Abstract
Importance
The Centers for Medicare & Medicaid Services national coverage determination for transcatheter aortic valve replacement (TAVR) includes volume requirements for surgical aortic valve replacement (SAVR) for hospitals seeking to initiate or continue TAVR programs. Evidence regarding the association between SAVR volume and TAVR outcomes is limited.
Objective
To examine the association of hospital SAVR and combined SAVR and TAVR volumes with patient outcomes of TAVR procedures performed within 1 year, 2 years, and for the entire period after initiation of TAVR programs.
Design, Setting, and Participants
This observational cohort study included 60 538 TAVR procedures performed in 438 hospitals between October 1, 2011, and December 31, 2015, among Medicare beneficiaries.
Main Outcomes and Measures
The associations between SAVR volume, SAVR and TAVR volumes, and risks of death, death or stroke, and readmissions within 30 days were determined using a hierarchical logistic regression model adjusting for patient and hospital characteristics. The association between SAVR and SAVR and TAVR volumes and 1-year and 2-year mortality after TAVR procedures was determined using a multivariable proportional hazard model with a robust variance estimator. The associations for procedures performed within 1 year, 2 years, and for the entire period after initiation of TAVR programs were examined.
Results
Among the 60 538 patients, 29 173 were women and 31 365 were men, with a mean (SD) age of 82.3 (8.0) years. Hospitals with high SAVR volume (mean annual volume, ≥97 per year) were more likely to adopt TAVR early and had a higher growth in TAVR volumes over time (median TAVR volume by hospitals with high SAVR volume and low SAVR volume: year 1, 32 vs 19; year 2, 48 vs 28; year 3, 82 vs 38; year 4, 118 vs 54; P < .001). In adjusted analysis, high hospital SAVR volume alone was not associated with better patient outcomes after TAVR. When hospital TAVR and SAVR volumes were jointly analyzed, patients treated in hospitals with high TAVR volume had lower 30-day mortality after TAVR (high TAVR and low SAVR vs low TAVR and low SAVR: odds ratio, 0.85; 95% CI, 0.72-0.99; high TAVR and high SAVR vs low TAVR and high SAVR: odds ratio, 0.81; 95% CI, 0.69-0.95), the effect of which was more pronounced when hospitals also had high SAVR volume. Patients treated in hospitals with high SAVR volume and high TAVR volume had the lowest 30-day mortality (vs hospitals with low SAVR volume and TAVR volume: odds ratio, 0.77; 95% CI, 0.66-0.89).
Conclusions and Relevance
Hospitals with high SAVR volume are most likely to be fast adopters of TAVR. Hospital SAVR volume alone is not associated with better TAVR outcomes. Accumulating high volumes of TAVR is associated with lower mortality after TAVR, particularly when hospitals have high SAVR volumes. Hospitals with high caseloads of both SAVR and TAVR are likely to achieve the best outcomes.
This cohort study examines the association of surgical aortic valve replacement with patient outcomes of transcatheter aortic valve replacement procedures performed within 1 year, 2 years, and for the entire period after initiation of transcatheter aortic valve replacement programs.
Key Points
Question
Is higher hospital volume of surgical aortic valve replacement associated with better short- and long-term patient outcomes after transcatheter aortic valve replacement?
Findings
This all-inclusive national cohort study found that hospitals with higher volumes of surgical aortic valve replacement were more likely to be faster adopters of transcatheter aortic valve replacement. When hospitals with high volume of surgical aortic valve replacement accumulate high volumes of transcatheter aortic valve replacement, they might have the best short- and long-term mortality after transcatheter aortic valve replacement, particularly when compared with hospitals with lower volumes for both procedures.
Meaning
Accumulating both surgical and transcatheter aortic valve replacement caseloads is likely to be associated with the best survival outcomes after transcatheter aortic valve replacement procedures.
Introduction
Since being approved by the US Food and Drug Administration in November 2011, transcatheter aortic valve replacement (TAVR) has become the main interventional option for patients with severe aortic stenosis who are ineligible or at very high risk to undergo surgical aortic valve replacement (SAVR).1,2,3,4,5,6 The Centers for Medicare & Medicaid Services set criteria to promote the conduct of higher-quality TAVR in the national coverage determination. One of the key criteria is the requirement of SAVR volumes for hospitals seeking to initiate and continue TAVR programs. The national coverage determination requirement was at least 50 SAVR procedures during the year prior to beginning a new TAVR program and at least 20 SAVRs per year for a continuing TAVR program.7
Procedural volume requirements for SAVR, TAVR, and percutaneous coronary intervention for TAVR programs have been continuously scrutinized and led to convening of Medicare Evidence Development & Coverage Advisory Committee panels, such as the one that was convened on July 25, 2018, to specifically address SAVR volume effect.8,9 Previous studies demonstrated that hospitals performing more TAVR procedures in a single year had fewer postoperative adverse events10,11 and that accumulated experience of TAVR by hospitals was associated with improved postoperative outcomes.12 However, none of the volume outcome studies investigated the association of hospital SAVR volume with adoption and outcomes of TAVR.
Professional societies released statements on operator and institutional recommendations for TAVR programs in 2012 and updated the recommendations in 2018; both emphasized the importance of the volume of related procedures in optimizing patient outcomes.13,14,15 With important clinical and policy implications, it is critical to understand the association between hospital SAVR volume and the outcomes of TAVR. The objective of this study is to examine the association between hospital SAVR volume and TAVR outcomes by evaluating the association of SAVR volume and combined SAVR and TAVR volumes with TAVR outcomes for procedures performed within 1 year, 2 years, and for the entire period after the initiation of TAVR programs.
