Key Points
Question
Does antidepressant treatment of depression shortly after acute coronary syndrome improve long-term cardiac outcomes?
Findings
In this randomized clinical trial that included 300 patients with recent acute coronary syndrome and depression, 24-week treatment with escitalopram compared with placebo resulted in an occurrence of major adverse cardiac outcomes of 40.9% vs 53.6% after a median follow-up of 8.1 years, a difference that was statistically significant.
Meaning
Treatment with escitalopram for depression following recent acute coronary syndrome may improve long-term cardiac outcomes.
Abstract
Importance
Depression has been associated with poorer medical outcomes in acute coronary syndrome (ACS), but there are few data on the effects of antidepressant treatment on long-term prognosis.
Objective
To investigate the effect on long-term major adverse cardiac events (MACE) of escitalopram treatment of depression in patients with recent ACS.
Design, Setting, and Participants
Randomized, double-blind, placebo-controlled trial conducted among 300 patients with recent ACS and depression enrolled from May 2007 to March 2013, with follow-up completed in June 2017, at Chonnam National University Hospital, Gwangju, South Korea.
Interventions
Patients were randomly assigned to receive either escitalopram in flexible dosages of 5, 10, 15, or 20 mg/d (n = 149) or matched placebo (n = 151) for 24 weeks.
Main Outcomes and Measures
The primary outcome was MACE, a composite of all-cause mortality, myocardial infarction (MI), and percutaneous coronary intervention (PCI). Four secondary outcomes were the individual MACE components of all-cause mortality, cardiac death, MI, and PCI. Cox proportional hazards models were used to compare the escitalopram and placebo groups by time to first MACE.
Results
Among 300 randomized patients (mean age, 60 years; 119 women [39.3%]), 100% completed a median of 8.1 (interquartile range, 7.5-9.0) years of follow-up. MACE occurred in 61 patients (40.9%) receiving escitalopram and in 81 (53.6%) receiving placebo (hazard ratio [HR], 0.69; 95% CI, 0.49-0.96; P = .03). Comparing individual MACE outcomes between the escitalopram and placebo groups, respectively, incidences for all-cause mortality were 20.8% vs 24.5% (HR, 0.82; 95% CI, 0.51-1.33; P = .43), for cardiac death, 10.7% vs 13.2% (HR, 0.79; 95% CI, 0.41-1.52; P = .48); for MI, 8.7% vs 15.2% (HR, 0.54; 95% CI, 0.27-0.96; P = .04), and for PCI, 12.8% vs 19.9% (HR, 0.58; 95% CI, 0.33-1.04; P = .07).
Conclusions and Relevance
Among patients with depression following recent acute coronary syndrome, 24-week treatment with escitalopram compared with placebo resulted in a lower risk of major adverse cardiac events after a median of 8.1 years. Further research is needed to assess the generalizability of these findings.
Trial Registration
ClinicalTrials.gov Identifier: NCT00419471
This randomized clinical trial compares the effect of escitalopram vs placebo on long-term major adverse cardiovascular events among patients in Korea with depression following acute coronary syndrome.
Introduction
Depression is frequently comorbid with acute coronary syndrome (ACS; including myocardial infarction [MI] and unstable angina)1 and associated with poor outcomes including increased mortality and nonfatal events.2,3 A clinically important question is whether antidepressant treatment mitigates these adverse effects. In randomized clinical trials of antidepressants in ACS, mostly evaluating selective serotonin reuptake inhibitors, improvement in depressive symptoms has been demonstrated repeatedly.4,5 However, effects on cardiac outcomes have not generally been found within the treatment period.6,7,8,9
Considering longer-term cardiac outcomes, in an 18-month follow-up of the Myocardial Infarction and Depression–Intervention Trial (MIND-IT), no difference was found in cardiac outcomes between 209 patients receiving antidepressants (8 weeks of mirtazapine and/or subsequent open-label citalopram) and 122 patients receiving usual care.10 In the 6.7-year follow-up of the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART), there was also no difference in mortality between 184 patients receiving 6 months of sertraline and 177 receiving placebo.11 Furthermore, the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) trial found no difference in cardiac outcomes over a 29-month follow-up between the intervention (9 months of cognitive behavioral therapy with or without 12 months of antidepressants) and usual care among 2481 patients with depression following MI.12 However, in a subanalysis, 301 patients receiving selective serotonin reuptake inhibitors showed better cardiac outcomes than 1388 not receiving them.13 Conversely, an observational study reported that 58 patients who received selective serotonin reuptake inhibitors had worse outcomes than 354 who did not receive them over a 3.6-year follow-up.14
Associations of antidepressant treatment with long-term cardiac outcomes in depression following ACS have therefore been inconclusive. Previous research has been limited by short follow-up periods,10,14 heterogeneous antidepressant regimes and samples,13,14 and limited evaluation of cardiac outcomes.11 This study aimed to investigate whether the effect of escitalopram vs placebo for treating acute-phase depression in patients with recent ACS resulted in benefits in longer-term cardiac outcomes.
