Abstract
Importance
Surgery represents the mainstay treatment of colorectal liver metastases. Indications for the laparoscopic approach in this setting have been widened and there is a need to confirm the benefits of minimally invasive liver surgery (MILS) in patients with complex disease states.
Objective
To compare outcomes of laparoscopic surgery with those of open surgery for liver metastases from colorectal cancer, focusing on the characteristics of modern MILS and therefore overcoming possible selection bias related to different policies for patients’ eligibility for MILS over time.
Design, Setting, and Participants
A cohort study of 885 resections performed for liver metastases from colorectal cancer between January 1, 2004, and June 30, 2017, at the Hepatobiliary Surgery Unit of San Raffaele Hospital, Milano, Italy, comprising 187 laparoscopic and 698 open resections. Procedures performed using the MILS approach with a ratio of MILS to total resections per year of more than 30% were considered and were matched by propensity scores (ratio of 1:4) to procedures performed using the open approach with a ratio of MILS to total resections per year of less than 30%.
Main Outcomes and Measures
The primary end point was short-term outcomes, including morbidity, mortality, functional recovery, and interval between surgery and adjuvant treatments; the secondary end point was long-term outcomes.
Results
Among this cohort (104 patients in the MILS group; 46 women and 58 men; median age, 62 years [range, 35-81 years]; and 412 patients in the open group; 181 women and 231 men; median age, 60 years [range, 37-80 years]), primary end-point data showed a significantly higher incidence of postoperative morbidity in patients who underwent open resections compared with those who underwent MILS (94 [22.8%] vs 21 [20.2%]; P = .04). Patients in the MILS group had fewer major complications (Dindo-Clavien grades III-V) compared with patients in the open group (Dindo-Clavien grades III-V; 7 [6.7%] vs 35 [8.5%]; P = .03) as well as shorter lengths of stay (median [range] duration, 3 [2-35] vs 5 [4-37] days; P = .02). Oncologic results were not compromised by the laparoscopic approach.
Conclusions and Relevance
In this study, the results of the propensity score matching analysis between modern laparoscopic surgery and previous open surgery appear to confer more comparable cohorts for complexity, further supporting the advantages of laparoscopy in the surgical treatment of liver metastases from colorectal cancer. The increase in use that laparoscopy has experienced appears to be based on increased feasibility, widening of eligibility criteria for patients, enhanced clinical effectiveness, and oncologic outcomes. All these elements together suggest that up to 70% of patients appear to be candidates for this minimally invasive surgical approach in high-volume centers.
This cohort study uses propensity score matching analysis to compare outcomes of laparoscopic surgery with those of open surgery among patients with liver metastases from colorectal cancer.
Key Points
Question
How do the outcomes of laparoscopy compare with those of open surgery for liver metastases due to colorectal cancer?
Findings
In this cohort study, results on the propensity score matching analysis between laparoscopic and open surgery conferred more comparable cohorts for complexity, further supporting the advantages of laparoscopy in the resection of liver metastases from colorectal cancer. Patients who underwent open resections had greater postoperative morbidity than those who underwent laparoscopy, while patients in the laparoscopy group had fewer major complications compared with those who underwent open resections, as well as shorter lengths of stay
Meaning
An increasing proportion of patients can benefit from the advantages of laparoscopic surgery for liver metastases from colorectal cancer, even those requiring procedures with a high degree of technical complexity.
Introduction
The only treatment with potentially curative intent for patients affected by colorectal liver metastases (CLMs) is liver resection. Oncologic and surgical advances, together with the implementation of the concept of multidisciplinary management,1 have allowed for a significant expansion of potential candidates for resection, maintaining an adequate profile of safety, and for the recent allocation of a higher proportion of patients able to undergo surgery with minimally invasive techniques.2 Benefits of the laparoscopic approach have been documented with a high degree of evidence by retrospective comparative studies, meta-analyses, and, more recently, results from the Oslo-CoMet (Oslo Randomized Laparoscopic Versus Open Liver Resection for Colorectal Metastases Study) trial that concluded that, in laparoscopic parenchymal-sparing resections, the rate of morbidity was reduced while oncologic adequacy was comparable to that seen with open surgical techniques3; currently, CLMs constitute one of the most frequent diagnoses in laparoscopic series.
Despite this fact, different policies for selecting patients for laparoscopy or open surgery have hindered the reliability and clinical applicability of the results of many retrospective studies; more complex and challenging cases were not considered for the laparoscopic technique in the past, especially in series including the first selected cases of limited metastatic disease.4,5,6,7
Determining the effect of the recent shift toward minimally invasive surgery by analyzing the ratio between laparoscopic surgery and open surgery for liver resections in high-volume institutions has never been specifically addressed in terms of short-term and long-term postoperative outcomes for patients with metastases. To our knowledge, there is no evidence in the literature describing how minimally invasive surgery manages to be representative of the whole group of patients with CLMs (especially in terms of disease burden, damage of liver parenchyma because of previous chemotherapy, and overall surgical complexity). Furthermore, wider recruitment of patients for minimally invasive surgery can help determine short-term outcomes and, therefore, jeopardize the favorable results of laparoscopy.
