Abstract
The Indian spice turmeric, in which the active and dominant biomolecule is curcumin, has been demonstrated to have significant medicinal properties, including anti‐inflammatory and anti‐neoplastic effects. This promise is potentially very applicable to musculoskeletal disorders, which are common causes of physician visits worldwide. Research at the laboratory, translational and clinical levels that supports the use of curcumin for various musculoskeletal disorders, such as osteoarthritis, osteoporosis, musculocartilaginous disorders, and sarcoma is here in comprehensively summarized. Though more phase I−III trials are clearly needed, thus far the existing data show that curcumin can indeed potentially be useful in treatment of the hundreds of millions worldwide who are afflicted by these musculoskeletal disorders.
Keywords: Curcumin, Osteoarthritis, Osteoporosis, Sarcoma, Turmeric
Background
Musculoskeletal conditions are substantial causes of loss of productivity and disability in all countries and economies. In the USA alone, an estimated 30% of American adults are affected by joint pain, swelling or limitation of movement1. In 2010, US‐Americans made 63 million visits to office‐based orthopedic surgeons, a number that would likely be much higher if visits to primary care physicians with musculoskeletal issues were included2. Arthritis, osteoporosis, musculocartilaginous injury and acute fractures are some of the commonest reasons for US‐Americans visiting orthopedic physicians3. Additionally, although much rarer, orthopedic cancers are important to consider because these musculoskeletal issues can be fatal.
Non‐operative management is the initial, if not primary treatment modality, for the majority of musculoskeletal complaints. The Centers for Disease Control have shown that acetaminophen and non‐steroidal anti‐inflammatory drugs (NSAIDs) are prescribed in half of orthopedic visits2. Use of these medications is limited by adverse effects in the gastrointestinal tract and kidney that can decrease patient compliance. For the much rarer orthopedic cancers, a combination of surgery, radiotherapy, and chemotherapy are first‐line treatments. Chemoradiotherapy has numerous adverse effects because of its effects on other rapidly dividing cells in the bone marrow, skin and gastrointestinal tract. In light of the wide variety of common musculoskeletal conditions, a low‐cost and efficacious pharmacologic compound with minimal side effects would be an important tool in the arsenal of patients and physicians alike.
Curcumin is an herbal compound that reportedly has potential benefit in a variety of orthopedic issues ranging from the common complaints to rarer sarcomas. The compound is a yellow extract of turmeric, which has ties to the Indian subcontinent where it has applications in customs, medicine, religious rituals and cuisines. Curcumin's numerous effects include decreasing concentrations of low‐density lipoprotein cholesterol, inhibiting platelet aggregation, reducing risk of thrombosis and myocardial infarction, controlling blood glucose, inhibiting HIV replication, protecting against cataracts and hepatoprotective effects4. It inhibits multiple processes in inflammatory cell proliferation, invasion, and angiogenesis, which gives it therapeutic potential in many musculoskeletal disorders with a significant inflammatory component. Moreover, it enhances natural cell apoptotic mechanisms in cells, which is potentially useful for cancer therapy. Curcumin effects these changes by interfering with gene expression, protein kinases, transcription factors, and enzymes involved in multiple steps of the nuclear factor κ‐light‐chain‐enhancer of activated B‐cells (NF‐κB), activator protein (AP)‐1, and mitogen‐activated protein kinase pathways, to name a few5, 6. This review article will summarize the current available molecular, translational, and clinical research on use of curcumin specifically for musculoskeletal disorders such as arthritis, osteoporosis, musculocartilaginous disorders and sarcoma.
Methods
We conducted a comprehensive search of published English‐language original research papers using the PubMed database. Keywords included combinations of the following: curcumin, turmeric and diferuloylmethane; and the following: orthopedic, joint, muscle, bone, cartilage, tendon, ligament, rheumatology, joint, sarcoma, osteoporosis and musculoskeletal. We excluded commentaries, editorials and articles that did not directly assess effects of curcumin. We completed all searches by May 2015. With the aim of searching comprehensively yet prioritizing recent data, we primarily searched for articles from 2000–2015 and, after screening for appropriateness, sought to primarily include most data from 2005–2015 in this review paper. Our searches resulted in 176 articles that met the aforementioned criteria; after removing redundant articles and articles with overlapping information, articles examining effects beyond the scope of this review, and adding non‐curcumin‐related articles for other citation purposes in this review (e.g. basic epidemiological studies of osteoarthritis), we have cited 85 articles.
Osteoarthritis
Osteoarthritis (OA) is a progressive, degenerative disease of joints involving the articular cartilage, subchondral bone, and synovium; joints in the fingers, hips, knees, and spine are most frequently affected7. In 2005, OA affected 13.9% of adults aged 25 years and older and 33.6% of adults aged over 65 years in the USA8. OA is associated with substantial morbidity and economic costs. OA of the knee is one of the five leading causes of disability among non‐institutionalized adults9. Job‐related costs of OA are $3.4 to $13.2 billion per year, and in 2009, hospital expenditures for total knee and hip replacements were estimated to be $28.5 billion and $13.7 billion, respectively10, 11.
Despite the high prevalence, morbidity, and healthcare costs of OA, current treatments cannot stop disease progression. The major classes of drugs currently used include acetaminophen, NSAIDs and intra‐articular glucocorticoids. The primary benefit of acetaminophen for OA patients is its analgesic effect; this drug does not control the inflammatory process. However, NSAIDs and glucocorticoids do have anti‐inflammatory properties. NSAIDs reversibly inhibit the cyclo‐oxygenase (COX) enzymes, COX‐1 and COX‐2. COX‐2 inhibition reduces production of prostaglandins (PG), including PGE2 and PGI2, which contribute to inflammation by promoting vasodilation and increasing vascular permeability. Glucocorticoids exert their anti‐inflammatory effects primarily by inhibiting key transcriptional factors such as AP‐1 and NF‐κB, which are responsible for production of a number of cytokines including interleukin (IL)‐1β, IL‐2, IL‐6 and IL‐812.
Laboratory Data
Curcumin has notable advantages over NSAIDs and glucocorticoids. Unlike NSAIDs, curcumin selectively inhibits COX‐2 but not COX‐113. By inhibiting COX‐1, NSAIDs reduce secretion of the protective mucus layer in the stomach and can cause clinically apparent adverse gastrointestinal effects such as peptic ulcers and subsequent risk of bleeding and perforation. The adverse gastrointestinal effects are especially significant for OA patients because these patients are often on lifelong treatment, which can result in a compounding of gastrointestinal mucosal damage. The absence of adverse gastrointestinal effects with curcumin treatment could be particularly beneficial in patients with history of ulcers and/or in those taking oral or subcutaneous anticoagulants. Regarding glucocorticoids, curcumin's suppression of matrix metalloproteinases (MMPs) that normally degrade the extracellular matrix (ECM) may clinically mean greater protection of the ECM and less abrupt catabolism of the joints' mesenchymal matrix14. Whether this results in clinically greater preservation of joint micro‐ (and macro‐) architecture remains to be seen.
