Key Points
Question
Can a plasma-based cardiac bridging integrator 1 (cBIN1) score distinguish patients with clinical heart failure with preserved ejection fraction (HFpEF) from healthy individuals and patients with risk factors but no heart failure?
Findings
In this study, the cBIN1 score outperformed N-terminal pro-B-type natriuretic peptide in differentiating 52 stable, ambulatory patients with HFpEF from age-matched and sex-matched healthy individuals and participants with heart failure risk factors but no HFpEF.
Meaning
If further validated, the cBIN1 score, a marker based not on preload but on maladaptive remodeling of cardiomyocytes, could potentially provide a novel index to identify stable, ambulatory patients with HFpEF.
This case-control study investigates whether if the normalized reciprocal of the plasma level of cardiac bridging integrator 1 protein differs in patients with heart failure with preserved ejection fraction, patients with risk factors for heart failure, and individuals with neither heart failure nor risk factors.
Abstract
Importance
Transverse tubule remodeling is a hallmark of heart failure. Cardiac bridging integrator 1 (cBIN1) is a circulating membrane scaffolding protein that is essential for transverse tubule health, and its plasma level declines with disease.
Objective
To determine if a cBIN1-derived score can serve as a diagnostic biomarker of heart failure with preserved ejection fraction (HFpEF).
Design, Setting, and Participants
In this cohort study, the cBIN1 score (CS) was determined from enzyme-linked immunoabsorbent assay–measured plasma cBIN1 concentrations from study participants in an ambulatory heart failure clinic at Cedars-Sinai Medical Center. Consecutive patients with a confirmed diagnosis of heart failure with preserved ejection fraction (HFpEF; defined by a left ventricular ejection fraction ≥50%) were recruited from July 2014 to November 2015 and compared with age-matched and sex-matched healthy volunteers with no known cardiovascular diagnoses and participants with risk factors for heart failure but no known HFpEF. Baseline characteristics and 1-year longitudinal clinical information were obtained through electronic medical records. Data analysis occurred from November 2016 to November 2017.
Main Outcomes and Measures
The analysis examined the ability of the CS and N-terminal pro-B-type natriuretic peptide (NT-proBNP) results to differentiate among patients with HFpEF, healthy control participants, and control participants with risk factors for heart failure. We further explored the association of the CS with future cardiovascular hospitalizations.
Results
A total of 52 consecutive patients with a confirmed diagnosis of HFpEF were enrolled (mean [SD] age, 57 [15] years; 33 [63%] male). The CS values are significantly higher in the patients with HFpEF (median [interquartile range (IQR)], 1.85 [1.51-2.28]) than in the 2 control cohorts (healthy control participants: median [IQR], −0.03 [−0.48 to 0.41]; control participants with risk factors only: median [IQR], −0.08 [−0.75 to 0.42]; P < .001). For patients with HFpEF, the CS outperforms NT-proBNP when the comparator group was either healthy control participants (CS: area under curve [AUC], 0.98 [95% CI, 0.96-1.00]; NT-proBNP level: AUC, 0.93 [95% CI, 0.88-0.99]; P < .001) or those with risk factors (CS: AUC, 0.98 [95% CI, 0.97-1.00]; NT-proBNP: AUC, 0.93 [95% CI, 0.88-0.99]; P < .001). Kaplan-Meier analysis of 1-year cardiovascular hospitalizations adjusted for age, sex, body mass index, and NT-proBNP levels reveals that patients with HFpEF with CS greater than or equal to 1.80 have a hazard ratio of 3.8 (95% CI, 1.3-11.2; P = .02) for hospitalizations compared with those with scores less than 1.80.
Conclusions and Relevance
If further validated, the plasma CS, a marker of transverse tubule dysfunction, may serve as a biomarker of cardiomyocyte remodeling that has the potential to aide in the diagnosis of HFpEF.
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a growing epidemic worldwide with a high burden of morbidity and mortality.1 Its incompletely understood pathophysiology has limited development of diagnostic tools to define and follow the disease progression.2 Cardiac bridging integrator 1 (cBIN1) is a membrane-scaffolding protein in cardiomyocytes that organizes the dyad-containing microdomains at the transverse tubules that are responsible for the initiation and regulation of systolic and diastolic calcium transients.3,4,5,6,7 The level of cBIN1 is reduced in animal models of heart failure, as well as in human biopsy samples from patients with end-stage cardiomyopathy.3,8,9,10,11 Its blood availability makes it an attractive biomarker of cardiomyocyte remodeling.12 In this study, we demonstrate the performance of the cBIN1 score (CS), a dimensionless index based on the normalized reciprocal of cBIN1 plasma levels, in a population of patients with HFpEF who are stable and ambulatory.