Methods
Study Design and Participants
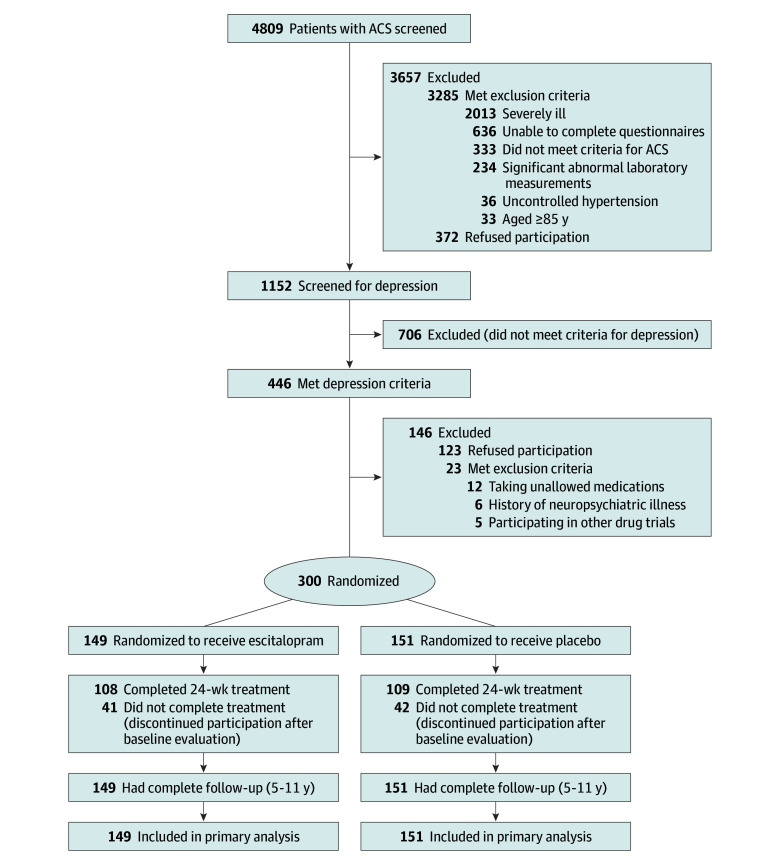
This article describes preplanned extended follow-up of the Escitalopram for Depression in Acute Coronary Syndrome (EsDEPACS) study, a 24-week randomized, placebo-controlled clinical trial of escitalopram for treating depression following ACS. The design of this trial has been published,15 and the trial protocol is available in Supplement 1. Written informed consent was collected, and the study was approved by the Chonnam National University Hospital (CNUH) Institutional Review Board. Participant recruitment and study flow are presented in Figure 1.
Figure 1. Participant Flow in the Escitalopram for Depression in Acute Coronary Syndrome Study.
ACS indicates acute coronary syndrome.
Baseline Evaluation
Participants were consecutively recruited from patients recently hospitalized with ACS at the Department of Cardiology of CNUH, Gwangju, South Korea. In 2005, this department was nominated by the Korean Circulation Society to serve as the central coordinating center for the Korea Acute Myocardial Infarction Registry (KAMIR).16 KAMIR is a nationwide prospective, multicenter, online registry (http://kamir5.kamir.or.kr/) designed as a surveillance platform to track clinical outcomes of patients with acute MI without exclusion criteria to reflect real-world practice; this enables prospective associations to be evaluated for a range of exposures or interventions with long-term cardiac outcomes. From November 2005 to December 2015, 53 966 individuals had been registered nationally and 44 758 (82.9%) were followed up for cardiac events at 1 year.16,17,18 Information was collected in a standardized proforma across sites. The study described herein focused solely on participants recruited from CNUH, which adopted a more intensive surveillance approach, with information gathered by personnel exclusively for this study, backed up by KAMIR network resources. This allowed complete follow-up for all 24-week trial participants. Preplanned monitoring of the obtained information was conducted by additional personnel at the Clinical Trial Centre of CNUH with expertise in data quality control and checked for completeness and accuracy once per year after registration. All missing or uncertain outcome data were complemented or amended through manual checks of electronic records and supplementary telephone calls where required.
Patients were treated by study cardiologists based on international guidelines for management of ACS.19 Inpatients who had had ACS in the previous 2 weeks (mean, 6.3 [SD, 2.4] days) and who met eligibility criteria (eAppendix 1 in Supplement 2) and agreed to participate were screened for depressive symptoms with the Beck Depression Inventory (BDI)20 at baseline and thereafter as outpatients every 4 weeks up to 12 weeks. Those with a BDI score greater than 10 on any of these occasions received a clinical evaluation by a study psychiatrist using the Mini International Neuropsychiatric Interview (MINI),21 a structured diagnostic psychiatric interview applying Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria, which yield diagnoses of major or minor depressive disorder.22
Information was collected on characteristics that could potentially affect cardiac outcomes.23 Demographic data were obtained on age, sex, education, marital status, living alone, accommodation, and employment status. For evaluating depressive symptoms, the self-completed BDI and psychiatrist-administered MINI diagnoses were ascertained as described, and history of depression was also recorded. Cardiovascular risk factors ascertained included diagnosed hypertension and diabetes mellitus, hypercholesterolemia by fasting serum total cholesterol level (>200 mg/dL [5.18 mmol/L]), obesity by measured body mass index, reported current smoking status, and personal and family histories of ACS. Cardiac severity status was identified using ACS diagnosis (MI or unstable angina), Killip classification,24 New York Heart Association functional classification,25 left ventricular ejection fraction, QTc duration, and serum levels of troponin I and creatine kinase MB.
24-Week Randomized Clinical Trial
Among participants with a baseline diagnosis of major or minor depressive disorder, those who met eligibility criteria and agreed to participate were randomized to a 24-week, double-blind, randomized clinical trial of escitalopram vs placebo. The first patient was enrolled in May 2007 and the last patient completed the 24-week follow-up evaluation in March 2013. Examinations were scheduled at baseline and at weeks 4, 8, 12, 16, 20, and 24 thereafter. The study drug dosage was 10 mg/d initially and could be changed (from 5 mg/d to 20 mg/d) according to the investigators’ clinical decision, taking into account response and tolerability after the second evaluation. Mean doses at the last visit were 7.6 (SD, 3.7) mg for the escitalopram group and 8.5 (SD, 3.9) mg for the placebo group. Adherence was checked by pill counts at every visit and was defined as acceptable if at least 75%. Adherence to medications was 93.3% and 95.4% in patients receiving escitalopram and placebo, respectively. At the end of 24 weeks of double-blind treatment, the study was completed, study medication was tapered down, and patients were unblinded.