Recent evidence from several in vitro studies suggests that curcumin is an effective therapeutic option for OA because it targets a number of pathways that are central to disease pathogenesis. First, curcumin can mitigate the inflammatory process by decreasing synthesis of inflammatory mediators such as IL‐6, IL‐8 and PGE215. Furthermore, curcumin reduces amounts of PGs, leukotrienes and thromboxanes through inhibition of phospholipase A2, cyclooxygenase‐2 and 5‐lipooxygenase16, 17. Second, curcumin can inhibit proliferation of synoviocytes, which are responsible for producing the inflammatory mediators that result in tissue destruction in subjects with OA16, 18. Third, curcumin can reduce reactive oxygen and nitrogen species formation in neutrophils19, 20, 21; reactive oxygen and nitrogen species can lead to cartilage degradation and depolymerization of hyaluronan, which can eventually lead to joint erosion22, 23. Fourth, curcumin possesses anti‐catabolic activity. MMPs secreted by chondrocytes in response to IL‐1β and oncostatin M catabolize the ECM in joints24. By inhibiting the AP‐1 pathway and NF‐κB activation, curcumin suppresses gene expression of a number of MMPs such as MMP‐1, MMP‐3, MMP‐9 and MMP‐13, preserving the integrity of the joint matrix15, 24, 25, 26, 27, 28. Thus, curcumin's potential for treatment of OA is supported by its action at the biochemical, molecular and cellular levels. These encouraging data have prompted translational and clinical studies as discussed subsequently.
Translational Data
Translational studies in animals have corroborated the beneficial effects of curcumin in OA. A study in mice with collagen‐induced arthritis by Moon et al. showed that following intraperitoneal injection of curcumin every other day for 2 weeks, proliferation of splenic T cells, expression of tumor necrosis factor (TNF)‐α and IL‐1β in the ankle joint, and serum immunoglobulin concentrations were downregulated compared with non‐curcumin‐treated mice28. Another study by Colitti et al. compared transcriptomes in peripheral white blood cells in OA‐affected dogs prior to and following treatment with curcumin or NSAIDs29. Following oral treatment with curcumin in a phytosome preparation (CurcuVET; Thorne Research, Dover, ID, USA) for 20 days, a variety of anti‐inflammatory changes were noted, including decreased macrophage proliferation, decreased IL‐18 and TNF‐α production, and inhibition of the inflammatory transcription factor NF‐κB29, 30. Furthermore, some of the aforementioned endpoints were affected only by curcumin and not by NSAIDs, suggesting that curcumin acts on more inflammatory pathways than do NSAIDs alone. Another key point of these reports is that the administration of curcumin can decrease inflammatory molecule levels in the synovium in vivo. Though the blood supply to joints and tendons/ligaments is not as liberal as to muscle or bone, the presence of decreased amounts of ILs in joints indicates that downstream targets of curcumin are indeed being affected at the appropriate locations (i.e., the locations of disease pathology).
Clinical Data
The few published clinical studies assessing treatment of OA with curcumin have shown promising results. A randomized, double‐blind, placebo‐controlled trial from Iran showed curcuminoid‐receiving patients had significantly lower scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and Lequesne's pain functional index than patients receiving a placebo. WOMAC scores more specifically reflect changes in pain, physical function and stiffness31. Two studies by Belcaro et al. have demonstrated the clinical efficacy and safety of Meriva (Thorne Research), a phosphatidylcholine complex with curcumin that is designed to enhance the latter's oral bioavailability32. The first study, which was a short‐term product registry study, showed that the group receiving Meriva had improvements in pain sensation, joint stiffness and physical function according to their WOMAC scores33. The second study aimed to assess the long‐term efficacy and safety of Meriva in subjects with OA. In this study, subjects in both the control group, which was defined as the “best available treatment” and treatment group, which was the “best available treatment plus Meriva” could medicate with NSAIDs during the study to control their pain if needed. These authors found that the treatment group had improvements in pain, stiffness and physical function according to their WOMAC scores, improved Karnofsky performance scores, and statistically significant reductions in serum concentrations of sCD40L, IL‐1β, IL‐6 and sV cell adhesion molecule‐1 and in erythrocyte sedimentation rate34. Additionally, the treatment group had a 63.4% decrease in use of NSAIDs and painkillers compared with 8% in the control group and a 63.5% decrease in management costs compared with 3.7% in the control group. Together with previous studies showing that curcumin acts on more inflammatory pathways than does NSAIDs, this study confirmed clinically that curcumin can be substituted for NSAIDs, which is a potential boon for patients who are intolerant of NSAIDs because of adverse gastrointestinal and renal effects or simply wish to take more natural medications.
Both the trial in Iran and the Meriva study showed improvements in pain and physical scores; however, only the Meriva study showed improvements in stiffness scores on the WOMAC scale. One possible explanation for this discrepancy is that the larger sample size in the Meriva study (n = 50 for treatment and n = 50 for control) compared with the study in Iran (n = 27 for treatment and n = 26 for placebo) could have more accurately captured the effects of the cucurmin‐based product. Another possible explanation is that the oral bioavailability of curcumin administered with Meriva is greater. These two studies used different strategies to enhance bioavailability. In the Iranian study, curcumin was co‐administered with piperine, whereas in Meriva curcumin is complexed with a phospholipid. The successes of these clinical trials prompts the question of how much clinical benefit is obtained by conjugating curcumin with bioavailability‐enhancing formulations. It would certainly change physician management if it was proved that curcumin given alone confers a significantly smaller clinical benefit in subjects with OA than bioavailability‐enhancing formulations.
The positive outcomes of the clinical trials suggest that curcumin is an effective therapeutic agent for OA. Future studies should investigate combinations of curcumin with other drugs such as glucocorticoids or NSAIDs. As mentioned above, mechanisms of action studied in vitro suggest that glucocorticoids and curcumin may have a synergistic effect. Additionally, alternative mechanisms for enhancing curcumin bioavailability should be considered. These could includes co‐administration with other substances, complexing curcumin with other molecules, making chemical modifications that do not reduce the intended clinical effects and using alternative routes of administration such as intra‐articular injection or sublingual administration. Measuring serum or synovial fluid concentrations of curcumin and correlating these with clinical outcomes would also be fruitful.
Additionally, future patient research should seek to ascertain whether curcumin can prevent the development of OA or whether it simply inhibits progression. To this end, curcumin would ideally be tested not only in patients with OA but also in symptom‐free subjects at high risk of developing OA (e.g. with risk factors such as obesity). It is hence important to begin questioning which stage(s) of OA curcumin benefits, and specifically at which stage maximal symptomatic benefit is attained. Other key future goals should be to delineate the patient characteristics associated with most benefits from prophylactic curcumin administration and to determine whether certain OA risk factors (e.g. diabetes mellitus, other rheumatological diseases) are more able to be modified by prophylactic curcumin therapy than others.
Osteoporosis
Bone growth and destruction are physiologic processes. An equilibrium is established between osteoclasts, which resorb bone, and osteoblasts, which build bone. In osteoporosis, this equilibrium is disturbed because of inadequate peak bone mass, excessive bone resorption or inadequate formation of new bone during remodeling. The pathogenesis involves the final common pathway that leads to increased osteoclast activation‐ligand for the receptor activator of NF‐κB (RANKL), which is expressed by osteoblasts and interacts with RANK to activate differentiation of progenitors into osteoclasts and maintain osteoclast function. This pathway is normally antagonized by osteoprotegerin, which is a decoy receptor. The Wnt signaling pathway also antagonizes this process by playing a crucial role in osteoblast growth and differentiation, this role involving an interaction between the canonical β‐catenin pathway and bone morphogenic protein‐2. Of note, specific defects in the production or activity of local and systemic growth factors such as insulin‐like growth factor‐1 and transforming growth factor‐β can contribute to impaired bone formation. Nitric oxide (NO) may inhibit bone resorption, possibly through osteoprotegerin interaction, whereas PGE2 predominantly stimulates bone turnover35.