In the present study, patients with CLMs who underwent laparoscopic surgery and patients with CLMs who underwent open surgery were compared after application of propensity score matching to the most recent laparoscopic series (ie, when, owing to wider indications and accomplishment of the learning curve, the proportion of minimally invasive cases were significantly increased). Our primary aim was to compare intraoperative and postoperative outcomes of patients, especially in terms of morbidity, mortality, functional recovery, and the interval between surgery and adjuvant treatments. Secondary end points were long-term outcomes.
Methods
Study Design
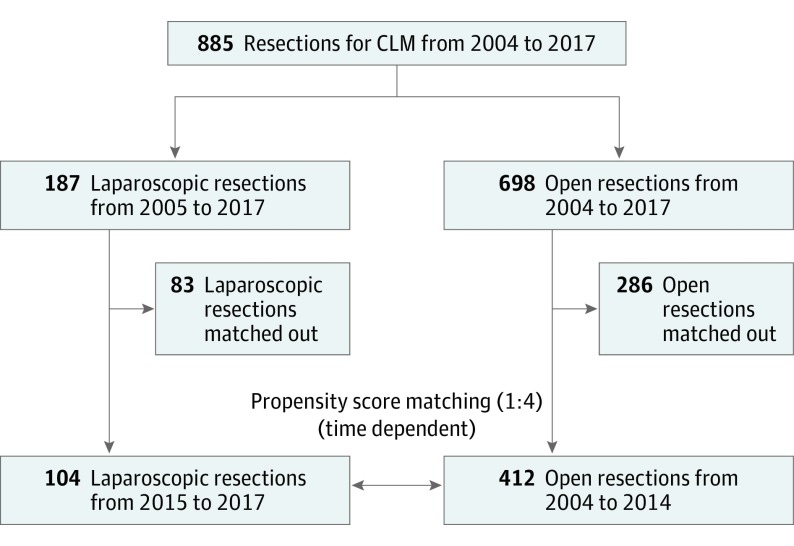
A total of 2418 hepatic resections were performed at the Hepatobiliary Surgery Division of San Raffaele Hospital, Milano, Italy, between January 1, 2004, and June 30, 2017. During the study period, 885 resections for CLMs (36.6%) were performed; of these, 187 were performed using the minimally invasive liver surgery (MILS) approach, constituting the study group, and 698 were performed using the open approach, constituting the control group (Figure 1). The inclusion trend of liver resections to either the MILS group or the open group was observed during the study period, and the ratio of MILS to total resections for CLMs was calculated per year. Approval to perform this retrospective study was obtained from the Institutional Review Board of San Raffaele Hospital, Milano, and written informed consent was obtained from the participants.
Figure 1. Study Design.
CLM indicates colorectal liver metastasis.
Time-dependent propensity score matching was used to compare the 2 groups; only procedures performed using the MILS approach with a ratio of MILS to total resections per year of more than 30% were considered and were matched to procedures performed using the open approach with a ratio of MILS to total resections per year of less than 30%. Within this propensity score matching, 104 procedures were included in the MILS group and 412 procedures were included in the open group, for a ratio of 1:4). Patient age, American Society of Anesthesiology score, number of chemotherapy cycles, primary tumor location and staging, number of liver lesions, and type of hepatectomy were used as covariates to achieve the propensity score matching.
Preoperative Workup
The indication for surgery and the characteristics (type and approach) of liver resections were discussed for every patient during weekly multidisciplinary tumor boards, with the participation of liver surgeons, radiologists, and medical oncologists. Indications for preoperative chemotherapy were initially unresectable liver metastases, until conversion to resectability, and borderline or easily resectable liver disease with neoadjuvant intent, unless none or only 1 of the following was detected: synchronous presentation, primary tumor nodal involvement, multiple liver metastases, carcinoembryonic antigen level higher than 200 ng/mL (to convert to micrograms per liter, multiply by 1.0), and metastases with a diameter larger than 5 cm.8,9
Procedures
For open procedures, right J-shaped incisions were performed. Features of lesions (number, size, and relationship with liver vascular structures) were assessed by intraoperative ultrasonography. As far as technically possible, primary vascular control of inflow was achieved before parenchymal transection, while vascular control of outflow was obtained transparenchymally. Technological ultrasonic-mediated dissection was used to perform parenchymal transection.
For laparoscopic resections, the French position was used to place the patient; the patient’s legs were apart with the first surgeon standing between the legs and 1 assistant on each side.10 Intraoperative ultrasonography was routinely performed, with the same end points as for the open procedures. Hepatic transection was performed using the SonoSurg system (Olympus). Vessels were coagulated or clipped or stapled according to their size. The Pringle maneuver was used systematically to control intraoperative bleeding.