Methods
Data Source and Study Population
We used Medicare Provider and Analysis Review and Master Beneficiary Summary Files data for this observational cohort study. Medicare Provider and Analysis Review files contain inpatient hospital and skilled nursing facility claims records for Medicare fee-for-service beneficiaries. Collected information includes patient demographic characteristics, diagnoses, procedures, admission type, discharge disposition, and payment and charge amounts. Master Beneficiary Summary Files contain information on patient enrollment and death. A unique identifier assigned to every beneficiary allows longitudinal follow-up of patients to ascertain death from the Master Beneficiary Summary Files as well as 30-day stroke and readmission events from the Medicare Provider and Analysis Review. This study used Medicare claims data and was determined to be exempt by Weill Cornell Medical College Institutional Review Board (protocol No. 1308014266). Patients were not involved in developing the research question, study outcome measures, study design, or conduct of the study and informed consent was waived.
We identified TAVR procedures performed between October 1, 2011, and December 31, 2015, using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and ICD-10-CM procedure codes (ICD-9-CM codes 35.05 and 35.06; ICD-10-CM codes 02RF37Z/H, 02RF38Z/H, 02RF3JZ/H, and 02RF3KZ/H) from the Medicare Provider and Analysis Review files. We excluded procedures performed at hospitals where there were only 1 or 2 cases recorded during the 5-year period. When a hospital had no case recorded for at least 1 year between a single case in earlier years and continuously recorded cases in later years, the single case in early years was excluded. In both situations, these procedures likely represented special cases that differed significantly from cases after the formal initiation of a TAVR program at the hospital.
Exposures and Outcomes
The exposure variable of interest is hospital SAVR volume. Surgical aortic valve replacement volume did not change significantly across years; therefore, annual SAVR volume was calculated, and averaged across all years for every hospital and dichotomized into high (≥97 per year) and low (<97 per year) categories based on the median of the entire cohort. We assessed the effect of hospital SAVR volume in 2 ways. First we evaluated the sole association between hospital SAVR volume and TAVR outcomes of patients, where hospital TAVR volume was treated as a covariate. Then, to account for the synergic effect of SAVR volume and accumulated TAVR performance, we also assessed the association of SAVR volume in combination with hospital TAVR volume with patient outcomes. Because there is a collinearity between hospital TAVR and SAVR volumes, we created a 4-category variable jointly defined by these 2 variables, with categories being low SAVR and low TAVR, high SAVR and low TAVR, low SAVR and high TAVR, and high SAVR and high TAVR.
As TAVR volume increased over time, we counted from the first TAVR procedure conducted in a specific hospital and calculated TAVR volume for every 365-day period. For hospitals that started TAVR programs in 2015 and had been performing TAVR for less than 1 year, the number of procedures per month was calculated to project TAVR volume in year 1. For the year periods being investigated, a hospital was determined to be high volume if the numbers of TAVR procedures it performed during these years were above the median (year 1 median, 35; year 2 median, 52; year 3 median, 84; and year 4 median, 137) for most of the time. For example, for analysis of procedures performed within 1 year after initiating TAVR programs, hospitals performing 35 TAVRs or more that year were considered to have a high TAVR volume. For analysis of procedures performed within 2 years after initiation of a TAVR program, hospitals performing 35 TAVRs or more in year 1 and 52 TAVRs or more in year 2 were considered to have a high TAVR volume. For analysis of the entire 4-year period, hospitals performing TAVR procedures above the median for at least 3 years were considered to have a high TAVR volume.
The primary outcome of the study was patient mortality within 30 days after their TAVR procedure. Secondary outcomes were patient 30-day mortality or stroke, 30-day hospital readmission, and 1-year and 2-year mortality. Mortality was ascertained from the Master Beneficiary Summary Files based on beneficiaries’ date of death. Stroke was identified both in the hospital and during readmission or skilled nursing facility stay within 30 days after the procedures, using ICD-9-CM and ICD-10-CM diagnosis codes. For postoperative stroke during the index hospitalization, stroke events were determined by intraoperative or postoperative stroke codes (ICD-9-CM code 997.02; and ICD-10-CM codes G97.32, I97.820, and I99.810), hemorrhagic stroke codes (ICD-9-CM codes 430 and 431; ICD-10-CM codes I60 and I61), and ischemic stroke or nonspecific stroke codes without a present-on-admission indicator (ICD-9-CM codes 433.x1, 434.x1, and 436; ICD-10-CM codes I63 and I67.89). Stroke events during follow-up were determined by any hemorrhagic, ischemic, and nonspecific stroke codes, regardless of present-on-admission status. This is a revised scheme developed based on previous literature reporting the use and validation of stroke codes.16,17 Readmission within 30 days was defined as readmission to hospitals, excluding skilled nursing facility stays.
Covariates
Patient characteristics included demographics (age, sex, and race/ethnicity), 1-year comorbidities, recent conditions (within 90 days prior to the procedure), previous interventions, and procedural variables. Comorbidities, recent conditions, and procedural variables were chosen based on variables included in the calculation of the EuroSCORE18 and the Elixhauser comorbidity index.19 Recent conditions were cardiovascular events (acute myocardial infarction, carotid stenosis, and endocarditis), atrial fibrillation or flutter, and renal failure. Previous interventions examined were previous valve interventions, previous coronary artery bypass graft or septal defect repair, and previous percutaneous coronary intervention. Procedural variables for the index procedure were calendar year when the procedure was performed, approach (transfemoral vs transapical), elective status, and concurrent percutaneous coronary intervention. Hospital characteristics were teaching status, region, mean annual volume of percutaneous coronary intervention (median, 330 per year), and cumulative hospital experience of TAVR. Cumulative hospital experience of TAVR was defined based on the case sequence approach used by Carroll et al12 and calculated as the number of procedures performed by the hospital up to the target procedure.