The primary outcome was remission of depressive symptoms, defined by a Hamilton Depression Rating Scale (HAM-D) score of 7 or lower at the trial end.26 In pretrial analyses, a sample size of 106 patients per group was estimated as sufficient to detect a mean difference in HAM-D scores of 2 points, assuming an SD of 5.5, 2-sided tests, α = .05, and 80% power; therefore, assuming 30% loss to follow-up, a total of 300 patients was specified as a target for recruitment. The presence of any antidepressant use after the trial was ascertained at 1 year after the index ACS. The results of this 24-week trial have been published; escitalopram was superior to placebo in reducing depressive symptoms.9
Long-term Follow-up for Cardiac Outcomes
Comprehensive evaluations for cardiac outcomes were possible for this study because KAMIR manages and records detailed data electronically on hospital admissions, deaths, recurrent MI, and percutaneous coronary intervention (PCI). Long-term follow-up was initiated after the patients were enrolled for this trial. Because previous studies in this field have shown conflicting results,10,11,12,13,14 there was no appropriate reference for power calculation within the designated sample size. The KAMIR study reported a 10.9% incidence of major adverse cardiac events (MACE) over 1 year16; therefore, approximately 50% MACE incidence was expected during a 5-year follow-up. Assuming 2-sided tests, α = .05, and a follow-up sample size of 300, the expected power was 70% and 96% for detecting 10% and 15% group differences, respectively. Each patient’s status was evaluated and recorded at every hospital visit using the KAMIR protocol administered by KAMIR researchers. At least 2 researchers were exclusively assigned to this study during the entire study period specifically to obtain accurate data on long-term cardiac outcomes. These researchers made telephone contact with patients or their family members the day before each expected hospital visit to facilitate continued participation and to maximize follow-up. For those transferred to other hospitals, the researchers verified the patient’s status through a telephone call to the hospital and using the KAMIR database shared between the hospitals. When reasons for loss to follow-up could not be identified in the hospital records, mainly deaths outside of the hospital (3% of all participants), deaths were confirmed through telephone contact with a family member and through death certification in the National Registration Records, with which it was confirmed that all patients were registered. Emigration did not occur for any cohort member. As described, assertive maximization of outcome information collection was carried out at the Clinical Trial Centre of CNUH, including checks for completeness and accuracy each year after the registration and manual supplementary data collection via records checks and telephone follow-up where indicated. Through these measures, all baseline participants were thus successfully and completely followed up for these outcomes. Because a patient could have more than 1 event, all patients were followed up to the present evaluation point or until death, and nonhierarchical end-point analyses were used.
Outcome Measures
The primary end point was MACE, which was a composite of all-cause mortality, MI, and PCI. Four secondary end points were (1) all-cause mortality; (2) cardiac death (defined as sudden death with no other explanation available, death due to arrhythmia or after MI or heart failure, or death caused by heart surgery or endocarditis); (3) MI; and (4) PCI. An independent end-point committee composed of study cardiologists adjudicated all potential events and was blinded to the participants’ randomization status.
Statistical Analysis
Baseline demographic and clinical characteristics were compared between the escitalopram and placebo groups using t tests or χ2 tests. The primary analysis included all randomized participants and was based on follow-up data at a median of 8.1 years after trial commencement. Kaplan-Meier models were used to calculate the cumulative proportion of participants experiencing a composite MACE according to the date of the first event for each patient. Cox proportional hazards models were used to compare the escitalopram and placebo groups by time to first composite MACE. Two sensitivity analyses were carried out: (1) excluding patients taking antidepressants at the 1-year post-ACS examination to exclude the possible medication effects of the 24-week trial on long-term cardiac outcomes and (2) restricting the analysis to those with impaired left ventricular ejection fraction (<55%) at baseline. Secondary analyses were performed for individual MACE components in the same analytic models. Schoenfeld residuals tests were carried out to test the proportional hazards assumptions for primary and secondary outcomes.
Post hoc analyses were conducted in the subsample who completed the trial. To evaluate the effects of treatment and remission status, Cox proportional hazards models were used to compare time to first MACE after the 24-week trial. Individual associations with treatment group (escitalopram vs placebo) and remission status (remission vs no remission) were estimated separately.
All analyses were repeated after adjustment for variables that have been associated with cardiac outcomes in previous research.23 All statistical tests were 2-sided with a significance level of P<.05. Statistical analyses were carried out using SPSS version 21.0 (IBM) and Stata version 12.0 (Stata Corp).
Results
Recruitment and Baseline Characteristics
Of 4809 patients with ACS screened, 1152 were recruited to undergo depression screening with the BDI and MINI, through which 446 participants were identified as having a baseline DSM-IV diagnosis of major (n = 202) or minor (n = 246) depressive disorder. Among these patients, 300 were included in the trial and randomized to receive either escitalopram (n = 149) or placebo (n = 151) (Figure 1). In the recruiting process, higher participation rates were noted in patients with more severe depressive symptoms, resulting in a frequency of major depressive disorder of 57.0% (85/149) among the escitalopram group and 55.6% (84/151) among the placebo group, compared with 22.6% (33/146) in patients who met inclusion criteria but were not randomized. Baseline demographic and clinical characteristics are described in the Table and were similar between the escitalopram and placebo groups. Severity of ACS was relatively mild; 81% of patients were in Killip class 1 and 89% were in New York Heart Association class I. Because randomization resulted in similar distributions of measured covariates between the allocation groups, analyses were reported without adjustment, consistent with other research in this field.27
Table. Baseline Characteristics by Randomization Group Among 300 Patients With Depression After Acute Coronary Syndrome.
