences over time (F4,47 = 7.59; P < .001), where baseline 1 (ie, day 0), or pre-infusion SAFTEE scores, were greater than scores at the 4 subsequent assessments (visit 1 vs baseline, t47 = 3.62; P < .001; visit 2 vs baseline, t47 = 4.00; P < .001; visit 3 vs baseline, t47 = 5.47; P < .001; and visit 4 vs baseline, t47 = 3.40; P < .001). The most frequently reported symptoms at the moderate or severe level on the SAFTEE are listed in eTable 3 in Supplement 2.
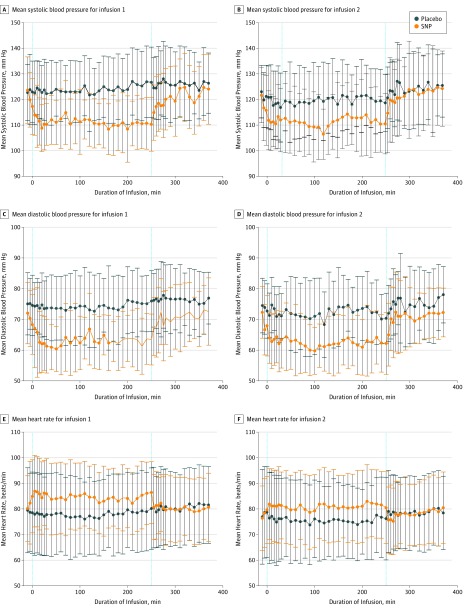
During both the first and second infusions, systolic and diastolic BP were significantly lower in the SNP groups compared with the placebo groups. After the infusions, BP returned to normal (Figure 3), but on average during the 2-hour follow-up period, BP remained detectably different for diastolic BP (both infusions) and, for the first infusion, for systolic BP. We also observed pre-infusion differences for diastolic BP (both infusions) and systolic BP (the first infusion only), but with greater uncertainty around these estimates, given that these estimates were based on only 2 observations per participant. For heart rate, although it was numerically different during both infusions (Figure 3), we did not find statistically significant differences before, during, or after the infusions.
Figure 3. Mean Blood Pressure and Heart Rate per Treatment Group for Each Infusion.
A, Mean systolic blood pressure for infusion 1. B, Mean systolic blood pressure for infusion 2. C, Mean diastolic blood pressure for infusion 1. D, Mean diastolic blood pressure for infusion 2. E, Mean heart rate for infusion 1. F, Mean heart rate for infusion 2. During phase 1 (ie, infusion 1), 18 patients received sodium nitroprusside (SNP) and 34 received placebo. During phase 2 (ie, infusion 2), 14 patients received SNP and 18 received placebo. Reference lines denote start and end of infusion.
There were no serious adverse events. One participant in the SNP group developed asymptomatic hypotension (which resolved) during the first infusion and withdrew from the study. Another participant in the SNP group became hypotensive during the second infusion and withdrew from the study.
There were no differences between groups on the Abnormal Involuntary Movement Scale total score as measured at baseline, 7 hours after the first infusion, 7 hours after the second infusion, and at the final follow-up visit, either as a main effect (F2,49 = 0.69; P = .51) or an interaction effect over time (F8,49 = 1.18; P = .33). There were also no statistically significant differences over time (F4,49 = 2.43; P = .06).
Among cognitive measures, there were no significant differences in Measurement and Treatment Research to Improve Cognition in Schizophrenia total change scores between the SNP and placebo groups (weighted β = 1.11; z = −0.98; P = .34). There were no statistically significant differences between the SNP and placebo groups for the University of California San Diego Performance-based Skills Assessment Brief total score (weighted β = 1.11; z = −0.98; P = .34).
Discussion
In this multicenter, randomized clinical trial of adjunctive intravenous SNP, we identified no evidence of efficacy for outpatients with schizophrenia who had previously failed to achieve resolution of psychotic symptoms after at least 1 trial of an antipsychotic medication. Neither primary nor secondary measures, including results of cognitive testing, demonstrated symptomatic improvement. Overall, SNP was relatively well tolerated.