Methods
Study Design
The study was approved by the institutional review board at Cedars-Sinai Medical Center. Full informed consent was obtained from all participants prior to study participation. Participants self-identified their race/ethnicity in the study.
Consecutive patients with ambulatory HFpEF were enrolled at the time of continuity visits at the Cedars-Sinai Advanced Heart Failure clinic. The diagnosis of HFpEF was established based on past evidence of objective signs of fluid overload in the absence of noncardiac contributors, as determined by an assessment by an advanced heart failure specialist, prior hospitalization for heart failure, or invasive hemodynamic data confirming presence of elevated cardiac filling pressures. Patients were followed up for at least 1 year by medical record review. Plasma from 2 age-matched and sex-matched control cohorts with detailed clinical histories was obtained from Innovative Research with full informed consent: a healthy cohort with no cardiovascular risk factors and a cohort with presence of HF risk factors but no HFpEF.
The CS was computed as the natural log of the inverse of measured cBIN1 plasma concentration. Plasma sample processing and storage from the heart failure and control cohorts are available in the eMethods in the Supplement.
Statistical Methods
Data distributions were assessed for normality with the quantile-quantile plot and the Kolmogorov-Smirnov test. Normal continuous variables were expressed as means and SDs and compared using a 2-sided t test. Nonnormal continuous variables were analyzed with medians and interquartile ranges (IQRs) and compared using the Mann-Whitney U test. Categorical variables were compared using χ2 tests. Receiver operating characteristic (ROC) analysis was performed to determine the sensitivity and specificity of CS and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels to identify HFpEF, adjusted for age, sex, and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared). Multiple linear and logistics regression analyses were utilized to determine if model is affected by age, BMI, left ventricular ejection fraction, blood pressure, heart rate, estimated glomerular filtration rate, sex, or race/ethnicity. Since NT-proBNP has a nonnormal distribution, log transformations were used in our analyses. Statistical analyses were conducted using SAS version 9.3.1 (SAS Institute Inc) and RStudio version 1.0.143 (RStudio Inc). Two-sided P values were reported, and a P value <.05 was considered significant.
Results
Study Cohorts
A total of 52 ambulatory patients with HFpEF were age matched and sex matched with 2 control cohorts: healthy individuals with no known cardiovascular risk factors and control participants with at least 1 risk factor (obesity, hypertension, or diabetes) but no heart failure (Table). In the cohort of patients with HFpEF, 38 patients (73.1%) had New York Heart Association class II or greater degree of symptoms (eTable 1 in the Supplement). Mean (SD) BMI was significantly higher in the control group with risk factors (34 [4.7]) than in the healthy cohort (24.4 [2.9]) or the cohort with HFpEF (29 [5.9]; P < .001; Table). Notably, in multivariate analyses, CS was not affected by age, sex, race/ethnicity, BMI, estimated glomerular filtration rate, blood pressure, or heart rate. Detailed laboratory, echocardiographic, and demographic characterizations of the cohort with HFpEF are shown in eTable 1 in the Supplement.
Table. Characteristics of Patients in the Study Cohorts.
| Characteristics | No. (%) | P Value | ||
|---|---|---|---|---|
| Patients With HFpEF (n = 52) | Healthy Control Participants (n = 52) | Control Participants With Risk Factors Only (n = 52) | ||
| Age, mean (SD), y | 57 (15) | 52 (6) | 52 (9) | .05 |
| Male | 33 (63) | 33 (63) | 33 (63) | >.99 |
| Race/ethnicity | ||||
| White | 30 (58) | 30 (58) | 29 (56) | .75 |
| Black | 10 (19) | 11 (21) | 7 (13) | |
| Hispanic | 8 (15) | 11 (21) | 16 (31) | |
| Asian | 4 (8) | 0 (0) | 0 (0) | |
| BMI, mean (SD) | 29 (5.9) | 24.4 (2.9) | 34 (4.7) | <.001 |
| Normal (≤24.9) | 13 (25) | 28 (54) | 2 (4) | |
| Overweight (25-29.9) | 20 (38) | 24 (46) | 9 (17) | |
| Obese (≥30) | 19 (37) | 0 (0) | 41 (79) | |
| Hypertension | 29 (57) | 0 (0) | 15 (29) | <.001 |
| Diabetes | 5 (10) | 0 (0) | 14 (27) | <.001 |
| Chronic kidney disease | 5 (10) | NA | NA | NA |
| Left ventricular ejection fraction, mean (SD) % | 58 (7.1) | NA | NA | NA |
| cBIN1 score, median (IQR) | 1.85 (1.51-2.28) |
−0.03 (−0.48 to 0.41) |
−0.08 (−0.75 to 0.42) |
<.001 |
| NT-proBNP level, median (IQR), ln(pg/mL) | 277 (99-1264) | 36 (19-72) | 21 (13-43) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HFpEF, heart failure with preserved ejection fraction; IQR, interquartile range; NA, not applicable; NT-proBNP, N-terminal prohormone of brain natriuretic peptide.