Statistical Analysis
Descriptive analyses were performed to examine hospital characteristics and trends in the use of TAVR by hospitals over years. For every calendar year, the numbers of hospitals starting to perform TAVR and those continuing to perform TAVR were calculated. To assess the differences in hospital characteristics associated with the time they started performing TAVR, the proportion of hospitals that had high SAVR volume was examined by year of first TAVR procedure. The Cochran-Armitage test was used to evaluate the trends over years. To assess the association between hospital SAVR volume and the use of TAVR and SAVR, we compared median hospital volumes of TAVR and SAVR during every subsequent year (365-day period) after the initiation. Differences were assessed using the Wilcoxon rank-sum test for each individual year. A quantile regression model was used to examine whether the trends in use of TAVR and SAVR over time were different between hospitals with low and high SAVR volumes.
Patient characteristics were examined and compared between groups defined by hospital SAVR volume and by combined SAVR and TAVR volumes. Differences between groups were assessed with t tests, 1-way analysis of variance, and χ2 tests. Numbers of events and percentages were calculated for 30-day outcomes by exposure groups. Longer-term mortality (1-year and 2-year) was analyzed as a time-to-event variable, censoring at the end of 2015. A Kaplan-Meier analysis was used to obtain estimated risks of death at the end of 1 and 2 years after the index TAVR procedure. We analyzed the outcomes for procedures performed within 1 year after initiation of TAVR programs, procedures performed within 2 years after initiation, and within all years after initiation.
To account for differences in baseline characteristics and clustering of patients within hospitals, generalized linear mixed models with a hospital random effect were used to compare 30-day outcomes between exposure groups, adjusting for patient demographics, comorbidities, procedure characteristics, and hospital factors. Patients who were discharged in December 2015 were excluded from the analysis of 30-day outcomes to ensure follow-up for all patients. Cox proportional hazards regression models with a robust sandwich variance estimate were used to compare 1-year and 2-year mortality between groups after index TAVR procedures.
A sensitivity analysis was performed to include only transfemoral TAVR. In this analysis, TAVR volume and experience were recalculated to include only transfemoral cases. Sensitivity analyses were also performed to test other definitions for high TAVR and SAVR volumes, including Leapfrog Group–recommended SAVR volume (120 per year, with estimated 70% being Medicare recipients), the 2018 recommendation for TAVR volume (50 per year or 100 in the prior 2 years), and the lowest or highest tertiles for TAVR volume definition. All analyses were performed using SAS, version 9.4 (SAS Institute Inc). All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Results
Hospital Characteristics and Trends in TAVR Use by Hospitals
This study included 60 538 TAVR procedures performed by 438 hospitals among Medicare beneficiaries between October 1, 2011, and December 31, 2015. Of the 438 hospitals that performed TAVR between 2011 and 2015, 167 (38.1%) were teaching hospitals (eTable 1 in the Supplement). There was a median of 83 (interquartile range, 34-182) TAVR procedures per site performed during 2011-2015.
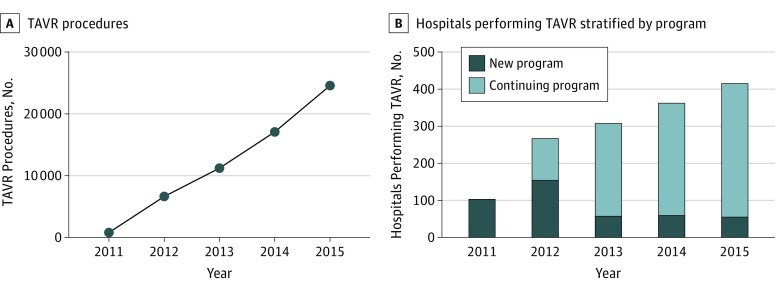
From 2012 to 2015, there was an increase in the number of TAVRs annually from 6672 to 24 651 cases (Figure 1). From 2011 to 2015, the number of hospitals performing TAVR increased from 113 to 415. Hospitals that started TAVR programs in earlier years were more likely to have high SAVR volume (55 [51.3%] in 2011 vs 4 [7.3%] in 2015; P < .001 for trend), and more likely to be teaching facilities (70 [61.9%] in 2011 vs 7 [12.7%] in 2015; P < .001 for trend), when compared with hospitals that started TAVR programs in later years.
Figure 1. Trends in Number of Transcatheter Aortic Valve Replacement (TAVR) Procedures and Number of Hospitals Performing TAVR Procedures, 2011-2015 Medicare Fee-for-Service.