Variables
The difficulty score of Ban et al,11 developed in the setting of laparoscopic resections and considering 5 preoperative factors (tumor location, extent of liver resection, tumor size, proximity to major vessels, and liver function), was calculated for every patient. Intraoperative and postoperative outcomes were evaluated, including morbidity and mortality. Postoperative complications were reviewed for 90 days after liver resection; the Dindo-Clavien classification of surgical complications was used to grade them.12 Time for functional recovery and length of stay were considered. The interval between surgery and resumption of chemotherapy was also calculated. Data regarding follow-up, including survival status, incidence, and type of recurrence, were recorded. Three-year and 5-year overall survival and disease-free survival were evaluated using the Kaplan-Meier method.
Statistical Analysis
Patients in the MILS group were selected according to propensity scores based on the covariates already mentioned in a ratio of 1:4 and were then matched to patients in the open surgery group. After matching, the χ2 or Fisher exact test for categorical data, the Mann-Whitney test for nonnormally distributed continuous data, and the t test for normally distributed continuous variables were used for comparison. All data were expressed as mean (SD) or median (range) values, as appropriate. Survival curves were generated and compared using the Kaplan-Meier method. Statistical significance was defined as P < .05 (2-sided). All analyses were performed using the statistical package SPSS, version 18.0 (SPSS Inc).
Results
Participants
Of the 2418 hepatic resections performed between January 2004 and June 2017, 885 (36.6%) were conducted for CLMs, comprising 698 open and 187 laparoscopic resections. eFigure 1 in the Supplement reports the number of inclusions per year according to treatment strategy (laparoscopic vs open), and eFigure 2 in the Supplement shows that the ratio of MILS to total procedures increased from 2.3% in 2005 (1 of 43) to a projected 71.2% in 2017 (74 of 104).
Short-term Outcomes: Primary End Point
As shown in Table 1, propensity score matching allowed us to obtain similar groups in terms of preoperative characteristics. The median difficulty score was 6 (range, 2-10) in the MILS group and 7 (range, 2-10) in the open group (P = .12) (Table 2). The difficulty score is based on the following 5 factors, obtained from preoperative information: tumor location, extent of liver resection, tumor size, proximity to major vessels, and liver function. The difficulty index was determined by the total score derived by the scores for the 5 factors. Based on the final score, each procedure can then classified into 3 different categories: low difficulty (score, 1-3), intermediate difficulty (score, 4-6), and high difficulty (score, 7-10). There were 111 major resections (26.9%) in the open group and 28 major resections (26.9%) in the MILS group; there were 301 minor resections (73.1%) in the open group and 76 minor resections (73.1%) in the MILS group.
Table 1. Characteristics of Patients and Disease According to Treatment Group After Time-Dependent Propensity Score Matching.
| Characteristic | Patients, No. (%) | P Value | |
|---|---|---|---|
| MILS (n = 104) | Open Surgery (n = 412) | ||
| Age, median (range), y | 62 (35-81) | 60 (37-80) | .10 |
| Sex | |||
| Male | 58 (55.8) | 231 (56.1) | .08 |
| Female | 46 (44.2) | 181 (43.9) | |
| ASA score | |||
| I and II | 70 (67.3) | 355 (86.2) | .16 |
| III and IV | 34 (32.7) | 57 (13.8) | |
| Neoadjuvant CT | 85 (81.7) | 336 (81.6) | .57 |
| Chemotherapy regimen | |||
| Oxaliplatin based | 51 (49.0) | 199 (48.3) | .46 |
| Irinotecan based | 34 (32.7) | 138 (33.5) | |
| Associated biologic therapy | 42 (40.4) | 162 (39.3) | |
| Associated comorbidities | 62 (59.6) | 251 (60.9) | .53 |
| Features of nontumorous parenchyma | |||
| Normal | 56 (53.8) | 213 (51.7) | .35 |
| Steatosis | 31 (29.8) | 129 (31.3) | |
| CALI | 17 (16.3) | 70 (17.0) | |
| Primary tumor location | |||
| Colon | 53 (51.0) | 236 (57.3) | .90 |
| Rectum | 51 (49.0) | 176 (42.7) | |
| Tumor stage | |||
| T1 | 4 (3.8) | 14 (3.4) | .40 |
| T2 | 34 (32.7) | 137 (33.3) | |
| T3 | 51 (49.0) | 200 (48.5) | |
| T4 | 15 (14.4) | 61 (14.8) | |
| Tumor grade | |||
| G1 | 3 (2.9) | 9 (2.2) | .55 |
| G2 | 86 (82.7) | 344 (83.5) | |
| G3 | 15 (14.4) | 59 (14.3) | |
| Nodal status | |||
| N0 | 49 (47.1) | 190 (46.1) | .46 |
| N1 | 43 (41.3) | 173 (42.0) | |
| N2 | 12 (11.5) | 49 (11.9) | |
| No. of liver lesions, median (range) | 2 (1-10) | 2 (1-44) | .38 |
| Nodularity | |||
| Monofocal | 39 (37.5) | 150 (36.4) | .52 |
| Multifocal | 75 (72.1) | 302 (73.3) | |
| Lobe distribution of metastases | |||
| Unilobar | 57 (54.8) | 219 (53.2) | .96 |
| Bilobar | 47 (45.2) | 193 (46.8) | |
| Repeated liver surgery | 19 (18.3) | 75 (18.2) | |
| Previous portal vein embolization or ligation | 6 (5.8) | 27 (6.6) | |
| Extrahepatic disease | 15 (14.4) | 58 (14.1) | |
| Liver metastasis diameter, median (range), cm | 3.4 (0.9-12) | 2.9 (0.5-11) | .41 |
| Fong score, median (range) | 3 (1-5) | 3 (1-5) | .32 |
| CEA level, median (range), ng/mL | 41.8 (3.1-590) | 35.6 (2-276) | .28 |
Abbreviations: ASA, American Society of Anesthesiologists; CALI, chemotherapy-related liver injury; CEA, carcinoembryonic antigen; CT, computed tomography; MILS, minimally invasive liver surgery.