| Characteristics | Escitalopram (n = 149) | Placebo (n = 151) |
|---|---|---|
| Demographics | ||
| Age, mean (SD), y | 60.0 (11.2) | 60.1 (10.5) |
| Men, No. (%) | 88 (59.1) | 93 (61.6) |
| Education, mean (SD), y | 9.4 (4.2) | 9.2 (4.1) |
| Unmarried, No. (%) | 18 (12.1) | 29 (19.2) |
| Living alone, No. (%) | 12 (8.1) | 16 (10.6) |
| Rented accommodation, No. (%) | 21 (14.1) | 34 (22.5) |
| Unemployed, No. (%) | 71 (47.7) | 73 (48.3) |
| Depression | ||
| Beck Depression Inventory scorea | 16 (13-22) | 17 (14-23) |
| Mean (SD) | 18.8 (8.3) | 19.2 (7.7) |
| Median (IQR) | 16 (13-22) | 17 (14-23) |
| DSM-IV diagnosis of major depressive disorder, No. (%) | 85 (57.0) | 84 (55.6) |
| Previous depression, No. (%) | 6 (4.0) | 7 (4.6) |
| Cardiac risk factors, No. (%) | ||
| Hypertension | 90 (60.4) | 94 (62.3) |
| Diabetes mellitus | 44 (29.5) | 41 (27.2) |
| Hypercholesterolemia | 73 (49.0) | 71 (47.0) |
| Obesity | 59 (39.6) | 65 (43.0) |
| Current smoker | 43 (28.9) | 42 (27.8) |
| Previous acute coronary syndrome | 8 (5.4) | 11 (7.3) |
| Family history of acute coronary syndrome | 9 (6.0) | 8 (5.3) |
| Current cardiac status | ||
| Acute coronary syndrome diagnosis of myocardial infarction, No. (%) | 92 (61.7) | 92 (60.9) |
| Killip class >1, No. (%)b | 24 (16.1) | 35 (23.2) |
| NYHA class >I, No. (%)c | 15 (10.1) | 18 (11.9) |
| LVEF, mean (SD), % | 60.4 (11.0) | 61.9 (10.4) |
| LVEF <55%, No. (%) | 34 (22.8) | 33 (21.9) |
| QTc duration, mean (SD), ms | 433.3 (39.7) | 438.9 (38.3) |
| Peak troponin I, mean (SD), mg/dL | 6.7 (8.5) | 7.7 (8.0) |
| Peak creatine kinase MB fraction, mean (SD), mg/dL | 17.2 (22.0) | 16.3 (20.8) |
Abbreviations: DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); IQR, interquartile range; LVEF, left ventricular ejection fraction.
The Beck Depression Inventory consists of 21 items with a total score ranging from 0 to 63, with higher scores indicating more severe depressive symptoms.
Killip class is ranked from 1 to 4, with higher rank indicating more severe clinical signs of heart failure.
New York Heart Association (NYHA) class is ranked from I to IV, with higher rank indicating more severe limitation of activity due to symptoms.
Primary Outcome
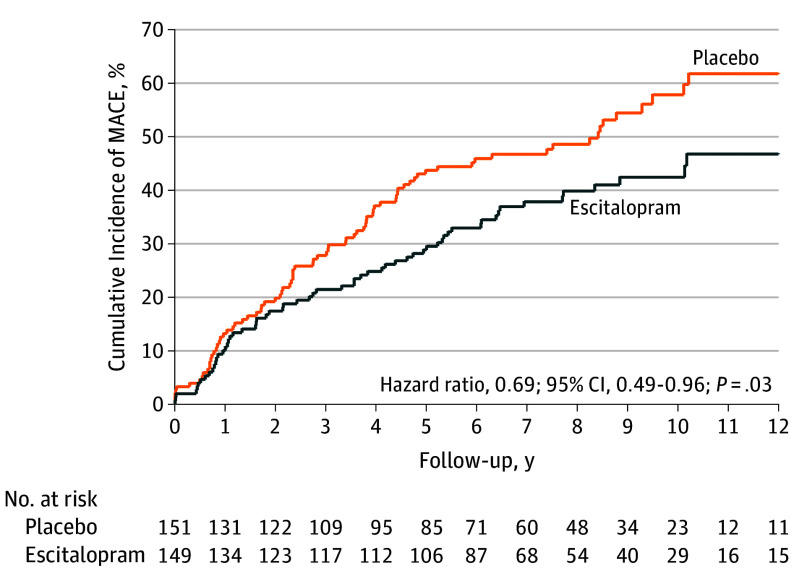
All participants were followed up for 5 to 11 years until death or June 2017 (median follow-up, 8.1 [interquartile range, 7.1-9.0] years; mean, 8.4 [SD, 1.2] years). Before the end of the 24-week trial, 12 patients experienced MACE. Figure 2 shows cumulative risk of the primary end point (composite MACE) in the escitalopram and placebo groups. A significant difference was found: composite MACE incidence was 40.9% (61/149) in the escitalopram group and 53.6% (81/151) in the placebo group (hazard ratio [HR], 0.69; 95% CI, 0.49-0.96; P = .03). The model assumption was met (Schoenfeld P = .48). The estimated statistical power to detect the observed difference in MACE incidence rates between the 2 groups was 89.7%. When the same analyses were repeated after excluding patients taking antidepressants at the 1-year point after ACS (n=5 for escitalopram and n=3 for placebo), the results were not changed substantially; composite MACE incidences were 40.3% (58/144) in the escitalopram group and 53.4% (79/148) in the placebo group (HR, 0.69; 95% CI, 0.48-0.96; P = .03). In patients with impaired left ventricular ejection fraction (<55%) at baseline, composite MACE occurred in 23/34 (55.8%) escitalopram participants and 24/33 (72.7%) placebo participants, which was not statistically significant either for this composite outcome (P = .12) or individual MACE outcomes (all P>.30).