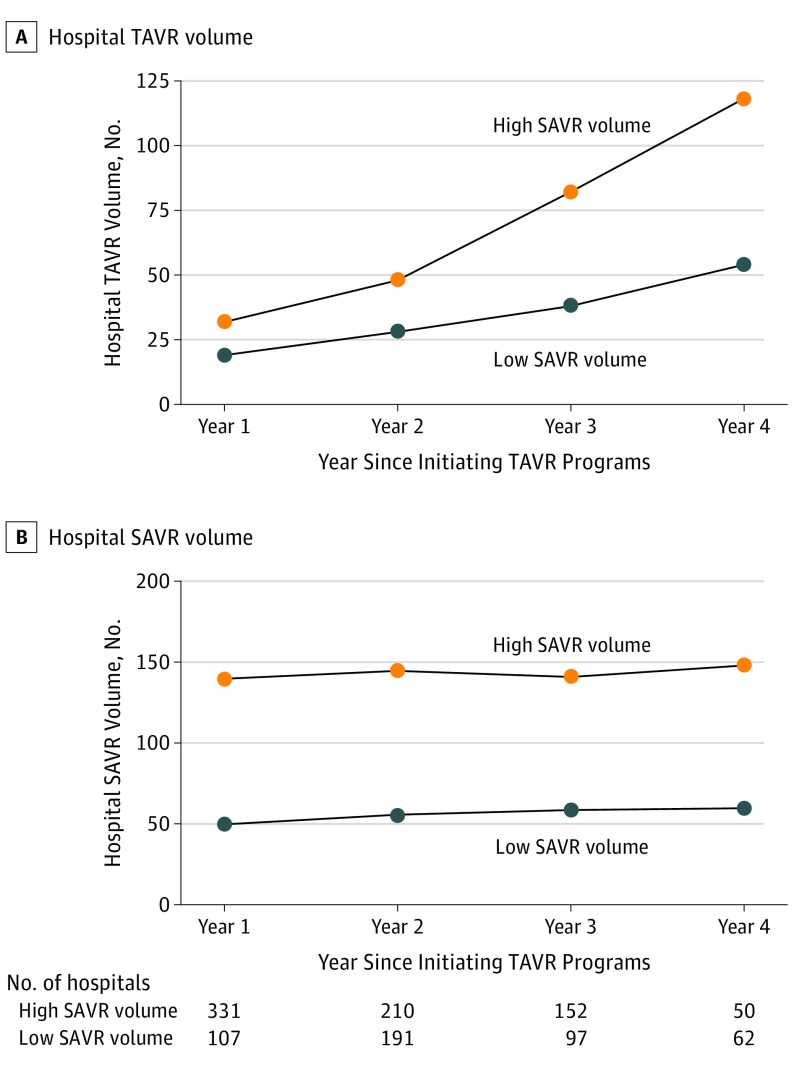
When compared with hospitals with low SAVR volume, those with high SAVR volume tended to perform more TAVR (median TAVR volume by hospitals with high SAVR volume and hospitals with low SAVR volume: year 1, 32 vs 19; year 2, 48 vs 28; year 3, 82 vs 38; year 4, 118 vs 54; P < .001 for all comparisons) and had higher growth of TAVR procedures over time (P < .001 from quantile regression, Figure 2A). There was no significant change in hospital SAVR volumes over time for hospitals with low SAVR volume and those with high SAVR volume after initiating TAVR programs (median SAVR volume: hospitals with high SAVR volume, 140 in year 1 to 149 in year 4; hospitals with low SAVR volume, 50 in year 1 to 60 in year 4; P = .60 from quantile regression, Figure 2B).
Figure 2. Trends in Median Volume of Transcatheter Aortic Valve Replacement (TAVR) and Surgical Aortic Valve Replacement (SAVR) Procedures of Facilities Since Initiating TAVR, Stratified by Category of SAVR Volume of the Hospital.
Patient Characteristics
The mean (SD) age of patients in the cohort was 82.3 (8.0) years (Table 1). Almost half the patients were women (29173 [48.2%]) and most were white (56201 of 60353 [93.1%]). When comparing patient characteristics between groups by SAVR volume, patients receiving the procedure in hospitals with high SAVR volume were more likely to have chronic heart failure (25485 of 31084 [82.0%] vs 23224 of 29454 [78.9%]; P < .001), atrial fibrillation or flutter (13553 of 31084 [43.6%] vs 12262 of 29454 [41.6%]; P < .001), and renal failure (3203 of 31084 [10.3%] vs 2830 of 29454 [9.6%]; P = .004). They were also more likely to be urgent procedures (7654 of 31084 [24.6%] vs 5683 of 29454 [19.3%]; P < .001). When examining patient characteristics stratified by hospital TAVR and SAVR volume (eTable 2 in the Supplement), patients undergoing the procedures at hospitals with high TAVR and high SAVR volumes had more comorbidities compared with others.
Table 1. Characteristics of Patients Undergoing TAVR Procedures by Hospital SAVR Volume.
| Characteristic | Patients, No. (%) | P Value | All Patients, No. (%) (N = 60 538) | |
|---|---|---|---|---|
| Low SAVR (n = 29 454) | High SAVR (n = 31 084) | |||
| Age, mean (SD), y | 82.1 (8.0) | 82.5 (8.1) | <.001 | 82.3 (8.0) |
| Female sex | 14 167 (48.1) | 15 006 (48.3) | .66 | 29 173 (48.2) |
| White race | 27 130/29354 (92.4) | 29 071/30999 (93.8) | <.001 | 56 201/60353 (93.1) |
| Comorbidities within 1 y | ||||
| Coronary artery disease | 23 379 (79.4) | 24 848 (79.9) | .09 | 48 227 (79.7) |
| Hypertension | 27 130 (92.1) | 28 729 (92.4) | .15 | 55 859 (92.3) |
| Chronic heart failure | 23 224 (78.9) | 25 485 (82.0) | <.001 | 48 709 (80.5) |
| Diabetes | 11 969 (40.6) | 12 323 (39.6) | .01 | 24 292 (40.1) |
| Chronic pulmonary disease | 11 780 (40.0) | 12 461 (40.1) | .81 | 24 241 (40.0) |
| Obesity | 5738 (19.5) | 5896 (19.0) | .11 | 11 634 (19.2) |
| Cerebrovascular disease | 2394 (8.1) | 2614 (8.4) | .21 | 5008 (8.3) |
| Peripheral artery disease | 8313 (28.2) | 8879 (28.6) | .35 | 17 192 (28.4) |
| Recent conditions within 90 d | ||||
| Cardiovascular eventsa | 4655 (15.8) | 4790 (15.4) | .18 | 9445 (15.6) |
| Atrial fibrillation or flutter | 12 262 (41.6) | 13 553 (43.6) | <.001 | 25 815 (42.6) |
| Renal failure | 2830 (9.6) | 3202 (10.3) | .004 | 6032 (10.0) |
| Previous valve intervention | 3543 (12.0) | 3936 (12.7) | .02 | 7479 (12.4) |
| Previous CABG or septal defect repair | 3524 (12.0) | 3748 (12.1) | .72 | 7272 (12.0) |
| Previous PCI | 6588 (22.4) | 6889 (22.2) | .54 | 13 477 (22.3) |
| Approach of procedure | <.001 | |||
| Transfemoral | 24 804 (84.2) | 27 157 (87.4) | 51 961 (85.8) | |
| Transapical | 4650 (15.8) | 3927 (12.6) | 8577 (14.2) | |
| Elective procedure | 23 771 (80.7) | 23 430 (75.4) | <.001 | 47 201 (78.0) |