Laboratory Data
Curcumin can improve multiple aspects of bone health in subjects with osteoporosis by acting on multiple steps in the activation and differentiation of osteoclasts, improving mineral density and mechanical properties. Mechanisms that have been proposed include inhibition of NF‐κB, RANKL, NO production, generation of reactive oxygen species and inflammatory cytokine synthesis36, 37, 38, 39, 40, 41, 42, 43. Through these mechanisms, curcumin can decrease osteoclast number, differentiation and activation. This makes curcumin an attractive means of shifting the balance between osteoblast and osteoclast activity in selected patients with an imbalance, such as those with osteoporosis. The next question now being investigated in animal studies is whether the changes in chemical mediators and osteoclast−osteoblast balance seen in the laboratory are manifested in gross improvements in indicators of bone health, physical strength and functional mobility.
Translational Data
Translational research studies have demonstrated that curcumin administration can lead to improvements in indicators of bone health and mechanical properties of bone and favorable changes in the structure of the bony matrix. They have shown that curcumin administration can reverse osteoporotic change, as indicated by achieving bone mineral density less than 2.5 standard deviations below the mean peak bone mass as measured by dual‐energy X‐ray absorptiometry. In one study, administration of oral curcumin to mice led to increases in trabecular bone mass in metaphyses and improved bone mineral density. The same study showed that curcumin was able to reverse the reduced osteoblast counts, increased osteoclast counts and increased eroded surface area in oophorectomized rats44. In non‐oophorectomized rats (with normal estrogen concentrations), curcumin decreased serum estradiol concentrations and slightly increased cancellous bone formation. However, the authors also noted that curcumin slightly improved some bone histomorphometric variables, but did not improve bone mineralization or mechanical properties in oophorectomized rats with low estrogen concentrations45. Thus, the precise relationship between curcumin and estrogen, which is ambiguous at present, needs to be better elucidated. Further translational research should also investigate whether slight structural modifications to curcumin may enable it to further improve bone structural and mechanical properties by an estrogen‐independent mechanism. Moreover, future research should also assess the effect on bone health of co‐administering curcumin with an estrogen agonist or selective agonist. Taken together, answering such questions could help to assess more fully whether pre‐ and post‐menopausal women at risk of osteoporosis should be treated differently if taking curcumin supplements.
Clinical Data
There are very few clinical studies in humans investigating curcumin's effects on osteoporosis. Because osteoporosis is asymptomatic and does not directly lead to mortality, clinical research may need to focus on fracture risk. Osteoporosis increases risk of fractures, which can cause debilitating symptoms and indirectly increase risk of mortality. Pilot clinical trial designs, for example, could start with simple administration of curcumin to a cohort of healthy or high‐risk patients and measure bone density radiologically after prolonged administration of curcumin.
Cartilaginous Disease
Although injuries to cartilage, menisci, tendons and ligaments are common reasons for patients visiting orthopedic surgeons, the role of curcumin in these areas has not been well studied. Research, although scarce, has primarily focused on tendons and ligaments. The pathogenesis of tendinitis and tendinopathy involves the same inflammatory mediators: IL‐1β and NF‐κB. Curcumin can suppress these processes in human tenocytes in vitro 46. Nevertheless, the effectiveness of curcumin for treating injuries to these tissues needs to be evaluated, even at the cellular and molecular levels. For ligaments specifically, co‐administration with other agents, such as growth factors, may be beneficial. While curcumin may not have specific benefits in treating cartilage, meniscus, tendon and ligament injuries, its analgesic and anti‐inflammatory properties may facilitate post‐operative repairs of these tissues. Injury to ligaments such as the anterior cruciate and medial collateral ligaments, and to menisci, have the potential for very high‐volume trials given the ubiquitousness of such injuries in sports medicine. It is important to consider delivery of curcumin in vivo to these tissues. Because of their tenuous blood supply, oral administration might not be effective. Other options like intra‐articular injection could be considered. Additionally, trials comparing intra‐articular administration of curcumin with steroid injections are warranted, as are trials examining the optimal time course of curcumin treatment during the often‐lengthy rehabilitation for some ligament tears; functional tests measuring strength and kinetic variables such as hop testing are common outcome measures that can be simply assessed. Additionally, it may be of interest to quantitate use of NSAIDs and other medications, especially in light of the often‐aggressive orthopedic rehabilitation for cartilaginous injuries.
Skeletal Muscle Pathology
Laboratory Data
Initial studies of curcumin's ability to treat skeletal muscle atrophy have been inconclusive. Atrophy of skeletal muscle has a number of different causes and it appears that curcumin's beneficial effect is dependent on the cause. Curcumin appears to prevent sepsis‐induced muscle wasting by inhibiting NF‐κB and p38 kinase activity, oxygen radical scavenging, and induction of a heat response; however, all of these mechanisms are not necessarily used47. While some studies have shown that curcumin exhibits protective effects against muscle wasting, the study described below did not show positive outcomes for treating atrophy induced by prolonged unloading, which is clinically seen in paralyzed patients or those with immobilized limb(s) following a fracture. The study showed that mice whose hindlimb muscles had been unloaded for 11 days, lost 51%−53% of soleus muscle weight and cross‐sectional area relative to freely‐ambulating controls48. It is known that NF‐κB activity increases following hindlimb unloading, leading to skeletal muscle breakdown49. Because curcumin inhibits NF‐κB, it was postulated that curcumin could prevent atrophy. However, curcumin neither reduced NF‐κB activity nor prevented atrophy. One possible reason is suggested by a prior study in which unloading‐induced NF‐κB activation seemed not to be triggered by the classical pathway, which is known to be the dominant target of curcumin inhibition49. The finding that curcumin reduced NF‐κB activity in peripheral tissues in the ambulatory animals further supports this possibility.
It is not clear why curcumin's efficacy in preventing skeletal muscle atrophy is variable. One possibility is that different underlying conditions such as sepsis, cancer and prolonged unloading elicit different molecular pathways, all of which converge on the common outcome of muscle atrophy. If this is the case, the molecular mechanisms that induce atrophy in each condition need to be further characterized. Another possibility is that the amount of curcumin in the muscle may impact its efficacy and the specific molecular pathways it inhibits. Measurement of amounts of curcumin in skeletal muscle and changes in efficacy with alternative routes of administration, such as i.m. injection, should be considered. Taken together, the available studies in muscle atrophy suggest that curcumin treatment is beneficial in certain cases, the nature of which is as yet undetermined.
Translational Data
Curcumin appears to improve exercise‐induced muscle fiber damage, inflammation and muscle soreness. Davis et al. studied changes in running performance and molecular markers of inflammation and muscle damage in mice following uphill and downhill running, with or without curcumin administration. They found that oral administration of curcumin to mice for 3 days prior to exercise reduced fatigue, increased subsequent running distance, reduced plasma creatine kinase concentrations, and blunted increases in IL‐1β, IL‐6 and TNF‐α in the soleus muscle following downhill running only. The greatest improvement was seen in downhill running, possibly because eccentric contractions have been shown to produce the most damage and delayed‐onset muscle soreness50.