SI conversion factor: To convert CEA to micrograms per liter, multiply by 1.0.
Table 2. Characteristics of Surgery According to Treatment Group After Time-Dependent Propensity Score Matching.
| Characteristic | Patients, No. (%) | P Value | |
|---|---|---|---|
| MILS (n = 104) | Open Surgery (n = 412) | ||
| Extent of liver resection | |||
| Major | 28 (26.9) | 111 (26.9) | >.99 |
| Minor | 76 (73.1) | 301 (73.1) | |
| Difficulty score, median (range) | 6 (2-10) | 7 (2-10) | .92 |
| Liver resection | |||
| Right hepatectomy | 16 (15.4) | 64 (15.5) | .57 |
| Left hepatectomy | 10 (9.6) | 40 (9.7) | |
| Right trisectionectomy | 2 (1.9) | 8 (1.9) | |
| Left trisectionectomy | 0 | 0 | |
| Left lateral sectionectomy | 11 (10.6) | 44 (10.7) | |
| Bisegmentectomy | 17 (16.3) | 68 (16.5) | |
| Segmentectomy | 18 (17.3) | 72 (17.5) | |
| Single wedge resection | 17 (16.3) | 68 (16.5) | |
| Multiple wedge resections | 9 (8.7) | 36 (8.7) | |
| Right posterior sectionectomy | 3 (2.9) | 12 (2.9) | |
| Right anterior sectionectomy | 1 (1.0) | 4 (1.0) | |
| Conversion | 16 (15.4) | NA | NA |
| Two-stage hepatectomy | 6 (5.8) | 34 (8.3) | .09 |
| Associated procedures | 27 (26.0) | 112 (27.2) | .49 |
Abbreviations: MILS, minimally invasive liver surgery; NA, not assessable.
Intraoperative and Postoperative Outcomes Analysis
Comparing the open and MILS groups, we found that the median operating time was 200 minutes (range, 70-600 minutes) in the open group vs 220 minutes (range, 150-540 minutes) in the MILS group (P = .22), the median blood loss was 350 mL (range, 100-950 mL) in the open group vs 250 mL (range, 100-900 mL) in the MILS group (P = .04), the Pringle maneuver was used for 385 patients (93.4%) in the open group vs 91 patients (87.5%) in the MILS group (P = .10), and the median duration of the Pringle maneuver was 30 minutes (range, 10-60 minutes) in the open group vs 45 minutes (range, 15-85 minutes) in the MILS group (P = .05) (Table 3).
Table 3. Intraoperative and Postoperative Outcomes According to Treatment Group After Time-Dependent Propensity Score Matching.