CS Values and Identification of HFpEF
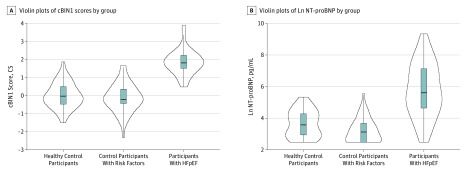
The distribution of CS values in the healthy control group, risk-factor control group, and the group with HFpEF is in Figure 1A. (Median [IQR] cBIN1 concentrations are available in eTable 2 in the Supplement.) The median (IQR) CS was similar in the healthy participants (−0.03 [IQR, −0.48 to 0.41]) and those with risk factors (−0.08 [IQR, −0.75 to 0.42]; P = .44). In contrast, the CS was significantly elevated in patients with HFpEF (median [IQR], 1.85 [1.51-2.28]; P < .001; Figure 1). Notably, the mean (SD) CS in the cohort with HFpEF (1.88 [0.69]) is more than 2 SDs higher than the mean (SD) CS in either control cohort (0.00 [0.62] in the healthy cohort and −0.15 [0.82] in the risk-factor control group; P < .001). The levels of NT-proBNP were used for comparison (Table). Although the natural logarithm of the median (IQR) NT-proBNP levels are significantly higher in the group with HFpEF (median [IQR], 5.62 [4.60-7.14] ln[pg/mL]; P < .001) compared with the 2 control groups (healthy control group: median [IQR], 3.58 [2.94-4.28] ln[pg/mL]; risk-factor control group, 3.04 [2.56-3.76] ln[pg/mL]), the distribution of ln NT-proBNP levels shows a considerable overlap with levels in both control groups (Figure 1B).
Figure 1. Violin Plots of Cardiac Bridging Integrator 1 (cBIN1) Scores and ln N-terminal Pro-B-Type Natriuretic Peptide (NT-proBNP) in the Study Cohorts.
A, Distribution of cBIN1 score values in the matched healthy control group (median [IQR], −0.03 [−0.48 to 0.41]), the risk-factor control group (median [IQR], −0.08 [−0.75 to 0.42) and the group with heart failure with preserved ejection fraction (HFpEF) (median [IQR], 1.85 [1.51-2.28]; P < .001). B, Distribution of ln NT-proBNP values in the matched healthy control cohort (median [IQR], 3.58 [2.94-4.28]), the risk-factor control cohort (median [IQR], 3.04 [2.56-3.76]) and patients with HFpEF (median [IQR], 5.62 [4.60-7.14]; P < .001).
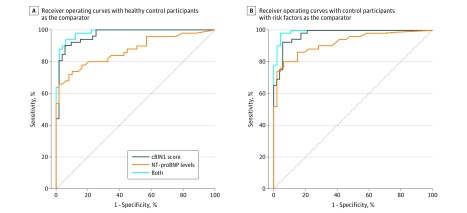
To assess the ability of CS and NT-proBNP levels to distinguish patients with HFpEF from those without heart failure, ROC curves were generated with adjustment for age, sex, and BMI. Using the healthy control cohort, CS values yielded an area under the curve (AUC) of 0.98 (95% CI, 0.96-1.00) compared with the AUC for NT-proBNP levels (0.93 [95% CI, 0.88-0.99]; P < .001), and the AUC for the 2 tests combined (0.99 [95% CI, 0.98-1.00]; Figure 2A). With the risk-factor control cohort as the comparator, CS values similarly outperformed NT-proBNP levels (CS: AUC, 0.98 [95% CI, 0.97-1.00]; P < .001; NT-proBNP level: AUC, 0.93 [95% CI, 0.88-0.99]; 2 tests combined, 0.99 [95% CI, 0.99-1.00; Figure 2B). Using a CS value of 1.80 as a diagnostic cutoff chosen from the ROC curves (Figure 2A and B), the CS has positive predictive values of 97% and 100%, and negative predictive values of 68% and 68% against the healthy and risk-factor control cohorts, respectively, for identifying HFpEF diagnosis (Figure 2A and B).