These data show that curcumin is potentially an effective agent for clinically reducing muscle soreness and improving recovery times following intense exercise. Curcumin use for this indication may be especially beneficial for athletes and military personnel, who are at higher risk of this type of muscle injury. These individuals may be able to take curcumin prophylactically prior to exercise to enhance recovery and prevent excessive damage. Curcumin may also be a much better long‐term solution for pain and inflammation reduction than NSAIDs, because of the aforementioned adverse effects of the latter. Clinical studies should follow to determine whether similar improvements occur in humans. Though i.m. injections may not be feasible after every exercise period, similar studies in humans could be devised. Pre‐administration of oral curcumin prior to intensive exercise and measuring subsequent pain/soreness could be an appropriate first step in determining curcumin's clinical efficacy in post‐exercise recovery.
Curcumin has anti‐diabetic properties that have also been found to modulate the glucose involved in the energy balance of skeletal muscle cells. This is relevant in that previous studies have established that chronic inflammation plays an important role in metabolic disorders51. Moreover, skeletal muscle is responsible for more than 75% of energy disposal52. Curcumin increases insulin sensitivity by basal translocation of glucose transporter‐4 and has been said to have potential in treating diabetes53. Curcumin has objectively been found to decrease blood glucose concentrations and diabetes‐induced complications such as diabetic retinopathy in rats54. Together, these data suggest that curcumin may function synergistically with exercise and anti‐diabetic agents in managing diabetes. It is not difficult to envision a simple human study involving administration of curcumin with a diet and exercise regimen and periodically measuring blood glucose concentrations and hemoglobin A1C at certain intervals.
Sarcoma
A major common mechanism in the spread of cancer and increasing of the disease burden is loss of cell cycle checkpoints and apoptotic mechanisms. The cell cycle progresses when phase‐specific cyclins bind to cyclin‐dependent kinase proteins. Tumor suppressor proteins such as p53 and Rb serve as checkpoints that prevent automatic progression of the cell cycle. When healthy cells undergo transformation into cancerous cells, apoptosis is the mechanism that destroys them. In the intrinsic pathway of apoptosis, changes in proportions of anti‐apoptotic factors, such as B‐cell lymphoma 2 regulator protein, and pro‐apoptotic factors, such as Bax, lead to increased mitochondrial permeability and release of cytochrome C. In the extrinsic pathway, Fas‐L binds to Fas‐R to form a multi‐protein death‐inducing signaling complex. In both cases, caspases are end‐effectors of the process55. Curcumin has been shown to be effective in multiple steps of this process, increasing apoptosis in cancerous cells.
Laboratory Data
Several studies in osteosarcoma have reported molecular evidence that supports enhancement of apoptosis by curcumin. Chang et al. showed that high concentrations of curcumin increase the concentration of reactive oxygen species, induce release of cytochrome C, activate the end‐effector caspase‐3 and decrease expression of cyclins and cyclin‐dependent kinases56. Another study added to these findings by demonstrating that curcumin promotes cleavage of specific procaspases, expression of the proapoptotic factors Fas, Bax and p53, expression of the DNA repair protein poly‐ADP‐ribose polymerase and downregulation of the antiapoptotic proteins Bid and B‐cell lymphoma 2 regulator protein57. Taken together, these findings show that curcumin works on all of the major steps that mediate apoptosis. Curcumin decreases expression of cyclins that promote advancement in the cell cycle, increases the ratio of pro‐apoptotic to anti‐apoptotic proteins and activates proteases that mediate apoptosis.
This evidence has been supplemented with evidence of apoptosis on microscopy, real‐time changes in mitochondrial membrane concentrations and assessments of the percentage of cells in each phase of the cell cycle. A study by Jin et al. used microscopy to assess cell shrinkage, nuclear fragmentation, apoptotic body formation and DNA fragmentation in the presence of curcumin. A block at G1/S phase was noted, 56.3% of cells being in G1 in the curcumin group as compared with 33.7% in the control group58. Similar findings were reported by Lee et al., who added that increasing concentrations of curcumin led to a more pronounced block at G2/M phases than at G1/S59. These studies suggest that cell cycle arrest—fundamental inhibition of which is intimately involved in carcinogenesis—can be reinstated at several different phases by curcumin. Given that pharmacologic anti‐cancer agents that affect the cell cycle are currently being developed, it could be useful to observe any additive or synergistic effects of curcumin.
However, for curcumin to be truly beneficial, it must have selective cytotoxicity for osteosarcoma cells as compared to healthy human osteoblast cells. Indeed, for concentrations between 5–25 μmol/L in 200 μL cell solution, one report seemed to indicate that this is indeed the case. At a dose of 5 μmol/L curcumin, osteosarcoma cell density decreased by a factor of 0.6 times, whereas there was no statistically significant decrease in numbers of healthy human osteoblast cells. At the higher concentration of 10 μmol/L curcumin, osteosarcoma cell density decreased 5.3‐fold, still without any statistically significant decrease in numbers of healthy human osteoblast cells60. This fact is especially important because osteosarcoma is a cancer of childhood and adolescence. Effective therapy would ideally target the rapidly proliferating cancerous cells and not the rapidly proliferating osteoblast cells that are involved in healthy bone growth in children.
Further research has shown that slight modifications to the structure of curcumin can create compounds that are 6.5 to 60 times as potent in inhibiting Wnt in the Wnt/β catenin pathway of osteosarcoma61. Fossey et al. demonstrated that the curcumin analog FLLL32 is more efficient than cucurmin itself at inhibiting the protein STAT3, and thus inducing apoptosis, in osteosarcoma cells62, 63. Ravindran et al. investigated whether chemical alterations in the hydroxyl, methoxy and phenyl groups of curcumin altered the activity of the compound, and found that various chemical manipulations did enhance curcumin's anti‐inflammatory activity and increase its anti‐proliferative activity64. This is particularly interesting from a standpoint of anti‐cancer drug design; though plants are the source of many common drugs, many currently available drugs have been chemically modified to render them more potent and/or specific for the target molecule of interest. Hence, as further information on this molecule is gleaned, it is certainly possible that candidate pharmacologic analogs of curcumin may prove to increase potency as well as other characteristics. This could in turn influence patient outcomes if traditional curcumin doses are translated to pharmacologic doses for human therapy.
While osteosarcoma remains the best studied malignant bone tumor, there are also data that support curcumin for Ewing sarcoma and chondrosarcoma. In Ewing sarcoma cell lines, 4 μmol/L of curcumin reportedly reduces cell viability by 51% and 8 μmol/L of curcumin reduces cell viability by 77%, suggesting a dose‐dependent response which could be worth exploiting pharmacologically65. Regarding Ewing sarcoma, in the pathogenesis of which p53 mutation plays a role, curcumin can increase the radiosensitivity of this cancer (radiosensitivity is discussed below)66. Additionally, curcumin has been observed to selectively induce apoptosis in chondrosarcoma cells through the extrinsic death receptor pathway67. While there is still a need for much more research evaluating curcumin in other tumors, there are data to support beneficial effects of curcumin in all three malignant bone tumors.
Translational Data
The most common cancer of bone is a metastasis from another organ; curcumin has also been beneficial for bone metastases. The curcumin analog UBS‐109 reportedly prevents metastasis‐induced bone loss by stimulating bone mineralization and suppressing osteoclastogenesis in mice that have been injected with metastatic cells68. Thamake et al. co‐encapsulated curcumin and the proteasome inhibitor bortezomib (which is now used in humans in selected circumstances) in alendronate‐coated poly‐lactic‐co‐glycolic acid particles as therapeutic agents targeting metastatic breast cancer. Although curcumin significant inhibited osteoclastogenic activity, there was no synergism in the anti‐osteoclastogenic activity of curcumin and bortezomib, suggesting these agents have different molecular pathways of action69. In prostate cancer, curcumin can reportedly block CC motif ligand‐2‐mediated effects on invasion, adhesion and motility to prevent metastasis to bone70. Curcumin inhibits the ligand‐stimulated autophosphorylation of epidermal growth factor and colony stimulating factor receptors that are crucially involved in the development of osteomimetic properties in the C4‐2B prostate cancer cell line71. Data on metastatic cancer suggest that though curcumin has not been proven to provide a cure (and may not be), it is possible that the particular tropism of some cancers to metastasize to bone could be decreased; furthermore, if cancer has invaded bone, curcumin may decrease osteoclast activation (see previous discussion of osteoclast inhibition) and bone destruction, which could translate to improvement in cancer‐related pain and decrease the risks of pathologic fracture.