Figure 2. Cumulative Incidence of MACE (Primary Outcome).
MACE indicates major adverse cardiac events, a composite of all-cause mortality, myocardial infarction, and percutaneous coronary intervention. Median follow-up was 8.1 (IQR, 7.0-9.0) years for the escitalopram group and 8.2 (IQR, 7.1-8.9) years for the placebo group.
Secondary Outcomes
Figure 3 shows cumulative risks in the escitalopram and placebo groups for secondary end points (individual MACE components). A significant difference was found in the incidence of MI at 8.7% in the escitalopram group and 15.2% in the placebo group (HR, 0.54; 95% CI, 0.27-0.96; P = .04). However, there were no significant differences in the incidences of other individual MACE components between the escitalopram and placebo groups: 20.8% and 24.5%, respectively, for all-cause mortality (HR, 0.82; 95% CI, 0.51-1.33; P= .43); 10.7% and 13.2% for cardiac death (HR, 0.79; 95% CI, 0.41-1.52; P= .48); and 12.8% and 19.9% for PCI (HR, 0.58; 95% CI, 0.33-1.04; P= .07). Model assumptions were all met (Schoenfeld P>.45).
Figure 3. Cumulative Incidence of the Individual MACE Components of All-Cause Mortality, Cardiac Death, Myocardial Infarction, and Percutaneous Coronary Intervention.
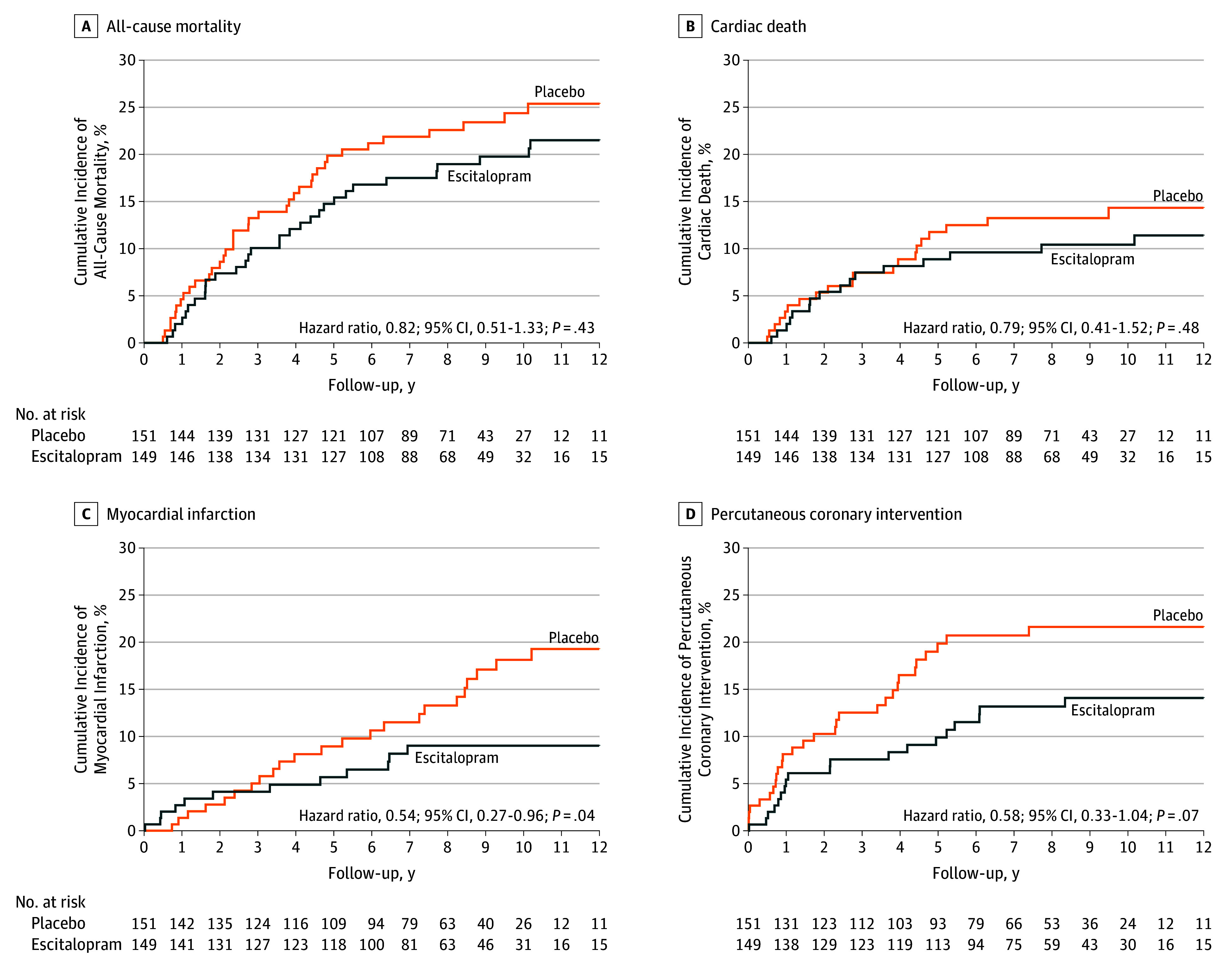
Cardiac death was defined as sudden death with no other explanation available, death due to arrhythmia or after myocardial infarction or heart failure, or death caused by heart surgery or endocarditis.