| Concurrent PCI | 1004 (3.4) | 1047 (3.4) | .78 | 2051 (3.4) |
| Teaching hospital | 13 313 (45.2) | 23 386 (75.2) | <.001 | 36 699 (60.6) |
| Hospital region | <.001 | |||
| Northeast | 4908 (16.7) | 11 187 (36.0) | 16 095 (26.6) | |
| Midwest | 6617 (22.4) | 7352 (23.6) | 13 969 (23.1) | |
| South | 11 521 (39.1) | 8145 (26.2) | 19 666 (32.5) | |
| West | 6408 (21.8) | 4400 (14.2) | 10 808 (17.9) | |
| Hospital TAVR volume (all years) | <.001 | |||
| Low | 21 113 (71.7) | 10 703 (34.4) | 31 816 (52.6) | |
| High | 8341 (28.3) | 20 381 (65.6) | 28 722 (47.4) | |
| Hospital PCI volume | <.001 | |||
| Low | 19 977 (67.8) | 10 258 (33.0) | 30 235 (49.9) | |
| High | 9477 (32.2) | 20 826 (67.0) | 30 303 (50.1) | |
Abbreviations: CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Recent cardiovascular conditions included acute myocardial infarction, carotid stenosis, and endocarditis.
Association Between Hospital SAVR Volume and Patient Outcomes
When assessing the association between hospital SAVR volume and TAVR outcomes without considering the collinearity between SAVR and TAVR volume, there was no significant difference in 30-day outcomes or 1-year and 2-year mortality between patients who underwent TAVR in hospitals with low SAVR volume and hospitals with high SAVR volume (Table 2), within 1 year, 2 years, or for the entire period after hospitals initiated TAVR programs. In this model, higher hospital TAVR volume was associated with better short- and long-term patient outcomes after TAVR, when SAVR volume was controlled for and data from the entire period were analyzed.
Table 2. Outcomes Among Medicare Beneficiaries Undergoing TAVR Procedures at Hospitals With Low and High SAVR or TAVR Volume.
| Characteristic | Procedures Performed in the First Year | Procedures Performed in the First 2 y | Procedures Performed in All Years | |||
|---|---|---|---|---|---|---|
| Events, No./Total No. (%) | Adjusted OR (95% CI) | Events, No./Total No. (%) | Adjusted OR (95% CI) | Events, No./Total No. (%) | Adjusted OR (95% CI) | |
| Patients, No. | 10 962 | 24 442 | 58 337 | |||
| 30-d Mortality | ||||||
| SAVR volume | ||||||
| Low | 450/6526 (6.9) | 1 [Reference] | 866/13924 (6.2) | 1 [Reference] | 1549/28263 (5.5) | 1 [Reference] |
| High | 294/4436 (6.6) | 0.96 (0.78-1.18) | 635/10518 (6.0) | 0.93 (0.80-1.09) | 1447/30074 (4.8) | 0.93 (0.83-1.04) |
| TAVR volume | ||||||
| Low | 369/5385 (6.9) | 1 [Reference] | 1332/21089 (6.3) | 1 [Reference] | 1715/30584 (5.6) | 1 [Reference] |
| High | 375/5577 (6.7) | 0.93 (0.77-1.12) | 169/3353 (5.0) | 0.82 (0.67-0.99) | 1281/27753 (4.6) | 0.83 (0.74-0.93) |
| 30-d Mortality and stroke | ||||||
| SAVR volume | ||||||
| Low | 626/6526 (9.6) | 1 [Reference] | 1195/13924 (8.6) | 1 [Reference] | 2203/28263 (7.8) | 1 [Reference] |
| High | 413/4436 (9.3) | 0.96 (0.81-1.13) | 905/10518 (8.6) | 0.97 (0.86-1.10) | 2206/30074 (7.3) | 0.98 (0.89-1.07) |
| TAVR volume | ||||||
| Low | 513/5385 (9.5) | 1 [Reference] | 1864/21089 (8.8) | 1 [Reference] | 2470/30584 (8.1) | 1 [Reference] |
| High | 526/5577 (9.4) | 0.96 (0.83-1.12) | 236/3353 (7.0) | 0.83 (0.71-0.97) | 1939/27753 (7.0) | 0.84 (0.77-0.93) |
| 30-d Readmission | ||||||
| SAVR volume | ||||||
| Low | 1690/6526 (25.9) | 1 [Reference] | 3470/13924 (24.9) | 1 [Reference] | 6522/28263 (23.1) | 1 [Reference] |
| High | 1191/4436 (26.9) | 1.00 (0.88-1.14) | 2668/10518 (25.4) | 1.01 (0.92-1.12) | 6565/30074 (21.8) | 0.98 (0.91-1.06) |
| TAVR volume | ||||||
| Low | 1376/5383 (25.6) | 1 [Reference] | 5293/21089 (25.1) | 1 [Reference] | 7175/30584 (23.5) | 1 [Reference] |
| High | 1505/5577 (26.9) | 1.04 (0.93-1.17) | 845/3353 (25.2) | 0.99 (0.88-1.13) | 5912/27753 (21.3) | 0.91 (0.84-0.98) |
| Estimated Risk, % | Adjusted HR (95% CI) | Estimated Risk, % | Adjusted HR (95% CI) | Estimated Risk, % | Adjusted HR (95% CI) | |