These results contrast with those of the original positive placebo-controlled trial by Hallak et al6 of SNP infusion in individuals with schizophrenia. In that single-site study of 20 inpatients, a 4-hour infusion of SNP at 0.5 μg/kg/min resulted in a significant decrease in psychotic symptoms, as measured by the Brief Psychiatric Rating Scale. A follow-up placebo-controlled study of 20 patients demonstrated improvement in impairments in cognitive function in patients with schizophrenia.8 There are some key differences between our study and the initial study by Hallak et al.6 In contrast to the initial study by Hallak et al,6 which included 20 inpatients at 1 site, our study included 52 outpatients at 4 sites, making the present results more generalizable and providing sufficient power to detect differences between groups. Another difference between the 2 studies is the age of the patients: those in our study are significantly older (SNP and SNP group: mean age, 47.1 years; SNP and placebo group: mean age, 45.9 years; and placebo and placebo group: mean age, 40.4 years) than those in the previous study (SNP group, 25.5 years; and placebo group, 25.6 years). Our results are similar to those of 2 subsequent studies that failed to show any benefit of intravenous SNP either in reducing psychotic symptoms or in improving cognition.9,20 Stone et al9 found no improvement in spatial working memory performance among patients with schizophrenia; like our study, that study also consisted of a significantly older population, which presumes a longer illness course. Another randomized, double-blind placebo-control study of 42 individuals with schizophrenia did not demonstrate any improvement in scores on the PANSS or Weschler Adult Intelligence Scale after a 4-hour intravenous SNP infusion, and also included a population with longer duration of illness.20 The patients in the study by Hallak et al6 were younger and likely earlier in the course of illness; we cannot exclude the possibility that SNP could be more effective for those with recent-onset psychosis. Moreover, as Maia-de-Oliveira and colleagues21 note, the study by Stone et al9 included 7 cannabis users and 12 cigarette smokers; both substances may affect the effectiveness of SNP. Although we did not include cannabis users in our study, we did include cigarette smokers (20 total), as there is a high prevalence of cigarette use among individuals with schizophrenia. We also note in our study, as in the study by Stone et al9 but not in the study by Hallak et al,6 there was a reduction in BP in the SNP group, but not in the placebo group. This decrease in BP may be owing to the older mean age of the participants; SNP may be better tolerated in a younger population.
Similar to the studies by Stone et al9 and Wang et al,20 our patient population had less severe negative symptoms than the population in the study by Hallak et al.6 Sodium nitroprusside may be most effective in decreasing severe negative symptoms; this effectiveness may be owing in part to its actions as an NMDA receptor modulator.22
In addition to the aforementioned randomized trials, 1 open-label study examining intravenous SNP treatment in 2 patients with clozapine-refractory (“ultra-treatment resistant”) schizophrenia demonstrated significant improvement of both positive and negative symptoms within 1 hour after infusion.23 Conversely, we did not detect any differences in PANSS scores between the SNP and placebo groups within our treatment-resistant (clozapine-treated) population. Finally, we used the PANSS-positive subscale to examine positive symptoms, differing from the initial study by Hallak et al6 in which the Brief Psychiatric Rating Scale was used. Participants in our study had a baseline mean PANSS-total score of 81.0 (SNP and SNP group, 83.6; placebo and SNP group, 77.6; and placebo and placebo group, 81.7), which clinically corresponds to between moderately and markedly ill.19 In the study by Hallak et al,6 participants had a Brief Psychiatric Rating Scale baseline score of approximately 22 (maximum score, 72), suggesting a less globally ill population. As such, we also cannot exclude the possibility that SNP treatment could be more effective in treating less severe positive psychotic symptoms, although this seems unlikely. We also consider the possibility that SNP may be more effective not as an adjunctive treatment, but as a single agent (ie, in patients not taking antipsychotic medications).