Post Hoc Outcomes
Associations of depression treatment and remission status with times to first MACE are summarized in eTable 1 in Supplement 2. Of 300 randomized patients, 217 (108 receiving escitalopram and 109 receiving placebo) completed the 24-week trial. The serum creatine kinase MB fraction was significantly higher in patients who did not complete the 24-week trial vs those who completed it (P= .04), but there were no significant differences in any other characteristic at baseline (all P>.05). Remission was achieved in 57 (52.3%) of 108 escitalopram patients and in 38 (34.9%) of 109 placebo participants who completed the 24-week trial. Of the completers, 7 experienced MACE before the end of the 24-week trial, so the remaining 210 were included in the long-term follow-up analysis. With respect to the association with treatment group, those randomized to escitalopram had significantly lower hazards of composite MACE, MI, and PCI compared with those randomized to placebo. With respect to the association with remission status, the group with remission had significantly lower hazards of composite MACE, all-cause mortality, and PCI compared with those without remission. Because all multiplicative interaction terms between treatment group and remission status on MACE were not statistically significant (all P>.12), the effect sizes should not be interpreted as different. Results of adjusted analyses are reported in eTables 2 and 3 in Supplement 2.
Discussion
In this median 8.1-year follow-up of a randomized 24-week clinical trial of treatment for depression in patients with recent ACS, MACE incidence was significantly lower in patients receiving escitalopram than those receiving placebo.
These results should be considered in relation to those from 2 previous randomized clinical trials with supplementary follow-up, MIND-IT and SADHART, which reported no significant difference between antidepressant and control treatment on MACE.10,11 In the present study, the beneficial effect of escitalopram over placebo was observed for composite MACE and MI outcomes but not for any mortality outcomes. In post hoc analyses, all-cause mortality was associated with nonremission status irrespective of treatment allocation. Findings from the SADHART 6.7-year follow-up and from the present 8.1-year follow-up were similar in that mortality differed not by treatment group but by remission status, although SADHART evaluated only all-cause mortality as an outcome.11 MIND-IT reported no significant difference in any composite or individual MACE measure between antidepressant intervention and care as usual; however, its follow-up duration was relatively short (18 months) and composite MACE incidence was low (13.4%).10 Although the present finding has not been reported previously in ACS, similar results were reported in a poststroke depression cohort; 12-week antidepressant treatment allocation was associated with improved survival over a 9-year follow-up.28
Several issues should be considered in the interpretation of apparent divergence between results of the current study and previous studies. First, treatment effects on depression differed in the previous 2 studies, which did not find significant differences between antidepressant and control groups in primary outcomes, but only in subgroups with recurrent or more severe depression11 or in only 1 of the secondary outcomes.7 This might account for the absence of an effect on longer-term cardiovascular outcomes, whereas it was reported that escitalopram had superiority over placebo in reducing depressive symptoms during the 24-week trial,9 sustained at 1-year follow-up.29,30 Citalopram, the racemate of which escitalopram is the S-(+) enantiomer, has also been found to be superior to placebo for reducing depressive symptoms in patients with ACS,8 although longer-term follow-up results have not yet been published. Therefore, escitalopram may have modifying effects on disease prognosis in ACS-associated depressive disorder through reduction of depressive symptoms. In addition, escitalopram may positively affect common mediators of ACS and depression including brain-derived neurotrophic factor and proinflammatory cytokines31,32 and may normalize autonomic and platelet dysfunction, which have adverse effects on cardiac outcomes.33,34
Second, the levels of depressive symptoms in this study’s participants were less severe (mean baseline HAM-D score, 15.9) than in MIND-IT or SADHART (mean baseline HAM-D scores, 18.1 and 19.6, respectively).10,11 Of potential relevance, previous research has indicated that even minor depressive symptoms may have significant negative effects on cardiac prognosis.35
Third, the antidepressant dosage was lower in this study (escitalopram had a mean dosage of 7.6 mg/d [fluoxetine-equivalent dosage, 16.9 mg/d]) than in the SADHART study (sertraline had a mean dosage of 68.8 mg/d [fluoxetine-equivalent dosage, 27.9 mg/d]).11 However, ethnic differences have been reported in response to psychotropic medications, and lower dosages have been suggested as sufficient for achieving similar responses in East Asian vs white patients.36 Fourth, initial antidepressant treatment duration in the present study (24 weeks) was the same as that of SADHART,11 but longer than that of MIND-IT (8 weeks)10 and thus potentially relevant for the latter difference. Fifth, considering severity of heart disease, antidepressants have been reported to be ineffective for reducing depressive symptoms37,38 and all-cause mortality39 in patients with heart failure, a more severe form of heart disease than ACS. Severity of ACS in the present study’s participants was relatively low, which might render it easier to demonstrate positive effects on both depressive and long-term cardiac outcomes. In the subgroup analysis with impaired left ventricular ejection fraction, no statistical group difference was observed in outcomes, although small numbers had diminished statistical power.
Limitations
This study has several limitations. First, recruitment was carried out at a single site, unlike previous multicenter studies.10,11,13 This may limit the generalizability of findings, although it facilitates consistency of evaluation and treatment. Findings were also drawn exclusively from a Korean population, and replication is needed in other ethnic groups. Second, differential attrition should be considered for the subgroup completing the 24-week trial. Higher serum creatine kinase MB levels were associated with attrition, potentially implicating more severe ACS pathology; however, there were no significant attrition associations with other baseline variables. Moreover, follow-up data on MACE are believed to be complete for the primary analysis. Third, the small numbers of participants experiencing individual MACE components (8.3% for cardiac death in particular) limit power and therefore generalizability, although the incidence of composite MACE (47.3%) was sufficient. Fourth, no attempt was made to investigate depression occurrence or antidepressant use after the 1-year follow-up, which could also affect long-term cardiac outcomes. However, the number of participants using any antidepressant was small at 1 year after ACS, and principal findings were not changed substantially after excluding this group.