| Patients, No. | 11 098 | 24 751 | 60 538 | |||
| 1-y Mortality | ||||||
| SAVR volume | ||||||
| Low | 23.1 | 1 [Reference] | 21.9 | 1 [Reference] | 20.3 | 1 [Reference] |
| High | 24.3 | 0.98 (0.85-1.12) | 22.0 | 0.96 (0.88-1.05) | 19.3 | 0.97 (0.91-1.04) |
| TAVR volume | ||||||
| Low | 23.6 | 1 [Reference] | 22.4 | 1 [Reference] | 20.8 | 1 [Reference] |
| High | 23.7 | 0.93 (0.84-1.03) | 19.1 | 0.85 (0.78-0.92) | 18.8 | 0.87 (0.81-0.94) |
| 2-y Mortality | ||||||
| SAVR volume | ||||||
| Low | 34.7 | 1 [Reference] | 33.9 | 1 [Reference] | 32.2 | 1 [Reference] |
| High | 35.8 | 0.97 (0.87-1.08) | 33.3 | 0.94 (0.87-1.01) | 30.9 | 0.97 (0.92-1.03) |
| TAVR volume | ||||||
| Low | 35.1 | 1 [Reference] | 34.0 | 1 [Reference] | 32.5 | 1 [Reference] |
| High | 35.3 | 0.94 (0.87-1.03) | 31.1 | 0.88 (0.83-0.93) | 30.5 | 0.89 (0.84-0.95) |
Abbreviations: HR, hazard ratio; OR, odds ratio; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
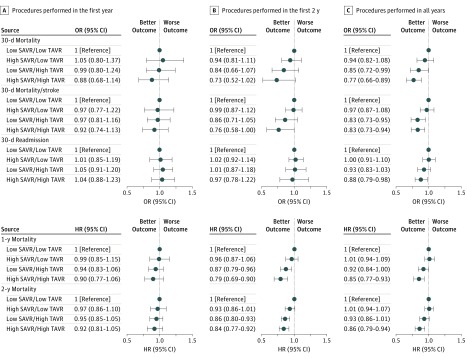
When jointly examining the association between hospital SAVR and TAVR volumes and TAVR outcomes (Figure 3 and eTable 3 in the Supplement), within the first 2 years after the hospitals initiated TAVR programs, patients treated at hospitals with high SAVR volume and high TAVR volume had the lowest short-term and long-term mortality of the 4 groups. When compared with patients undergoing TAVR in hospitals with low SAVR volume and low TAVR volume, those treated in hospitals with high SAVR volume and high TAVR volume had a 27% decrease in 30-day mortality (odds ratio, 0.73; 95% CI, 0.52-1.02; P = .06) and a 21% decrease in 1-year mortality (hazard ratio, 0.79; 95% CI, 0.69-0.90; P < .001). During the entire period after initiation of TAVR programs, patients treated in hospitals with high SAVR volume and high TAVR volume had the lowest 30-day mortality (odds ratio, 0.77; 95% CI, 0.66-0.89; P < .001), when compared with those treated in hospitals with low SAVR volume and low TAVR volume. Higher hospital TAVR volume was associated with lower 30-day patient mortality for hospitals with low SAVR volume (odds ratio, 0.85; 95% CI, 0.72-0.99; P = .04) and those with high SAVR volume (odds ratio, 0.81; 95% CI, 0.69-0.95; P = .009). The reduction in mortality was slightly more prominent for hospitals with high SAVR volume.
Figure 3. Outcomes Among Medicare Beneficiaries Undergoing Transcatheter Aortic Valve Replacement (TAVR) Procedures by Hospital Surgical Aortic Valve Replacement (SAVR) and TAVR Volume Categories.
HR indicates hazard ratio; OR, odds ratio.
Results from sensitivity analysis of transfemoral cases only were consistent with results from the main analysis (eTables 4 and 5 in the Supplement). Sensitivity analyses using different volume category definitions consistently demonstrated that patients treated at hospitals with high SAVR volume and high TAVR volume had the lowest postoperative and long-term mortality (eTable 6 in the Supplement). Hospital TAVR volume was more strongly associated with patient mortality than hospital SAVR volume was. The reduction in postoperative patient mortality associated with higher hospital TAVR volume was more pronounced when hospitals had high SAVR volume.
Discussion
In this nationwide study, we found that hospitals with high SAVR volume were more likely to adopt TAVR early and had faster growth in TAVR volume. Hospital SAVR volume alone was not associated with postoperative outcomes and 1-year and 2-year mortality after TAVR. However, when jointly assessing the effect of SAVR and TAVR volumes, patients undergoing TAVR at hospitals with high SAVR volume and high TAVR volume had the lowest 30-day, 1-year, and 2-year mortality. This effect was observed when evaluating procedures performed within at least 2 years after the initiation of TAVR programs.