A particularly important aspect of sarcoma treatment with curcumin involves its interaction with radiotherapy and/or chemotherapy. One of the most commonly used chemotherapeutic drugs for sarcomas, doxorubicin, has been shown to synergize with curcumin in liver cancer cells72. Furthermore, the most common serious adverse effect of doxorubicin, cardiac toxicity, is also reportedly attenuated by curcumin73. Additionally, curcumin has been postulated to act as a radioprotector of normal organs and a radiosensitizer for multiple tumor types74. Specifically, in Ewing sarcoma, curcumin enhances radiocytotoxicity66, which in clinical terms means that there is an overlooked aspect of use of curcumin in conjunction with other therapies for multiple cancer types; namely, whether a cancer's therapeutic index (the delicate balance between tumor cell death and normal tissue cell death) is increased. Normally, higher doses of chemoradiation for a given tumor mean increased killing of both tumor and normal tissue cells. With further research on chemoradioprotection and chemoradiosensitization mediated by curcumin, the in vitro models of increasing chemoradiosensitization for tumors and decreasing chemoradioprotection of normal tissue could be validated, thus enabling maximizing of the therapeutic index.
Lastly, whether curcumin can act on cancer cells that are resistant to drugs—so‐called “multidrug resistant cancers”—is an emerging area of oncological medicine. It has been postulated that the presence of multidrug resistant cancers could explain a lack or no response of tumors in general to chemotherapy; this phenomenon may be related to expression of the P‐glycoprotein efflux transporter, which pumps chemotherapeutic drugs out of cancer cells75. Indeed, important recent data have revealed that curcumin can downregulate and inhibit this transporter both in vitro and in vivo 76. Though the data need to be corroborated by other research, this result is a tantalizing example of the curcumin's incredible untapped potential. Heretofore, very little progress has been made on therapy for multidrug resistant cancers; however, with more data the use of curcumin could potentially open up an entire new frontier on any pathology involving multidrug resistance, possibly including autoimmune rheumatic diseases77.
Practical Considerations
To better understand curcumin's pharmacologic potential, it is important to consider pharmacokinetic factors such as half‐life, minimal effective concentration and potential toxic effects. The liver is the major organ in which curcumin is metabolised, the byproducts being glucuronides of tetrahydrocurcumin and hexahydrocurcumin. There are not enough data to fully ascertain whether these metabolites are more or less active than curcumin. Curcumin concentrations can decrease quickly, reaching unquantifiable levels 3–6 hours after feeding32. Future data to consider, particularly for bringing curcumin into clinical trials, include dose‐response relationships, synergy with other compounds and vehicles for drug delivery.
Dose‐response animal studies in osteoporosis suggest that higher concentrations of curcumin have better effects. One study has shown that, in ovariectomized rats, high doses of curcumin (50 mg/kg) lead to greater increases in bone mineral and cortical bone mineral densities in the fourth lumbar vertebra 4 weeks after surgery than low dose curcumin (10 mg/kg). High dose curcumin was associated with greater mechanical strength than in controls, whereas low dose curcumin was not78. It is appropriate to question how much this difference is due to bioavailability and how much related to efficacy. Studies have showed that in humans, curcumin doses of 8000–12,000 mg/day for many months are well‐tolerated with essentially no adverse effects79. We therefore recommend that clinical trials testing curcumin escalate the doses liberally to determine what dose achieves maximal benefit with minimal adverse effects.
It is also important to consider curcumin's relationships with other agents. For example, in osteoporosis curcumin and the bisphosphonate alendronate reportedly have a synergistic antiresorptive effect on bone remodeling and improvement in bone mechanical strength80. Another study showed a synergistic effect of adding curcumin to etidronate in ovariectomized rats81. These studies show that curcumin's benefit in osteoporosis is not additive, but multiplicative. Cucurmin can have tremendous benefit when used as a stand‐alone agent, but even more when combined with other medications.
There are also many considerations regarding curcumin's absorption and metabolism that are important when considering the clinical use of curcumin. One major impediment is bioavailability—serum concentrations are extremely low, especially if curcumin is taken orally. In one animal study, 10 mg/kg i.v. curcumin in rats resulted in a plasma concentration of 0.36 μg/mL, whereas an oral dose 50 times higher resulted in a plasma concentration six times lower at 0.06 μg/mL82. Though intravenous curcumin has not been tested extensively in humans, there is currently no reason to believe that i.v. administration of purified curcumin would be detrimental. Other options based on the structure of curcumin suggest that bioavailability increases if curcumin is in a lipophilic milieu, such as that provided by micelles, liposomes and phospholipid complexes32. Hence, many clinicians instructing their patients to take curcumin suggest mixing it with oil or a lipophilic substance, these agents often being ingested as components of normal meals.
Areas that are currently being explored with the aim of improving curcumin bioavailability include co‐administration of adjuvants that block curcumin degradation, modulation of the route and mechanism of curcumin administration and novel delivery strategies. Piperine, an adjuvant inhibitor of hepatic and intestinal glucuronidation, has been shown to increase bioavailability by 2000%32. In addition to liposomes, micelles and phospholipid complexes, nanoparticles are also currently being explored83. Researchers have demonstrated that sustained drug release occurs when curcumin is embedded in calcium sulfate hemihydrate based bone graft substitutes84, which could be important for surgical options in which bone grafts are used (this approach may decrease postoperative inflammation and fibrosis).
It is easily apparent that foremost, there is a need for clinical trials of curcumin in humans. This raises several key questions of whom to treat for various conditions: whether cucurmin trials should be limited to patients with diseases or whether prophylaxis should also be investigated (although clinical outcomes may be more difficult to assess with prophylactic treatments). Dosing questions always remain, especially whether daily megadoses should be taken in certain circumstances, or lower doses at regular intervals. The main barrier to use of curcumin in the clinic is the lack of clinical data; however, some clinicians use them empirically, given they have minimal adverse effects. We sincerely encourage clinicians to not only assess curcumin in clinical trials, but also to recommend oral curcumin taken with lipophilic foods empirically to a variety of patients and assess their responses. Thus, we could continue to move forward in treatment of certain diseases for which there is no good therapy, let alone cure.
Conclusions
Curcumin is extremely useful for a large number of musculoskeletal pathologies. Cellular and molecular data have been very encouraging and led to important translational studies in animals that have proven that curcumin does indeed have in vivo salubrious effects. The clinical trials that have been performed for certain conditions such as OA have also been fruitful; however, trials for other musculoskeletal conditions lag behind (Fig. 1). Buoyed by positive and encouraging data, we sincerely hope that future studies on human subjects and drug delivery will lead to curcumin being used clinically more often for both common and serious ailments.
Figure 1.