Conclusions
Among patients with depression following recent ACS, 24-week treatment with escitalopram compared with placebo resulted in a lower risk of MACE after a median of 8.1 years. Further research is needed to assess the generalizability of these findings.
DEPACS Study Protocol
eAppendix 1. Eligibility Criteria for the K-DEPACS and EsDEPACS Participants
eAppendix 2. Associations With Long-term Cardiac Outcomes After Adjustment for Potential Covariates
eTable 1. Associations of Depression Treatment and Remission Status With Composite and Individual Components of Major Adverse Cardiac Events (MACE) in 210 of 217 Patients (pts) With Acute Coronary Syndrome (ACS) Who Completed the 24 Week Randomized Clinical Trial
eTable 2. Associations of Escitalopram vs Placebo on Composite and Individual Components of Major Adverse Cardiac Events (MACE) in Patients With Acute Coronary Syndrome (ACS), After Adjustment
eTable 3. Associations of Depression Treatment and Remission Status With Composite and Individual Components of Major Adverse Cardiac Events (MACE) in 210 of 217 Patients (pts) With Acute Coronary Syndrome (ACS) Who Completed the 24 Week Randomized Clinical Trial, After Adjustment
References
- 1.Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry. 2003;54(3):227-240. doi: 10.1016/S0006-3223(03)00587-0 [DOI] [PubMed] [Google Scholar]
- 2.Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction: impact on 6-month survival. JAMA. 1993;270(15):1819-1825. doi: 10.1001/jama.1993.03510150053029 [DOI] [PubMed] [Google Scholar]
- 3.Lespérance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105(9):1049-1053. [DOI] [PubMed] [Google Scholar]
- 4.Thombs BD, de Jonge P, Coyne JC, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300(18):2161-2171. doi: 10.1001/jama.2008.667 [DOI] [PubMed] [Google Scholar]
- 5.Pizzi C, Rutjes AW, Costa GM, et al. Meta-analysis of selective serotonin reuptake inhibitors in patients with depression and coronary heart disease. Am J Cardiol. 2011;107(7):972-979. [DOI] [PubMed] [Google Scholar]
- 6.Glassman AH, O’Connor CM, Califf RM, et al. ; Sertraline Antidepressant Heart Attack Randomized Trial Group . Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701-709. doi: 10.1001/jama.288.6.701 [DOI] [PubMed] [Google Scholar]
- 7.Honig A, Kuyper AM, Schene AH, et al. ; MIND-IT Investigators . Treatment of post-myocardial infarction depressive disorder: a randomized, placebo-controlled trial with mirtazapine. Psychosom Med. 2007;69(7):606-613. [DOI] [PubMed] [Google Scholar]
- 8.Lespérance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297(4):367-379. doi: 10.1001/jama.297.4.367 [DOI] [PubMed] [Google Scholar]
- 9.Kim JM, Bae KY, Stewart R, et al. Escitalopram treatment for depressive disorder following acute coronary syndrome: a 24-week double-blind, placebo-controlled trial. J Clin Psychiatry. 2015;76(1):62-68. doi: 10.4088/JCP.14m09281 [DOI] [PubMed] [Google Scholar]
- 10.van Melle JP, de Jonge P, Honig A, et al. ; MIND-IT Investigators . Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190(6):460-466. doi: 10.1192/bjp.bp.106.028647 [DOI] [PubMed] [Google Scholar]
- 11.Glassman AH, Bigger JT Jr, Gaffney M. Psychiatric characteristics associated with long-term mortality among 361 patients having an acute coronary syndrome and major depression: seven-year follow-up of SADHART participants. Arch Gen Psychiatry. 2009;66(9):1022-1029. doi: 10.1001/archgenpsychiatry.2009.121 [DOI] [PubMed] [Google Scholar]
- 12.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289(23):3106-3116. doi: 10.1001/jama.289.23.3106 [DOI] [PubMed] [Google Scholar]
- 13.Taylor CB, Youngblood ME, Catellier D, et al. ; ENRICHD Investigators . Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792-798. doi: 10.1001/archpsyc.62.7.792 [DOI] [PubMed] [Google Scholar]
- 14.Rieckmann N, Kronish IM, Shapiro PA, Whang W, Davidson KW. Serotonin reuptake inhibitor use, depression, and long-term outcomes after an acute coronary syndrome: a prospective cohort study. JAMA Intern Med. 2013;173(12):1150-1151. doi: 10.1001/jamainternmed.2013.910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JM, Stewart R, Bae KY, et al. Effects of depression co-morbidity and treatment on quality of life in patients with acute coronary syndrome: the Korean depression in ACS (K-DEPACS) and the escitalopram for depression in ACS (EsDEPACS) study. Psychol Med. 2015;45(8):1641-1652. [DOI] [PubMed] [Google Scholar]
- 16.Lee KH, Jeong MH, Kim HM, et al. ; Korea Acute Myocardial Infarction Registry Investigators . Benefit of early statin therapy in patients with acute myocardial infarction who have extremely low low-density lipoprotein cholesterol. J Am Coll Cardiol. 2011;58(16):1664-1671. doi: 10.1016/j.jacc.2011.05.057 [DOI] [PubMed] [Google Scholar]
- 17.Lee JM, Rhee TM, Hahn JY, et al. ; KAMIR Investigators . Multivessel percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2018;71(8):844-856. doi: 10.1016/j.jacc.2017.12.028 [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Chae SC, Oh DJ, et al. ; Korea Acute Myocardial Infarction–National Institutes of Health Registry Investigators . Multicenter cohort study of acute myocardial infarction in Korea. Circ J. 2016;80(6):1427-1436. doi: 10.1253/circj.CJ-16-0061 [DOI] [PubMed] [Google Scholar]