The exact mechanisms through which SNP could decrease psychotic symptoms is not fully understood. Sodium nitroprusside, which in clinical practice is used as an antihypertensive agent, is converted to NO, resulting in vascular smooth muscle relaxation and vasodilation. The NO may act to increase cerebral perfusion to regions that have decreased areas of blood flow in patients with schizophrenia, perhaps exerting an antipsychotic effect.24 It has also been postulated that the NO acts a neural modulator; SNP releases NO, activating soluble guanylate cyclase, producing cyclic guanosine monophosphate.25 As mentioned, activation of the NMDA receptor also produces intracellular NO release through activation of nNOS.7 The NMDA-nNOS-cyclic guanosine monophosphate pathway is involved in long-term potentiation and neuroplasticity, mediated partially by cyclic guanosine monophosphate phosphorylating cAMP response element-binding protein and protein kinase B. Nitric oxide is a signaling molecule that is also downstream from the NMDA receptor; there is evidence that hypofunction of the NMDA receptor plays a role in the pathogenesis of schizophrenia.26 Finally, in vitro studies of neurons derived from patients with schizophrenia provide evidence that NO production is decreased, suggesting that NO is also decreased in brains of living patients.27 Conversely, overproduction of NO has also been associated with neurotoxic effects and may play a role in some neurodegenerative disorders.28,29 Thus, there may be a critical balance of NO that must be maintained, suggesting that any benefit of interventions such as SNP could be dose specific.
Limitations
We note several important limitations in interpreting our results. As mentioned, our participants were on average older and likely to have experienced multiple episodes of psychosis, so our results are not perfectly comparable with the original results of Hallak et al.6 We used intravenous SNP only at 1 dose and 1 duration of treatment, and therefore cannot investigate dose dependence of effects (eg, if older individuals with a longer illness course require a higher dose and longer treatment duration). A further limitation is that we did not exclude cigarette smokers from our study; nicotine in cigarettes can interfere with NO, potentially diminishing the efficacy of SNP.30 Finally, we did not use a precision-based medicine approach to stratify groups; this method could potentially be used in future studies to identify SNP responders.
Conclusions
Antipsychotic-treated patients with schizophrenia with some evidence of treatment resistance did not show significant improvement in psychotic symptoms or cognition after an infusion of SNP across any of the measured outcomes. As with any treatment, particular subgroups may respond differently; if further studies of NO donors are pursued, they might focus on a younger, nonsmoking population earlier in the course of illness, with alternate dosing and duration of drug delivery.
Trial Protocol
eFigure 1. Sequential Parallel Comparison Design (SPCD)
eFigure 2. Study Schema
eTable 1. Positive and Negative Syndrome Scale (PANSS) Scores Stratified by Treatment Status
eTable 2. Positive and Negative Syndrome Scale (PANSS) Scores Stratified by PANSS-Negative Subscores
eTable 3. Percentage of Participants Reporting Experiencing SAFTEE Symptoms at “Moderate” or “Severe” Level for the 10 Most Frequently Reported Symptoms Post Randomization
Data Sharing Statement
References
- 1.Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063-2071. doi: 10.1016/S0140-6736(12)60239-6 [DOI] [PubMed] [Google Scholar]
- 2.Green MF. Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J Clin Psychiatry. 2016;77(suppl 2):8-11. doi: 10.4088/JCP.14074su1c.02 [DOI] [PubMed] [Google Scholar]
- 3.Mousavi SG, Rostami H, Sharbafchi MR, Boroujeni AS, Mahaki B. Onset of action of atypical and typical antipsychotics in the treatment of acute psychosis. J Res Pharm Pract. 2013;2(4):138-144. doi: 10.4103/2279-042X.128142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777. doi: 10.1177/0706743717718167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elkis H, Buckley PF. Treatment-resistant schizophrenia. Psychiatr Clin North Am. 2016;39(2):239-265. doi: 10.1016/j.psc.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 6.Hallak JE, Maia-de-Oliveira JP, Abrao J, et al. Rapid improvement of acute schizophrenia symptoms after intravenous sodium nitroprusside: a randomized, double-blind, placebo-controlled trial. JAMA Psychiatry. 2013;70(7):668-676. doi: 10.1001/jamapsychiatry.2013.1292 [DOI] [PubMed] [Google Scholar]