Our study demonstrated that assessing hospital SAVR volume alone is not adequate and potentially misleading given the tendency for these hospitals to accumulate TAVR volume more quickly. It was within hospitals with high SAVR volume that the association of accumulated TAVR volume with better outcomes became very strong. Previous studies have reported the association between hospital volume of single-year TAVR procedures and improved postoperative outcomes.10,11 These studies did not assess the effect of hospital SAVR volume or consider the potential interacting role of hospital SAVR volume when assessing the effect of hospital TAVR volume. Our study has substantially advanced the knowledge in this area. We assessed the association of hospital SAVR volume and combined SAVR and TAVR volumes with TAVR outcomes and demonstrated the additional benefit of high SAVR volume after the accumulation of TAVR experience.
Surgical aortic valve replacement was the standard treatment for high-risk aortic stenosis during the pre-TAVR era. Hospitals with higher SAVR volume are likely to be more experienced in managing patients with severe aortic stenosis. After initial approval, TAVR was offered mostly to patients who were at a very high risk of complications of open surgery or were ineligible for SAVR.20 It is possible that hospitals’ accumulated experience in managing high-risk cases led to improved postoperative outcomes of the new procedure. The joint effect of hospital SAVR and TAVR volumes indicated that better outcomes are achieved when frequent use of TAVR is combined with surgical expertise. In addition, these hospitals with high SAVR and TAVR volume are often hospitals involved in early clinical trials and are more likely to have access to newer technologies, contributing to improved outcomes. The lower 1-year and 2-year mortality of patients treated at hospitals with high SAVR and TAVR volume suggests that these hospitals may also have made better candidate selection and offered better continuing care after discharge. More important, the SAVR volume effect becomes clear when accounting for procedures performed at least 2 years after initiation, which may be attributed to the enhanced benefit of extensive prior experience with SAVR in high-volume hospitals that are past the TAVR learning curve. Further examination of hospital process and patient management may help provide insights into the factors that were associated with better patient outcomes in hospitals with high SAVR and TAVR volumes.
To our knowledge, this is the first study that comprehensively evaluated the association of SAVR volume and the use and outcomes of TAVR, accounting for its collinearity with TAVR volume. Previous research has shown that there is wide variation in outcomes after TAVR in the commercial era.21,22 With the expansion of TAVR indications,23 it is critical to ensure high-quality care for the growing patient population. Our analyses emphasized the role of both hospital SAVR and TAVR volumes, which has important policy implications when considering the benefit of having volume requirements for hospitals that seek to initiate or continue TAVR programs. Policy decisions to determine the necessity of volume requirement need to take into account the higher likelihood of hospitals with higher SAVR volume to frequently perform TAVR and the combined benefit of hospitals’ surgical and accumulated TAVR experience for patients undergoing TAVR.
Limitations
Our study has limitations. We relied on Medicare claims data for the current study. Transcatheter aortic valve replacement might be performed for patients younger than 65 years who were not enrolled in Medicare. However, based on queries using the Healthcare Cost and Utilization Project website (https://hcupnet.ahrq.gov; 2011-2014), Medicare covers approximately 90% of all TAVR procedures. It is unlikely that some hospitals disproportionately performed TAVR among younger patients. Thus, the dichotomization of volume categories was unlikely to be affected if procedures performed for young patients were proportional to total cases. In addition, claims data were not able to provide detailed clinical information, such as Society of Thoracic Surgeons score or high-risk anatomical features, and there might be residual confounding by patient conditions. However, it can be inferred from patient characteristics and previous studies10,11 that hospitals with higher TAVR and SAVR volumes tended to treat sicker patients. For this reason, bias in our study was expected to be toward the null and the real benefit of undergoing TAVR at hospitals with high SAVR and TAVR volumes was expected to be more pronounced. As hospitals with high SAVR and TAVR volumes are presumably more likely to be those involved in early clinical trials with access to mature and newer technologies, our findings should be interpreted as associations and not causal. Data from years beyond 2015 were not available at the time of the study. With TAVR becoming more mature and being performed among patients with lower risk of complications, the association between hospital SAVR and TAVR volume and patient outcomes may change. Future studies are needed to continuously assess the effect of hospital SAVR and TAVR volumes.
Conclusions
Hospitals with high SAVR volume are most likely to be fast adopters of TAVR. Hospital SAVR volume alone is not associated with better TAVR outcomes. Accumulating high volumes of TAVR is associated with lower mortality after TAVR, particularly when hospitals have high SAVR volumes. Hospitals with high caseloads of both SAVR and TAVR are likely to achieve best outcomes.
eTable 1. Hospital Characteristics
eTable 2. Patient Characteristics (N/%) by Hospital SAVR and TAVR Volume
eTable 3. Unadjusted 30-day, 1-Year, and 2-Year Outcomes Among Medicare Beneficiaries Undergoing TAVR Procedures at Hospitals With Low and High SAVR Volume
eTable 4. Sensitivity Analysis of 30-Day, 1-Year, and 2-Year Outcomes Among Medicare Beneficiaries Undergoing Transfemoral TAVR Procedures at Hospitals with Low and High SAVR Volume
eTable 5. Sensitivity Analysis of 30-Day, 1-Year, and 2-Year Outcomes Among Medicare Beneficiaries Undergoing Transfemoral TAVR Procedures at Hospitals With Low and High SAVR/TAVR Volumes
eTable 6. Sensitivity Analysis of 30-Day, 1-Year, and 2-Year Outcomes Among Medicare Beneficiaries Undergoing Transfemoral TAVR Procedures at Hospitals, Using Different Volume Thresholds
References
- 1.Leon MB, Smith CR, Mack M, et al. ; PARTNER Trial Investigators . Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-1607. doi: 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, et al. ; PARTNER Trial Investigators . Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187-2198. doi: 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 3.Mack MJ, Leon MB, Smith CR, et al. ; PARTNER 1 trial investigators . 5-Year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477-2484. doi: 10.1016/S0140-6736(15)60308-7 [DOI] [PubMed] [Google Scholar]
- 4.Kapadia SR, Leon MB, Makkar RR, et al. ; PARTNER trial investigators . 5-Year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2485-2491. doi: 10.1016/S0140-6736(15)60290-2 [DOI] [PubMed] [Google Scholar]
- 5.Popma JJ, Adams DH, Reardon MJ, et al. ; CoreValve United States Clinical Investigators . Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63(19):1972-1981. doi: 10.1016/j.jacc.2014.02.556 [DOI] [PubMed] [Google Scholar]
- 6.Adams DH, Popma JJ, Reardon MJ, et al. ; US CoreValve Clinical Investigators . Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790-1798. doi: 10.1056/NEJMoa1400590 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Medicare & Medicaid Services National Coverage Determination (NCD) for transcatheter aortic valve replacement (TAVR) (20.32). https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=355&ncdver=1&NCAId=293&type=Open&bc=ACAAAAAAQAAA&. Accessed September 10, 2018.