- 19.Anderson JL, Adams CD, Antman EM, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction. J Am Coll Cardiol. 2013;61(23):e179-e347. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561-571. doi: 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 21.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33. [PubMed] [Google Scholar]
- 22.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 23.Jaffe AS, Krumholz HM, Catellier DJ, et al. Prediction of medical morbidity and mortality after acute myocardial infarction in patients at increased psychosocial risk in the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) study. Am Heart J. 2006;152(1):126-135. doi: 10.1016/j.ahj.2005.10.004 [DOI] [PubMed] [Google Scholar]
- 24.Killip T III, Kimball JT. Treatment of myocardial infarction in a coronary care unit: a two-year experience with 250 patients. Am J Cardiol. 1967;20(4):457-464. doi: 10.1016/0002-9149(67)90023-9 [DOI] [PubMed] [Google Scholar]
- 25.Criteria Committee of the New York Heart Association . Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Little Brown & Co; 1994:253-256. [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galløe AM, Thuesen L, Kelbaek H, et al. Comparison of paclitaxel- and sirolimus-eluting stents in everyday clinical practice: the SORT OUT II randomized trial. JAMA. 2008;299(4):409-416. doi: 10.1001/jama.299.4.409 [DOI] [PubMed] [Google Scholar]
- 28.Jorge RE, Robinson RG, Arndt S, Starkstein S. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829. [DOI] [PubMed] [Google Scholar]
- 29.Kim JM, Bae KY, Kang HJ, et al. Design and methodology for the Korean observational and escitalopram treatment studies of depression in acute coronary syndrome: K-DEPACS and EsDEPACS. Psychiatry Investig. 2014;11(1):89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang HJ, Stewart R, Bae KY, et al. Effects of depression screening on psychiatric outcomes in patients with acute coronary syndrome: findings from the K-DEPACS and EsDEPACS studies. Int J Cardiol. 2015;190:114-121. [DOI] [PubMed] [Google Scholar]
- 31.Polyakova M, Stuke K, Schuemberg K, et al. BDNF as a biomarker for successful treatment of mood disorders: a systematic and quantitative meta-analysis. J Affect Disord. 2015;174:432-440. doi: 10.1016/j.jad.2014.11.044 [DOI] [PubMed] [Google Scholar]
- 32.Rana P, Sharma AK, Jain S, et al. Comparison of fluoxetine and 1-methyl-L-tryptophan in treatment of depression-like illness in bacillus Calmette-Guerin–induced inflammatory model of depression in mice. J Basic Clin Physiol Pharmacol. 2016;27(6):569-576. doi: 10.1515/jbcpp-2015-0120 [DOI] [PubMed] [Google Scholar]
- 33.Kemp AH, Brunoni AR, Santos IS, et al. Effects of depression, anxiety, comorbidity, and antidepressants on resting-state heart rate and its variability: an ELSA-Brasil Cohort baseline study. Am J Psychiatry. 2014;171(12):1328-1334. [DOI] [PubMed] [Google Scholar]
- 34.Dawood T, Barton DA, Lambert EA, et al. Examining endothelial function and platelet reactivity in patients with depression before and after SSRI therapy. Front Psychiatry. 2016;7:18. doi: 10.3389/fpsyt.2016.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88(4):337-341. doi: 10.1016/S0002-9149(01)01675-7 [DOI] [PubMed] [Google Scholar]
- 36.Yoon J-S. Measuring ethnic differences in response to psychotropic drugs. In: Hindmarch I, Stonier PD, eds. Human Psychopharmacology: Measures and Methods, Vol 5. Chichester, England: John Wiley & Sons Ltd; 1995:31-51. [Google Scholar]
- 37.Gottlieb SS, Kop WJ, Thomas SA, et al. A double-blind placebo-controlled pilot study of controlled-release paroxetine on depression and quality of life in chronic heart failure. Am Heart J. 2007;153(5):868-873. doi: 10.1016/j.ahj.2007.02.024 [DOI] [PubMed] [Google Scholar]
- 38.O’Connor CM, Jiang W, Kuchibhatla M, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angermann CE, Gelbrich G, Störk S, et al. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315(24):2683-2693. doi: 10.1001/jama.2016.7635 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DEPACS Study Protocol
eAppendix 1. Eligibility Criteria for the K-DEPACS and EsDEPACS Participants
eAppendix 2. Associations With Long-term Cardiac Outcomes After Adjustment for Potential Covariates
eTable 1. Associations of Depression Treatment and Remission Status With Composite and Individual Components of Major Adverse Cardiac Events (MACE) in 210 of 217 Patients (pts) With Acute Coronary Syndrome (ACS) Who Completed the 24 Week Randomized Clinical Trial
eTable 2. Associations of Escitalopram vs Placebo on Composite and Individual Components of Major Adverse Cardiac Events (MACE) in Patients With Acute Coronary Syndrome (ACS), After Adjustment
eTable 3. Associations of Depression Treatment and Remission Status With Composite and Individual Components of Major Adverse Cardiac Events (MACE) in 210 of 217 Patients (pts) With Acute Coronary Syndrome (ACS) Who Completed the 24 Week Randomized Clinical Trial, After Adjustment