| Variable | MILS (n = 104) | Open Surgery (n = 412) | P Value |
|---|---|---|---|
| Operating time, median (range), min | 220 (150-540) | 200 (70-600) | .22 |
| Blood loss, median (range), mL | 250 (100-900) | 350 (100-950) | .04 |
| Pringle maneuver, No. (%) | 91 (87.5) | 385 (93.4) | .10 |
| Duration of Pringle maneuver, median (range), min | 45 (15-85) | 30 (10-60) | .05 |
| Intraoperative blood transfusion, No. (%) | 8 (7.7) | 31 (7.5) | .22 |
| R1 liver resection, No. (%) | 6 (5.8) | 24 (5.8) | .50 |
| Depth of liver margin, median (range), mm | 4 (0-11) | 4 (0-12) | .44 |
| Time to first flatus, median (range), d | 3 (2-6) | 5 (2-7) | .04 |
| Return to diet, median (range), d | 1 (0-4) | 1 (1-8) | .69 |
| Morbidity, No. (%) | 21 (20.2) | 94 (22.8) | .04 |
| Hemorrhage | 1 (1.0) | 8 (1.9) | .27 |
| Liver failure | 3 (2.9) | 17 (4.1) | .03 |
| Biliary fistula | 6 (5.8) | 28 (6.8) | .44 |
| Abdominal abscess | 4 (3.8) | 17 (4.1) | .36 |
| Fever | 10 (9.6) | 35 (8.5) | .24 |
| Wound infection | 3 (2.9) | 11 (2.7) | .28 |
| Pneumonia | 2 (1.9) | 6 (1.5) | .19 |
| Pleural effusion | 14 (13.5) | 35 (8.5) | .04 |
| Arrhythmia | 4 (3.8) | 8 (1.9) | .03 |
| Other cardiac complications | 2 (1.9) | 5 (1.2) | .22 |
| DVT or pulmonary embolism | 1 (1.0) | 3 (0.7) | .12 |
| Minor morbidity (Dindo-Clavien grades I-II) | 14 (13.5) | 59 (14.3) | .07 |
| Major morbidity (Dindo-Clavien grades III-V) | 7 (6.7) | 35 (8.5) | .03 |
| Mortality, No. (%) | 1 (1.0) | 3 (0.7) | .94 |
| Postoperative transfusions, No. (%) | 12 (11.5) | 64 (15.5) | .03 |
| Total transfusions, No. (%) | 16 (15.4) | 78 (18.9) | .05 |
| Length of postoperative stay, median (range), d | 3 (2-35) | 5 (4-37) | .02 |
Abbreviations: DVT, deep vein thrombosis; MILS, minimally invasive liver surgery; R1, positive resection margin.
The morbidity rate in the open group was 22.8% (n = 94) and 20.2% (n = 21) in the MILS group (P = .04). Both groups had a similar rate of Dindo-Clavien grades I and II complications (14.3% [n = 59] in the open group and 13.4% [n = 14] in the MILS group), but the open group had a higher rate of Dindo-Clavien grades III and V complications than did the MILS group (8.5% [n = 35] vs 6.7% [n = 7]; P = .03). Mortality rates were similar between the groups (3 [0.7%] in the open group and 1 [1.0%] in the MILS group), while the median length of postoperative stay was 5 days (range, 4-37 days) in the open group vs 3 days (range, 2-35 days) in the MILS group (P = .02).
Long-term Outcome: Secondary End Points
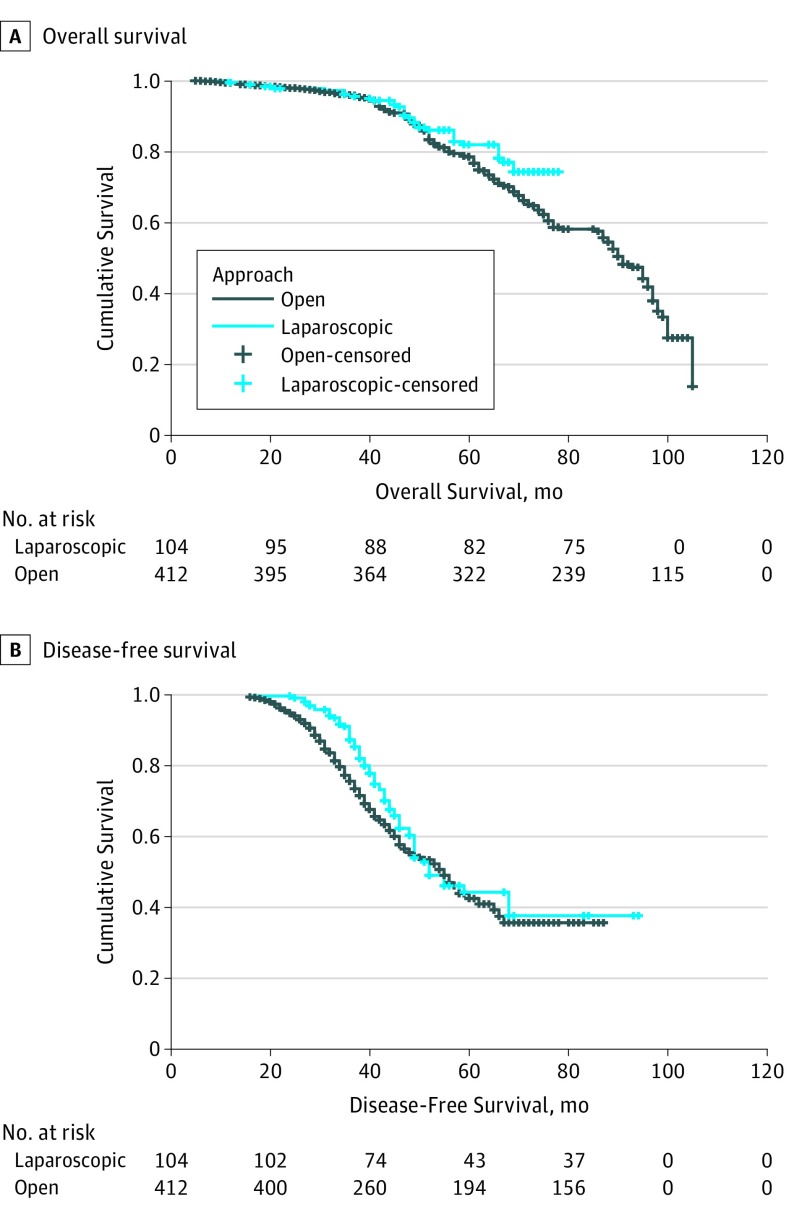
Long-term outcomes of overall and disease-free survival are represented in Figure 2A and B. Comparison of data after propensity score matching analysis showed a median overall survival of 58 months in the open group vs 60 months in the MILS group (P = .07). Median disease-free survival was 40 months in the open group vs 42 months in the MILS group (P = .22), while disease recurred in 198 of 412 patients in the open group (48.1%) vs 40 of 104 patients (38.5%) in the MILS group (P = .04).