- 7.Szabadits E, Cserép C, Szonyi A, et al. NMDA receptors in hippocampal GABAergic synapses and their role in nitric oxide signaling. J Neurosci. 2011;31(16):5893-5904. doi: 10.1523/JNEUROSCI.5938-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maia-de-Oliveira JP, Abrao J, Evora PR, et al. The effects of sodium nitroprusside treatment on cognitive deficits in schizophrenia: a pilot study. J Clin Psychopharmacol. 2015;35(1):83-85. doi: 10.1097/JCP.0000000000000258 [DOI] [PubMed] [Google Scholar]
- 9.Stone JM, Morrison PD, Koychev I, et al. The effect of sodium nitroprusside on psychotic symptoms and spatial working memory in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. Psychol Med. 2016;46(16):3443-3450. doi: 10.1017/S0033291716002245 [DOI] [PubMed] [Google Scholar]
- 10.Fava M, Evins AE, Dorer DJ, Schoenfeld DA. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom. 2003;72(3):115-127. doi: 10.1159/000069738 [DOI] [PubMed] [Google Scholar]
- 11.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 12.Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22(2):343-381. [PubMed] [Google Scholar]
- 13.Munetz MR, Benjamin S. How to examine patients using the Abnormal Involuntary Movement Scale. Hosp Community Psychiatry. 1988;39(11):1172-1177. [DOI] [PubMed] [Google Scholar]
- 14.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery Manual. Los Angeles, CA: MATRICS Assessment, Inc; 2006. [Google Scholar]
- 16.Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull. 2007;33(6):1364-1372. doi: 10.1093/schbul/sbm014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamura RN, Huang X. An examination of the efficiency of the sequential parallel design in psychiatric clinical trials. Clin Trials. 2007;4(4):309-317. doi: 10.1177/1740774507081217 [DOI] [PubMed] [Google Scholar]
- 18.Ivanova A, Qaqish B, Schoenfeld DA. Optimality, sample size, and power calculations for the sequential parallel comparison design. Stat Med. 2011;30(23):2793-2803. doi: 10.1002/sim.4292 [DOI] [PubMed] [Google Scholar]
- 19.Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res. 2005;79(2-3):231-238. doi: 10.1016/j.schres.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Zhao J, Hu Y, et al. Sodium nitroprusside treatment for psychotic symptoms and cognitive deficits of schizophrenia: a randomized, double-blind, placebo-controlled trial. Psychiatry Res. 2018;269:271-277. doi: 10.1016/j.psychres.2018.08.079 [DOI] [PubMed] [Google Scholar]
- 21.Maia-de-Oliveira JP, Baker GB, Dursun SM, Hallak JE. Letter to the Editor: Sodium nitroprusside for schizophrenia: could methodological variables account for the different results obtained? Psychol Med. 2017;47(5):981-982. doi: 10.1017/S003329171600307X [DOI] [PubMed] [Google Scholar]
- 22.Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 2011;25(10):859-885. doi: 10.2165/11586650-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 23.Maia-de-Oliveira JP, Belmonte-de-Abreu P, Bressan RA, et al. Sodium nitroprusside treatment of clozapine-refractory schizophrenia. J Clin Psychopharmacol. 2014;34(6):761-763. doi: 10.1097/JCP.0000000000000217 [DOI] [PubMed] [Google Scholar]
- 24.Pinkham A, Loughead J, Ruparel K, et al. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res. 2011;194(1):64-72. doi: 10.1016/j.pscychresns.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hottinger DG, Beebe DS, Kozhimannil T, Prielipp RC, Belani KG. Sodium nitroprusside in 2014: a clinical concepts review. J Anaesthesiol Clin Pharmacol. 2014;30(4):462-471. doi: 10.4103/0970-9185.142799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26(4-6):365-384. doi: 10.1007/s10571-006-9062-8 [DOI] [PubMed] [Google Scholar]
- 27.Brennand KJ, Simone A, Jou J, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473(7346):221-225. doi: 10.1038/nature09915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Contestabile A, Ciani E. Role of nitric oxide in the regulation of neuronal proliferation, survival and differentiation. Neurochem Int. 2004;45(6):903-914. doi: 10.1016/j.neuint.2004.03.021 [DOI] [PubMed] [Google Scholar]
- 29.Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996;10(3-4):179-190. doi: 10.1016/0891-0618(96)00148-2 [DOI] [PubMed] [Google Scholar]
- 30.Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. 2013;10(4):219-230. doi: 10.1038/nrcardio.2013.8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Sequential Parallel Comparison Design (SPCD)
eFigure 2. Study Schema
eTable 1. Positive and Negative Syndrome Scale (PANSS) Scores Stratified by Treatment Status
eTable 2. Positive and Negative Syndrome Scale (PANSS) Scores Stratified by PANSS-Negative Subscores
eTable 3. Percentage of Participants Reporting Experiencing SAFTEE Symptoms at “Moderate” or “Severe” Level for the 10 Most Frequently Reported Symptoms Post Randomization
Data Sharing Statement