- 8.Centers for Medicare & Medicaid Services MEDCAC Meeting 7/25/2018—transcatheter aortic valve replacement (TAVR). https://www.cms.gov/medicare-coverage-database/details/medcac-meeting-details.aspx?MEDCACId=75. Accessed June 29, 2018.
- 9.Wendling P. CMS panel takes first look at volume requirements for TAVR centers. https://www.medscape.com/viewarticle/899832. Published July 28, 2018. Accessed September 5, 2018.
- 10.Kim LK, Minutello RM, Feldman DN, et al. Association between transcatheter aortic valve implantation volume and outcomes in the United States. Am J Cardiol. 2015;116(12):1910-1915. doi: 10.1016/j.amjcard.2015.09.040 [DOI] [PubMed] [Google Scholar]
- 11.Badheka AO, Patel NJ, Panaich SS, et al. Effect of hospital volume on outcomes of transcatheter aortic valve implantation. Am J Cardiol. 2015;116(4):587-594. doi: 10.1016/j.amjcard.2015.05.019 [DOI] [PubMed] [Google Scholar]
- 12.Carroll JD, Vemulapalli S, Dai D, et al. Procedural experience for transcatheter aortic valve replacement and relation to outcomes: the STS/ACC TVT Registry. J Am Coll Cardiol. 2017;70(1):29-41. doi: 10.1016/j.jacc.2017.04.056 [DOI] [PubMed] [Google Scholar]
- 13.Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59(13):1200-1254. doi: 10.1016/j.jacc.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 14.Tommaso CL, Bolman RM III, Feldman T, et al. Multisociety (AATS, ACCF, SCAI, and STS) expert consensus statement: operator and institutional requirements for transcatheter valve repair and replacement, part 1: transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59(22):2028-2042. doi: 10.1016/j.jacc.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 15.Bavaria JE, Tommaso CL, Brindis RG, et al. 2018 AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutional recommendations and requirements for transcatheter aortic valve replacement: a joint report of the American Association for Thoracic Surgery, the American College of Cardiology, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons [published online July 2018]. J Am Coll Cardiol. doi: 10.1016/j.jacc.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 16.Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29(8):1602-1604. doi: 10.1161/01.STR.29.8.1602 [DOI] [PubMed] [Google Scholar]
- 17.Gialdini G, Nearing K, Bhave PD, et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA. 2014;312(6):616-622. doi: 10.1001/jama.2014.9143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roques F, Michel P, Goldstone AR, Nashef SA. The logistic EuroSCORE. Eur Heart J. 2003;24(9):881-882. doi: 10.1016/S0195-668X(02)00799-6 [DOI] [PubMed] [Google Scholar]
- 19.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 20.Nishimura RA, Otto CM, Bonow RO, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):2438-2488. doi: 10.1016/j.jacc.2014.02.537 [DOI] [PubMed] [Google Scholar]
- 21.O’Brien SM, Cohen DJ, Rumsfeld JS, et al. ; STS/ACC TVT Registry . Variation in hospital risk-adjusted mortality rates following transcatheter aortic valve replacement in the United States: a report from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circ Cardiovasc Qual Outcomes. 2016;9(5):560-565. doi: 10.1161/CIRCOUTCOMES.116.002756 [DOI] [PubMed] [Google Scholar]
- 22.Murugiah K, Wang Y, Desai NR, Nuti SV, Krumholz HM. Hospital variation in outcomes for transcatheter aortic valve replacement among Medicare beneficiaries, 2011 to 2013. J Am Coll Cardiol. 2015;66(23):2678-2679. doi: 10.1016/j.jacc.2015.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70(2):252-289. doi: 10.1016/j.jacc.2017.03.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Hospital Characteristics
eTable 2. Patient Characteristics (N/%) by Hospital SAVR and TAVR Volume
eTable 3. Unadjusted 30-day, 1-Year, and 2-Year Outcomes Among Medicare Beneficiaries Undergoing TAVR Procedures at Hospitals With Low and High SAVR Volume
eTable 4. Sensitivity Analysis of 30-Day, 1-Year, and 2-Year Outcomes Among Medicare Beneficiaries Undergoing Transfemoral TAVR Procedures at Hospitals with Low and High SAVR Volume
eTable 5. Sensitivity Analysis of 30-Day, 1-Year, and 2-Year Outcomes Among Medicare Beneficiaries Undergoing Transfemoral TAVR Procedures at Hospitals With Low and High SAVR/TAVR Volumes
eTable 6. Sensitivity Analysis of 30-Day, 1-Year, and 2-Year Outcomes Among Medicare Beneficiaries Undergoing Transfemoral TAVR Procedures at Hospitals, Using Different Volume Thresholds