Figure 2. Survival After Propensity Score Matching.
A, Overall survival stratified according to treatment strategy after propensity score matching. B, Disease-free survival stratified according to treatment strategy after propensity score matching.
Discussion
The present study reports a single institution’s experience with patients affected by CLMs, treated during a 14-year time period. Previous series from the literature5,6,13 revealed the advantages of laparoscopy in terms of postoperative morbidity and its superiority for functional recovery, but most of these studies are not fully representative of existing differences within the study population, given the differing policies of patient suitability for the MILS or open approach.
The key point of the present study, instead, was to identify a different perspective to deepen the issue of comparison: this need comes from the observation that the period from 2015 to 2017 marked the point during which the number of patients with CLMs treated laparoscopically was equivalent to the number of patients with CLMs treated with an open surgical approach. Since that moment, biases inherent to the achievement of a learning curve and to the establishment of an institutional policy on eligibility for MILS lapsed because patients with more complex conditions, who had previously been excluded from MILS, were instead deemed eligible for laparoscopy. Since 2015, an increase in this trend persisted in favor of the laparoscopic approach. Thus, it was proposed to compare, through a propensity score matching time-dependent analysis, patients who had been treated laparoscopically since 2015 with those treated by open surgery before 2015, allowing cases of similar complexity to be compared.
Furthermore, propensity score matching to compare patients undergoing laparoscopic resection and those undergoing open resection during 2015-2017 is not feasible anymore because using the same covariates for propensity score matching—chosen as representative of patient and disease characteristics—reduces the statistical power of the analysis dramatically, thus failing to achieve a good match between the laparoscopic and open groups. This infeasibility resulted in the radical shift in indication toward MILS, resulting in 2 different populations of patients, with no overlap for comparison. Hence, the choice to compare patients who had been treated laparoscopically in 2015-2017 with patients treated with open surgery in a previous period was an attempt to reproduce the same policy of recruitment for each technique and therefore to point out the benefits of laparoscopy. There were 104 patients who underwent MILS and 412 patients who underwent open surgery, in a 1:4 ratio, who were homogeneous for their characteristics and medical complexity, thus conferring enhanced statistical power to the propensity score matching analysis.14
Improvement in both technological and technical skills is the reason for this increase in the use of laparoscopic procedures. This increase has focused the discussion on 4 cardinal points: feasibility, widening of enrollment criteria, clinical effectiveness, and oncologic outcomes.
Feasibility
Although the approach to CLMs at the beginning of laparoscopic liver surgery programs was reserved for cases needing minor resections (eg, wedge resections, subsegmentectomies, or resection of laparoscopic segments), the spectrum of possible resections has become wider in recent years; the possibility of laparoscopy has extended to bisegmentectomies, nonlaparoscopic segments (ie, liver segments 1, 4a, 8, and 7), major hepatic resections, and composite and more complex procedures (2-stage hepatectomies, and laparoscopic ALPPS [associating liver partition and portal vein ligation for staged hepatectomy]). Thus, many more procedures for more complex cases are now feasible with laparoscopy, as depicted by Aldrighetti et al15 in the context of a national registry for laparoscopic liver surgery: among 1678 cases of MILS performed in 48 centers during a 31-month period, CLMs constituted 31.2% of the cases for malignant tumors, thus becoming the second most frequent malignant liver neoplasm after hepatocellular carcinoma to be treated laparoscopically. This wide diffusion is emblematic of how MILS has been adopted in several different clinical settings.