Figure 2. Receiver Operating Characteristic Curves for N-terminal Pro-B-Type Natriuretic Peptide (NT-proBNP) Levels, Cardiac Bridging Integrator 1 (cBIN1) Scores, and Both Assays.
A, The cBIN1 scores yielded an area under the curve (AUC) of 0.98 (95% CI, 0.96-1.00), while the AUC for NT-proBNP is 0.93 (95% CI, 0.88-0.99), and the AUC for the 2 tests combined is 0.99 (95% CI, 0.98-1.00). B, The AUC for cBIN1 scores is 0.98 (95% CI, 0.97-1.00), the AUC for NT-proBNP is 0.93 (95% CI, 0.88-0.99), and the AUC for the 2 tests combined is 0.99 (95% CI, 0.99-1.00).
CS Values and Cardiovascular Hospitalization in Patients With HFpEF
We explored the prognostic ability of the CS for future cardiovascular events in patients with HFpEF. eFigure 1 in the Supplement demonstrates the restricted cubic spline fit for the cardiovascular hospitalizations hazard rate ratio (HRR) compared with CS values. The HRR changes little until the CS value reaches approximately 1.70, at which point it increases and tapers off at a value of approximately 2.29 (P = .04). A CS of 1.80, the median CS in the HFpEF cohort, is close to an HRR of unity, and it occurs in the rapid linear rise of the plot. Hence, a CS of 1.80 is used as a cutoff value for outcomes risk stratification. eFigure 2 in the Supplement illustrates cardiovascular hospitalizations for the cohort with HFpEF within 1 year of follow-up, stratified by CS values greater than or less than 1.80, adjusted for age, sex, BMI, and NT-proBNP level. Patients with CS values equal to or greater than 1.80 have an HRR of 3.8 (95% CI, 1.3-11.2; P = .02) for cardiovascular hospitalization compared with those with CS less than 1.80. The high morbidity and mortality burden of the individuals with HFpEF is illustrated by their 1-year follow-up period, in which there were 27 any-cause hospitalizations, 26 cardiovascular hospitalizations, 5 major adverse cardiovascular events, 3 automatic implantable cardioverter defibrillator discharges, 2 cardiovascular event–associated deaths, 4 heart transplantations, and 1 left ventricular assist device implantation.
Discussion
The prevalence of HFpEF is expected to rise precipitously over the next few decades.13 Understanding this syndrome has been challenged by the lack of diagnostic tools that can assay cardiac health independent of fluid status. The CS is a cardiac-specific biomarker of transverse tubule remodeling occurring with HF progression.3,14,15 In this study, we show that the CS, a biomarker based on the normalized reciprocal of plasma cBIN1 concentration, is more than 2 SDs higher in stable ambulatory patients with HFpEF compared with age-matched, sex-matched healthy volunteers, as well as individuals with heart failure risk factors but no HFpEF (Figure 1A). Notably, CS values are markedly increased even in those patients with New York Heart Association class I and II symptoms, whose NT-proBNP levels are within the age-appropriate normal ranges. Using cubic spline analysis (eFigure 1 in the Supplement) and ROC curve estimate of the test’s diagnostic accuracy (eFigure 2 in the Supplement), a cBIN1 cutoff value of 1.80 was chosen for its high positive predictive value for HFpEF diagnosis, as well as its prognostic value for cardiovascular hospitalizations during 1 year of follow-up.
Limitations
Limitations of this study include the heterogeneity of the HFpEF cohort and cBIN1 testing in a single center, without a separate validation cohort. Additionally, the plasma samples from the 2 control cohorts were purchased from a third party. However, our laboratory confirmed the stability of the cBIN1 assay under freeze-thaw conditions. Furthermore, we purposefully focused this study of CS values on stable patients in the ambulatory setting, as opposed to patients with acute dyspnea who were presenting to the emergency department, where assessment of NT-proBNP level has proven diagnostic utility.