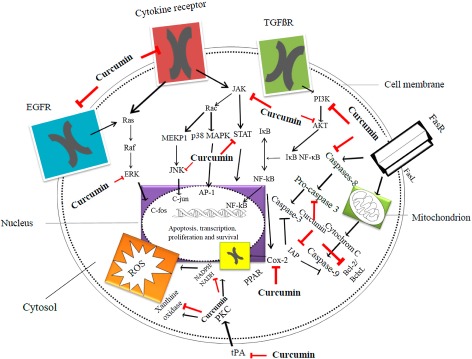
Diagrammatic representation of the major inflammatory and apoptotic pathways inhibited at various steps by curcumin. Red arrows indicate inhibition and black arrows indicate stimulation. Reproduced with permission from Terlikowska et al.85 AKT, serine/threonine‐specific protein kinase; transcriptional factor; BclxL, B‐cell lymphoma‐extra large antiapoptotic protein; c‐fos, c‐fos protein, proto‐oncogen; c‐jun, c‐jun protein, proto‐oncogene; EGFR, epidermal growth factor receptor; ERK, extracellular‐signal‐regulated kinases; FasR, death receptor; FasL, type‐II transmembrane protein; IAP, inhibitor of apoptosis protein family; IκB, inhibitor of κB; JAK, Janus kinase; JNK, c‐jun N‐terminal kinases; MEKP1, mitogen‐activated protein kinase 1; NADH, reduced nicotinamide adenine dinucleotide; NADPH, reduced nicotinamide adenine dinucleotide phosphate; p38 MAPK, p38 mitogen‐activated protein kinases; PI3K, phosphatidylinositol‐4,5‐bisphosphate 3‐kinase; PKC, protein kinase C; PPAR, peroxisome proliferator‐activated receptor; Rac, subfamily of the Rho family of GTPases; Raf, Raf family kinases; Ras, Ras family kinases; STAT, signal transducer and activator of transcription protein family; TGFβR, transforming growth factor β receptor; tPA, tissue plasminogen activator.
Disclosure: The authors all declare that there are no conflicts of interest. All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. There was no independent funding involved in performance of this study.
References
- 1. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ, 2003, 81: 646–656. [PMC free article] [PubMed] [Google Scholar]
- 2. (CDC) CfDCaP . National ambulatory medical care survey factsheet 2015. Available from: http://www.cdc.gov/nchs/data/ahcd/NAMCS_2010_factsheet_orthopedic_surgery.pdf (accessed 14 March 2015).
- 3. Schappert SM. Office visits to orthopedic surgeons: United States, 1995–96 1998. (accessed 14 March 2015). [PubMed]
- 4. Pari L, Tewas D, Eckel J. Role of curcumin in health and disease. Arch Physiol Biochem, 2008, 114: 127–149. [DOI] [PubMed] [Google Scholar]
- 5. Jurenka JS. Anti‐inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev, 2009, 14: 141–153. [PubMed] [Google Scholar]
- 6. Shehzad A, Rehman G, Lee YS. Curcumin in inflammatory diseases. Biofactors, 2013, 39: 69–77. [DOI] [PubMed] [Google Scholar]
- 7. van Saase JL, van Romunde LK, Cats A, Vandenbroucke JP, Valkenburg HA. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis, 1989, 48: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lawrence RC, Felson DT, Helmick CG, et al Estimates of the prevalence of arthritis and other rheumatic conditions in the United States, part II. Arthritis Rheum, 2008, 58: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guccione AA, Felson DT, Anderson JJ, et al The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health, 1994, 84: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res, 2004, 427 (Suppl.): S6–S15. [DOI] [PubMed] [Google Scholar]
- 11. Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population‐health perspective: a population‐based review of the fourth most common cause of hospitalization in U.S. adults. Orthop Nurs, 2012, 31: 85–91. [DOI] [PubMed] [Google Scholar]
- 12. van der Velden VH. Glucocorticoids: mechanisms of action and anti‐inflammatory potential in asthma. Mediators Inflamm, 1998, 7: 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase‐2 (COX‐2) expression by dietary curcumin in HT‐29 human colon cancer cells. Cancer Lett, 2001, 172: 111–118. [DOI] [PubMed] [Google Scholar]
- 14. Young L, Katrib A, Cuello C, et al Effects of intraarticular glucocorticoids on macrophage infiltration and mediators of joint damage in osteoarthritis synovial membranes: findings in a double‐blind, placebo‐controlled study. Arthritis Rheum, 2001, 44: 343–350. [DOI] [PubMed] [Google Scholar]
- 15. Mathy‐Hartert M, Jacquemond‐Collet I, Priem F, Sanchez C, Lambert C, Henrotin Y. Curcumin inhibits pro‐inflammatory mediators and metalloproteinase‐3 production by chondrocytes. Inflamm Res, 2009, 58: 899–908. [DOI] [PubMed] [Google Scholar]
- 16. Lev‐Ari S, Strier L, Kazanov D, et al Curcumin synergistically potentiates the growth‐inhibitory and pro‐apoptotic effects of celecoxib in osteoarthritis synovial adherent cells. Rheumatology, 2006, 45: 171–177. [DOI] [PubMed] [Google Scholar]
- 17. Hong J, Bose M, Ju J, et al Modulation of arachidonic acid metabolism by curcumin and related beta‐diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5‐lipoxygenase. Carcinogenesis, 2004, 25: 1671–1679. [DOI] [PubMed] [Google Scholar]
- 18. Jackson JK, Higo T, Hunter WL, Burt HM. The antioxidants curcumin and quercetin inhibit inflammatory processes associated with arthritis. Inflamm Res, 2006, 55: 168–175. [DOI] [PubMed] [Google Scholar]
- 19. Jancinova V, Perecko T, Nosal R, Kostalova D, Bauerova K. Drabikova K. Decreased activity of neutrophils in the presence of diferuloylmethane (curcumin) involves protein kinase C inhibition. Eur J Pharmacol, 2009, 612: 161–166. [DOI] [PubMed] [Google Scholar]
- 20. Sreejayan N, Rao MN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol, 1997, 49: 105–107. [DOI] [PubMed] [Google Scholar]
- 21. Sreejayan N, Rao MN. Free radical scavenging activity of curcuminoids. Arzneimittelforschung, 1996, 46: 169–171. [PubMed] [Google Scholar]
- 22. Cross A, Barnes T, Bucknall RC, Edwards SW, Moots RJ. Neutrophil apoptosis in rheumatoid arthritis is regulated by local oxygen tensions within joints. J Leukoc Biol, 2006, 80: 521–528. [DOI] [PubMed] [Google Scholar]
- 23. Edwards SW, Hallett MB. Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today, 1997, 18: 320–324. [DOI] [PubMed] [Google Scholar]
- 24. Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin‐1‐stimulated MAP kinases, activating protein‐1 (AP‐1) and nuclear factor kappa B (NF‐kappa B) transcription factors down‐regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol, 2002, 21: 251–262. [DOI] [PubMed] [Google Scholar]
- 25. Shakibaei M, John T, Schulze‐Tanzil G, Lehmann I, Mobasheri A. Suppression of NF‐kappaB activation by curcumin leads to inhibition of expression of cyclo‐oxygenase‐2 and matrix metalloproteinase‐9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol, 2007, 73: 1434–1445. [DOI] [PubMed] [Google Scholar]
- 26. Schulze‐Tanzil G, Mobasheri A, Sendzik J, John T, Shakibaei M. Effects of curcumin (diferuloylmethane) on nuclear factor kappaB signaling in interleukin‐1beta‐stimulated chondrocytes. Ann N Y Acad Sci, 2004, 1030: 578–586. [DOI] [PubMed] [Google Scholar]