Widening of Enrollment Criteria for MILS
Implementation of technological and technical skills led to the possibility of complete resections with higher grades of complexity, thus developing the concept of parenchymal-sparing resection for CLMs. The further extension of indications arises from the possibility of achieving a positive resection margin (R1), which is considered an acceptable oncologic result for the purpose of parenchymal-sparing resections for CLMs in otherwise unresectable disease.16
Extension of the laparoscopic approach to CLMs has recently been endorsed by guidelines for laparoscopic liver surgery,17 confirming that parenchymal-sparing resections should continue to be the basis of treatment of CLMs. Furthermore, in the spectrum of parenchymal-sparing procedures, interventional radiologic procedures associated with laparoscopy offer further extensions. Microwave ablations are now considered as effective as surgical resections for nodules of CLMs up to 2 cm. Nevertheless, the use of thermal ablation for lesions in close proximity to hollow viscous organs (eg, gallbladder, colon, or duodenum) and located on the glissonian surface is contraindicated by the procedure itself. These limitations can often be overcome by the adjunct use of a laparoscopic approach during intraoperative ablative procedures.
Clinical Effectiveness
Since the beginning of its development, laparoscopy has been associated with decreased postoperative morbidity and mortality, key points that have been detected in its role to confer lower biological effect. As demonstrated by Fretland et al,18 laparoscopic resection of CLMs reduced the inflammatory response compared with open resection in the context of a substudy within the Oslo CoMet study.3 Furthermore, laparoscopy resulted in a lower rate of postoperative complications (19% vs 31%) and a shorter hospital stay compared with open surgery, as observed in the Oslo CoMet randomized clinical trial.3 The clinical enforcement of this is reflected in the need for patients affected by CLMs to return to their homeostatic status as soon as possible, for the opportunity to proceed with (possibly) already scheduled further chemotherapy lines. Therefore, because the open surgical approach put such patients at higher risk of perioperative complications with consecutively increased morbidity, the chance to resume chemotherapy in a timely manner is threatened. A recent study by Tohme et al,19 specifically addressing this topic, reported that patients undergoing laparoscopic liver resection have a shorter interval to postoperative chemotherapy compared with those undergoing open surgery; furthermore, univariable analysis confirmed that the surgical approach was associated with the timing of chemotherapy. In the setting of laparoscopic liver resection, the implementation of enhanced recovery after surgery programs has resulted in patients having less blood loss and in lower rates of postoperative morbidity.20
Oncologic Outcome
In the present series, laparoscopic surgery appeared to be effective in terms of oncologic validity expressed as resection margins, overall survival, and disease-free survival, giving overlapping results with open surgery outcomes. If a faster recovery was demonstrated as an independent variable, thus allowing patients an earlier return to chemotherapy, laparoscopy’s oncologic validity would be further supported.
Limitations
Recurrence of disease was observed in a lower percentage of patients undergoing MILS, although this finding could still be jeopardized by case selection prior to laparoscopy. It can also be speculated that long-term comparative outcomes of MILS and open resections are still hindered by the shorter follow-up time of laparoscopic series, while the effect of a more favorable biological scenario in minimally invasive surgery has yet to be investigated and should be the subject of future targeted investigations. Previous laparoscopic approaches favored repeated resections as the treatment of choice in cases of intrahepatic and extrahepatic recurrence, confirming the superiority of MILS to allow further surgical treatment.
Conclusions
It is expected that an increasing number of patients affected by CLMs will be treated laparoscopically in the future owing to the implementation of the new technology, the parenchymal-sparing techniques of the surgery, and their association with techniques of interventional radiology. Since 2015, an estimated 60% to 70% of patients in the present series have been suitable for a minimally invasive approach. A further increase of 15% to 20% in the future is expected. Nevertheless, there is another 15% to 20% of patients who will never be suitable for laparoscopic surgery owing to exclusion criteria that will always contraindicate minimally invasive surgery. Nevertheless, the four-fifths of patients with metastatic disease treated laparoscopically represents a trend in surgery for CLMs, indicating a more minimally invasive approach in the future.