Conclusions
The potential power of CS is in diagnosing remodeled heart muscle, independent of fluid overload. We are encouraged by the synergistic performance of the CS and NT-proBNP level, indicating different mechanistic origins (cardiac muscle health vs volume status, respectively). The receiver operating characteristic curves for the diagnostic performance of the CS and NT-proBNP level demonstrate that combining the 2 assays results in superior discriminating power between patients with heart failure and patients with risk factors but no manifestation of the clinical syndrome. Future directions will further validate CS values for the diagnosis of HFpEF, assess cBIN1 levels in patients with heart failure and reduced ejection fraction, and explore the role of CS in early subclinical HFpEF detection.
eMethods. Supplemental methods.
eTable 1. Detailed characteristics of the HFpEF cohort.
eTable 2. Median cBIN1 concentration in the three studied cohorts.
eFigure 1. Cubic spline fit and CV hospitalization incidence according to CS level
eFigure 2. Kaplan-Meier curves for CV hospitalization free survival
eReferences.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292. doi: 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Upadhya B, Haykowsky MJ, Kitzman DW. Therapy for heart failure with preserved ejection fraction: current status, unique challenges, and future directions. Heart Fail Rev. 2018. doi: 10.1007/s10741-018-9714-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong T, Yang H, Zhang SS, et al. Cardiac BIN1 folds t-tubule membrane, controlling ion flux and limiting arrhythmia. Nat Med. 2014;20(6):624-632. doi: 10.1038/nm.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong TT, Smyth JW, Chu KY, et al. BIN1 is reduced and Cav1.2 trafficking is impaired in human failing cardiomyocytes. Heart Rhythm. 2012;9(5):812-820. doi: 10.1016/j.hrthm.2011.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong TT, Smyth JW, Gao D, et al. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010;8(2):e1000312. doi: 10.1371/journal.pbio.1000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu Y, Shaw SA, Naami R, et al. Isoproterenol promotes rapid ryanodine receptor movement to bridging integrator 1 (BIN1)-organized dyads. Circulation. 2016;133(4):388-397. doi: 10.1161/CIRCULATIONAHA.115.018535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong T, Shaw RM. Cardiac t-tubule microanatomy and function. Physiol Rev. 2017;97(1):227-252. doi: 10.1152/physrev.00037.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah SJ, Aistrup GL, Gupta DK, et al. Ultrastructural and cellular basis for the development of abnormal myocardial mechanics during the transition from hypertension to heart failure. Am J Physiol Heart Circ Physiol. 2014;306(1):H88-H100. doi: 10.1152/ajpheart.00642.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidel T, Navankasattusas S, Ahmad A, et al. Sheet-like remodeling of the transverse tubular system in human heart failure impairs excitation-contraction coupling and functional recovery by mechanical unloading. Circulation. 2017;135(17):1632-1645. doi: 10.1161/CIRCULATIONAHA.116.024470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frisk M, Ruud M, Espe EK, et al. Elevated ventricular wall stress disrupts cardiomyocyte t-tubule structure and calcium homeostasis. Cardiovasc Res. 2016;112(1):443-451. doi: 10.1093/cvr/cvw111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong TT, Cogswell R, James CA, et al. Plasma BIN1 correlates with heart failure and predicts arrhythmia in patients with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2012;9(6):961-967. doi: 10.1016/j.hrthm.2012.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu B, Fu Y, Liu Y, et al. The ESCRT-III pathway facilitates cardiomyocyte release of cBIN1-containing microparticles. PLoS Biol. 2017;15(8):e2002354. doi: 10.1371/journal.pbio.2002354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137-e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 14.Lyon AR, MacLeod KT, Zhang Y, et al. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci U S A. 2009;106(16):6854-6859. doi: 10.1073/pnas.0809777106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Y, Hong T. BIN1 regulates dynamic t-tubule membrane. Biochim Biophys Acta. 2016;1863(7, pt B):1839-1847. doi: 10.1016/j.bbamcr.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental methods.
eTable 1. Detailed characteristics of the HFpEF cohort.
eTable 2. Median cBIN1 concentration in the three studied cohorts.
eFigure 1. Cubic spline fit and CV hospitalization incidence according to CS level
eFigure 2. Kaplan-Meier curves for CV hospitalization free survival
eReferences.