- 27. Li WQ, Dehnade F, Zafarullah M. Oncostatin M‐induced matrix metalloproteinase and tissue inhibitor of metalloproteinase‐3 genes expression in chondrocytes requires Janus kinase/STAT signaling pathway. J Immunol, 2001, 166: 3491–3498. [DOI] [PubMed] [Google Scholar]
- 28. Moon DO, Kim MO, Choi YH, Park YM, Kim GY. Curcumin attenuates inflammatory response in IL‐1beta‐induced human synovial fibroblasts and collagen‐induced arthritis in mouse model. Int Immunopharmacol, 2010, 10: 605–610. [DOI] [PubMed] [Google Scholar]
- 29. Colitti M, Gaspardo B, Della Pria A, Scaini C, Stefanon B. Transcriptome modification of white blood cells after dietary administration of curcumin and non‐steroidal anti‐inflammatory drug in osteoarthritic affected dogs. Vet Immunol Immunopathol, 2012, 147: 136–146. [DOI] [PubMed] [Google Scholar]
- 30. Futani H, Okayama A, Matsui K, et al Relation between interleukin‐18 and PGE2 in synovial fluid of osteoarthritis: a potential therapeutic target of cartilage degradation. J Immunother, 2002, 25 (Suppl. 1): 61–64. [DOI] [PubMed] [Google Scholar]
- 31. Panahi Y, Rahimnia AR, Sharafi M, Alishiri G, Saburi A. Sahebkar A. Curcuminoid treatment for knee osteoarthritis: a randomized double‐blind placebo‐controlled trial. Phytother Res, 2014, 28: 1625–1631. [DOI] [PubMed] [Google Scholar]
- 32. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm, 2007, 4: 807–818. [DOI] [PubMed] [Google Scholar]
- 33. Belcaro G, Cesarone MR, Dugall M, et al Product‐evaluation registry of Meriva®, a curcumin‐phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med, 2010, 52: 55–62. [PubMed] [Google Scholar]
- 34. Belcaro G, Cesarone MR, Dugall M, et al Efficacy and safety of Meriva®, a curcumin‐phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev, 2010, 15: 337–344. [PubMed] [Google Scholar]
- 35. Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest, 2005, 115: 3318–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oh S, Kyung TW, Choi HS. Curcumin inhibits osteoclastogenesis by decreasing receptor activator of nuclear factor‐kappaB ligand (RANKL) in bone marrow stromal cells. Mol Cells, 2008, 26: 486–489. [PubMed] [Google Scholar]
- 37. von Metzler I, Krebbel H, Kuckelkorn U, et al Curcumin diminishes human osteoclastogenesis by inhibition of the signalosome‐associated I kappaB kinase. J Cancer Res Clin Oncol, 2009, 135: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ozaki K, Kawata Y, Amano S, Hanazawa S. Stimulatory effect of curcumin on osteoclast apoptosis. Biochem Pharmacol, 2000, 59: 1577–1581. [DOI] [PubMed] [Google Scholar]
- 39. Bharti AC, Takada Y, Aggarwal BB. Curcumin (diferuloylmethane) inhibits receptor activator of NF‐kappa B ligand‐induced NF‐kappa B activation in osteoclast precursors and suppresses osteoclastogenesis. J Immunol, 2004, 172: 5940–5947. [DOI] [PubMed] [Google Scholar]
- 40. Moran JM, Roncero‐Martin R, Rodriguez‐Velasco FJ, et al Effects of curcumin on the proliferation and mineralization of human osteoblast‐like cells: implications of nitric oxide. Int J Mol Sci, 2012, 13: 16104–16118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moon HJ, Ko WK, Han SW, et al Antioxidants, like coenzyme Q10, selenite, and curcumin, inhibited osteoclast differentiation by suppressing reactive oxygen species generation. Biochem Biophys Res Commun, 2012, 418: 247–253. [DOI] [PubMed] [Google Scholar]
- 42. Kim WK, Ke K, Sul OJ, et al Curcumin protects against ovariectomy‐induced bone loss and decreases osteoclastogenesis. J Cell Biochem, 2011, 112: 3159–3166. [DOI] [PubMed] [Google Scholar]
- 43. Yang MW, Wang TH, Yan PP, et al Curcumin improves bone microarchitecture and enhances mineral density in APP/PS1 transgenic mice. Phytomedicine, 2011, 18: 205–213. [DOI] [PubMed] [Google Scholar]
- 44. Hussan F, Ibraheem NG, Kamarudin TA, Shuid AN, Soelaiman IN, Othman F. Curcumin protects against ovariectomy‐induced bone changes in rat model. Evid Based Complement Alternat Med, 2012, 2012 Article ID 174916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Folwarczna J, Zych M, Trzeciak HI. Effects of curcumin on the skeletal system in rats. Pharmacol Rep, 2010, 62: 900–909. [DOI] [PubMed] [Google Scholar]
- 46. Buhrmann C, Mobasheri A, Busch F, et al Curcumin modulates nuclear factor kappaB (NF‐kappaB)‐mediated inflammation in human tenocytes in vitro: role of the phosphatidylinositol 3‐kinase/Akt pathway. J Biol Chem, 2011, 286: 28556–28566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jin B, Li YP. Curcumin prevents lipopolysaccharide‐induced atrogin‐1/MAFbx upregulation and muscle mass loss. J Cell Biochem, 2007, 100: 960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Farid M, Reid MB, Li YP, Gerken E, Durham WJ. Effects of dietary curcumin or N‐acetylcysteine on NF‐kappaB activity and contractile performance in ambulatory and unloaded murine soleus. Nutr Metab, 2005, 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hunter RB, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest, 2004, 114: 1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Davis JM, Murphy EA, Carmichael MD, et al Curcumin effects on inflammation and performance recovery following eccentric exercise‐induced muscle damage. Am J Physiol Regul Integr Comp Physiol, 2007, 292: R2168–R2173. [DOI] [PubMed] [Google Scholar]
- 51. Park J, Choe SS, Choi AH, et al Increase in glucose‐6‐phosphate dehydrogenase in adipocytes stimulates oxidative stress and inflammatory signals. Diabetes, 2006, 55: 2939–2949. [DOI] [PubMed] [Google Scholar]
- 52. DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes, 1981, 30: 1000–1007. [DOI] [PubMed] [Google Scholar]
- 53. Kang C, Kim E. Synergistic effect of curcumin and insulin on muscle cell glucose metabolism. Food Chem Toxicol, 2010, 48: 2366–2373. [DOI] [PubMed] [Google Scholar]
- 54. Cheng TC, Lin CS, Hsu CC, Chen LJ, Cheng KC, Cheng JT. Activation of muscarinic M‐1 cholinoceptors by curcumin to increase glucose uptake into skeletal muscle isolated from Wistar rats. Neurosci Lett, 2009, 465: 238–241. [DOI] [PubMed] [Google Scholar]
- 55. Danial NN. BCL‐2 family proteins: critical checkpoints of apoptotic cell death. Clin Cancer Res, 2007, 13: 7254–7263. [DOI] [PubMed] [Google Scholar]
- 56. Chang Z, Xing J, Yu X. Curcumin induces osteosarcoma MG63 cells apoptosis via ROS/Cyto‐C/Caspase‐3 pathway. Tumour Biol, 2014, 35: 753–758. [DOI] [PubMed] [Google Scholar]