eFigure 1. Number of Procedures Performed for Colorectal Liver Metastasis, Stratified According to Open or Laparoscopic Approach
eFigure 2. Trend in the Ratio of Laparoscopic/Total Resections for Colorectal Liver Metastasis Over Years
References
- 1.Fiorentini G, Sarti D, Aliberti C, Carandina R, Mambrini A, Guadagni S. Multidisciplinary approach of colorectal cancer liver metastases. World J Clin Oncol. 2017;8(3):190-202. doi: 10.5306/wjco.v8.i3.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berardi G, Van Cleven S, Fretland ÅA, et al. . Evolution of laparoscopic liver surgery from innovation to implementation to mastery: perioperative and oncologic outcomes of 2,238 patients from 4 European specialized centers. J Am Coll Surg. 2017;225(5):639-649. doi: 10.1016/j.jamcollsurg.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 3.Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, et al. ; Oslo-CoMet study group . Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg. 2018;267(2):199-207. doi: 10.1097/SLA.0000000000002353 [DOI] [PubMed] [Google Scholar]
- 4.Langella S, Russolillo N, D’Eletto M, Forchino F, Lo Tesoriere R, Ferrero A. Oncological safety of ultrasound-guided laparoscopic liver resection for colorectal metastases: a case-control study. Updates Surg. 2015;67(2):147-155. doi: 10.1007/s13304-015-0325-0 [DOI] [PubMed] [Google Scholar]
- 5.Cipriani F, Rawashdeh M, Ahmed M, Armstrong T, Pearce NW, Abu Hilal M. Oncological outcomes of laparoscopic surgery of liver metastases: a single-centre experience. Updates Surg. 2015;67(2):185-191. doi: 10.1007/s13304-015-0308-1 [DOI] [PubMed] [Google Scholar]
- 6.O’Rourke N, Shaw I, Nathanson L, Martin I, Fielding G. Laparoscopic resection of hepatic colorectal metastases. HPB (Oxford). 2004;6(4):230-235. doi: 10.1080/13651820410023978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection—2,804 patients. Ann Surg. 2009;250(5):831-841. doi: 10.1097/SLA.0b013e3181b0c4df [DOI] [PubMed] [Google Scholar]
- 8.Passot G, Soubrane O, Giuliante F, et al. . Recent advances in chemotherapy and surgery for colorectal liver metastases. Liver Cancer. 2016;6(1):72-79. doi: 10.1159/000449349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanat O. Current treatment options for patients with initially unresectable isolated colorectal liver metastases. World J Clin Oncol. 2016;7(1):9-14. doi: 10.5306/wjco.v7.i1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorentini G, Ratti F, Cipriani F, et al. . Minimally invasive approach to intrahepatic cholangiocarcinoma: technical notes for a safe hepatectomy and lymphadenectomy. Ann Laparosc Endosc Surg. 2017;2:68. doi: 10.21037/ales.2017.03.05 [DOI] [Google Scholar]
- 11.Ban D, Tanabe M, Ito H, et al. . A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21(10):745-753. doi: 10.1002/jhbp.166 [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cipriani F, Rawashdeh M, Stanton L, et al. . Propensity score–based analysis of outcomes of laparoscopic versus open liver resection for colorectal metastases. Br J Surg. 2016;103(11):1504-1512. doi: 10.1002/bjs.10211 [DOI] [PubMed] [Google Scholar]
- 14.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25(1):1-21. doi: 10.1214/09-STS313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aldrighetti L, Ratti F, Cillo U, et al. ; Italian Group of Minimally Invasive Liver Surgery (I GO MILS) . Diffusion, outcomes and implementation of minimally invasive liver surgery: a snapshot from the I Go MILS (Italian Group of Minimally Invasive Liver Surgery) Registry. Updates Surg. 2017;69(3):271-283. doi: 10.1007/s13304-017-0489-x [DOI] [PubMed] [Google Scholar]
- 16.Laurent C, Adam JP, Denost Q, Smith D, Saric J, Chiche L. Significance of R1 resection for advanced colorectal liver metastases in the era of modern effective chemotherapy. World J Surg. 2016;40(5):1191-1199. doi: 10.1007/s00268-016-3404-6 [DOI] [PubMed] [Google Scholar]
- 17.Abu Hilal M, Aldrighetti L, Dagher I, et al. . The Southampton Consensus Guidelines for laparoscopic liver surgery: from indication to implementation [published online October 23, 2017]. Ann Surg. doi: 10.1097/SLA.0000000000002524 [DOI] [PubMed] [Google Scholar]
- 18.Fretland AA, Sokolov A, Postriganova N, et al. . Inflammatory response after laparoscopic versus open resection of colorectal liver metastases: data from the Oslo-CoMet trial. Medicine (Baltimore). 2015;94(42):e1786. doi: 10.1097/MD.0000000000001786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tohme S, Goswami J, Han K, et al. . Minimally invasive resection of colorectal cancer liver metastases leads to an earlier initiation of chemotherapy compared to open surgery. J Gastrointest Surg. 2015;19(12):2199-2206. doi: 10.1007/s11605-015-2962-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratti F, Cipriani F, Reineke R, et al. . Impact of Enhanced Recovery After Surgery (ERAS) approach and minimally-invasive techniques on outcome of patients undergoing liver surgery for hepatocellular carcinoma: a comparative study from a single institution. Clin Nutr ESPEN. 2016;12:e41. doi: 10.1016/j.clnesp.2016.02.036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Number of Procedures Performed for Colorectal Liver Metastasis, Stratified According to Open or Laparoscopic Approach
eFigure 2. Trend in the Ratio of Laparoscopic/Total Resections for Colorectal Liver Metastasis Over Years