- 57. Yang SJ, Lee SA, Park MG, et al Induction of apoptosis by diphenyldifluoroketone in osteogenic sarcoma cells is associated with activation of caspases. Oncol Rep, 2014, 31: 2286–2292. [DOI] [PubMed] [Google Scholar]
- 58. Jin S, Xu HG, Shen JN, Chen XW, Wang H, Zhou JG. Apoptotic effects of curcumin on human osteosarcoma U2OS cells. Orthop Surg, 2009, 1: 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee DS, Lee MK, Kim JH. Curcumin induces cell cycle arrest and apoptosis in human osteosarcoma (HOS) cells. Anticancer Res, 2009, 29: 5039–5044. [PubMed] [Google Scholar]
- 60. Chang R, Sun L, Webster TJ. Short communication: selective cytotoxicity of curcumin on osteosarcoma cells compared to healthy osteoblasts. Int J Nanomedicine, 2014, 9: 461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leow PC, Bahety P, Boon CP, et al Functionalized curcumin analogs as potent modulators of the Wnt/beta‐catenin signaling pathway. Eur J Med Chem, 2014, 71: 67–80. [DOI] [PubMed] [Google Scholar]
- 62. Fossey SL, Bear MD, Lin J, et al The novel curcumin analog FLLL32 decreases STAT3 DNA binding activity and expression, and induces apoptosis in osteosarcoma cell lines. BMC Cancer, 2011, 11: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Onimoe GI, Liu A, Lin L, et al Small molecules, LLL12 and FLLL32, inhibit STAT3 and exhibit potent growth suppressive activity in osteosarcoma cells and tumor growth in mice. Invest New Drugs, 2012, 30: 916–926. [DOI] [PubMed] [Google Scholar]
- 64. Ravindran J, Subbaraju GV, Ramani MV, Sung B, Aggarwal BB. Bisdemethylcurcumin and structurally related hispolon analogues of curcumin exhibit enhanced prooxidant, anti‐proliferative and anti‐inflammatory activities in vitro. Biochem Pharmacol, 2010, 79: 1658–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Singh M, Pandey A, Karikari CA, Singh G, Rakheja D. Cell cycle inhibition and apoptosis induced by curcumin in Ewing sarcoma cell line SK‐NEP‐1. Med Oncol, 2010, 27: 1096–1101. [DOI] [PubMed] [Google Scholar]
- 66. Veeraraghavan J, Natarajan M, Herman TS, Aravindan N. Curcumin‐altered p53‐response genes regulate radiosensitivity in p53‐mutant Ewing's sarcoma cells. Anticancer Res, 2010, 30: 4007–4015. [PubMed] [Google Scholar]
- 67. Lee HP, Li TM, Tsao JY, Fong YC, Tang CH. Curcumin induces cell apoptosis in human chondrosarcoma through extrinsic death receptor pathway. Int Immunopharmacol, 2012, 13: 163–169. [DOI] [PubMed] [Google Scholar]
- 68. Yamaguchi M, Zhu S, Weitzmann MN, Snyder JP, Shoji M. Curcumin analog UBS109 prevents bone marrow osteoblastogenesis and osteoclastogenesis disordered by coculture with breast cancer MDA‐MB‐231 bone metastatic cells in vitro. Mol Cell Biochem, 2015, 401: 1–10. [DOI] [PubMed] [Google Scholar]
- 69. Thamake SI, Raut SL, Gryczynski Z, Ranjan AP, Vishwanatha JK. Alendronate coated poly‐lactic‐co‐glycolic acid (PLGA) nanoparticles for active targeting of metastatic breast cancer. Biomaterials, 2012, 33: 7164–7173. [DOI] [PubMed] [Google Scholar]
- 70. Herman JG, Stadelman HL, Roselli CE. Curcumin blocks CCL2‐induced adhesion, motility and invasion, in part, through down‐regulation of CCL2 expression and proteolytic activity. Int J Oncol, 2009, 34: 1319–1327. [PMC free article] [PubMed] [Google Scholar]
- 71. Dorai T, Dutcher JP, Dempster DW, Wiernik PH. Therapeutic potential of curcumin in prostate cancer—V: interference with the osteomimetic properties of hormone refractory C4‐2B prostate cancer cells. Prostate, 2004, 60: 1–17. [DOI] [PubMed] [Google Scholar]
- 72. Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D'Alessandro N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF‐kB activation levels and in IAP gene expression. Cancer Lett, 2005, 224: 53–65. [DOI] [PubMed] [Google Scholar]
- 73. Mohamad RH, El‐Bastawesy AM, Zekry ZK, et al The role of Curcuma longa against doxorubicin (adriamycin)‐induced toxicity in rats. J Med Food, 2009, 12: 394–402. [DOI] [PubMed] [Google Scholar]
- 74. Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer, 2010, 62: 919–930. [DOI] [PubMed] [Google Scholar]
- 75. Niero EL, Rocha‐Sales B, Lauand C, et al The multiple facets of drug resistance: one history, different approaches. J Exp Clin Cancer Res, 2014, 33: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Si M, Zhao J, Li X, Tian JG, Li YG, Li JM. Reversion effects of curcumin on multidrug resistance of MNNG/HOS human osteosarcoma cells in vitro and in vivo through regulation of P‐glycoprotein. Chin Med J, 2013, 126: 4116–4123. [PubMed] [Google Scholar]
- 77. Garcia‐Carrasco M, Mendoza‐Pinto C, Macias Diaz S, et al P‐glycoprotein in autoimmune rheumatic diseases. Autoimmun Rev, 2015, 14: 594–600. [DOI] [PubMed] [Google Scholar]
- 78. Cho DC, Jung HS, Kim KT, Jeon Y, Sung JK, Hwang JH. Therapeutic advantages of treatment of high‐dose curcumin in the ovariectomized rat. J Korean Neurosurg Soc, 2013, 54: 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cheng AL, Hsu CH, Lin JK, et al Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high‐risk or pre‐malignant lesions. Anticancer Res, 2001, 21: 2895–2900. [PubMed] [Google Scholar]
- 80. Cho DC, Kim KT, Jeon Y, Sung JK. A synergistic bone sparing effect of curcumin and alendronate in ovariectomized rat. Acta Neurochir, 2012, 154: 2215–2223. [DOI] [PubMed] [Google Scholar]
- 81. French DL, Muir JM, Webber CE. The ovariectomized, mature rat model of postmenopausal osteoporosis: an assessment of the bone sparing effects of curcumin. Phytomedicine, 2008, 15: 1069–1078. [DOI] [PubMed] [Google Scholar]
- 82. Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC‐MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci, 2007, 853: 183–189. [DOI] [PubMed] [Google Scholar]
- 83. Kumar A, Ahuja A, Ali J, Baboota S. Conundrum and therapeutic potential of curcumin in drug delivery. Crit Rev Ther Drug Carrier Syst, 2010, 27: 279–312. [DOI] [PubMed] [Google Scholar]
- 84. Orellana BR, Thomas MV, Dziubla TD, Shah NM, Hilt JZ, Puleo DA. Bioerodible calcium sulfate/poly(beta‐amino ester) hydrogel composites. J Mech Behav Biomed Mater, 2013, 26: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Terlikowska KM, Witkowska AM, Zujko ME, Dobrzycka B, Terlikowski SJ. Potential application of curcumin and its analogues in the treatment strategy of patients with primary epithelial ovarian cancer. Int J Mol Sci, 2014, 15: 21703–21722. [DOI] [PMC free article] [PubMed] [Google Scholar]


