Abstract
Objective
To determine the features of discs in spinal cord injury without radiographic abnormality (SCIWORA) by intraoperative disc contrast injection (IODCI) and to subsequently treat the responsible discs operatively.
Methods
From January 2007 to December 2011, 16 adult cases of cervical SCIWORA were enrolled in this study. The average preoperative Japanese Orthopaedic Association (JOA) score was 9.1 ± 1.8. Although preoperative imaging showed no obvious fracture or dislocation, spinal cord compression was evident in all cases. High spinal cord signals on MRI T2WI and cervical disc degeneration were present in all cases and swollen soft tissue anterior to the cervical spine in nine cases. All patients underwent anterior cervical surgeries for spinal cord compression, IODCI being performed after exposure of suspicious discs. Patients with only one ruptured disc underwent anterior cervical discectomy and fusion; those with more complex injuries underwent anterior cervical corpectomy and fusion with fixation of the ruptured segment. JOA scores, X‐rays and CT scans were checked at specified intervals over an average of 24.4 months.
Results
Of 32 discs suspected preoperatively of being injured, 19 were identified as ruptured by IODCI. Anterior annulus fibrosus rupture was proved in 11 patients whereas the anterior longitudinal ligament was intact in all. JOA scores at 2 weeks, 3 months and last follow‐up postoperatively were 13.3 ± 1.5, 14.5 ± 1.6 and 15.1 ± 1.5 respectively. The recovery rates were 53.2%, 68.3% and 75.9%, respectively.
Conclusion
IODCI helps to determine the segment responsible for cervical SCIWORA.
Keywords: Cervical spine, Diagnosis, Disc injection, SCIWORA
Introduction
Burke first reported spinal cord injury without radiographic abnormality (SCIWORA) in 19741 whereas Pang and Wilberger were the first to define SCIWORA as spinal cord injury without fracture or dislocation on X‐ray or CT scan2. Because the cervical spine is the most mobile part of the human spine, the incidence SCIWORA is much higher in the cervical spine than in the rest of the spine. SCIWORA is always caused by acute trauma to the head and neck; edema of the cord generally occurs within 6 hours of trauma and reaches a peak after about a week. Degenerative changes such as disc herniation, ossification of the posterior longitudinal ligament (OPLL) and stenosis have usually been present before the onset of SCIWORA symptoms. Because ruptured discs decrease the stability of the cervical spine, abnormal intervertebral translation is likely to aggravate the injury to the spinal cord. If appropriate decompression and fixation are not performed, the function of spinal cord will not recover and may even deteriorate. An accurate diagnosis is crucial to determining the appropriate surgical intervention. With the development of radiological techniques, especially MRI, more and more cases of SCIWORA are now being diagnosed. Cervical spinal cord injury and rupture of the annulus fibrosus and posterior longitudinal ligament (PLL) can be demonstrated on MRI. However, it has been reported that the accuracy of diagnosis of internal rupture of a cervical disc by MRI is poor3. Moreover, static MRI is not able to identify instability of the cervical spine caused by internal disc rupture. Flexion‐extension MRI has been utilized to reveal spinal cord compression4; however, because the cervical spine is likely unstable in patients with SCIWORA, dynamic tests (X‐ray or MRI) are contraindicated because of the high risk of aggravating neurological symptoms. In this study, we performed intraoperative disc contrast injection (IODCI) during anterior cervical surgery to identify the injured disc(s), thus determining the responsible segments that required surgical intervention.
Materials and Methods
General Information
Inclusion criteria were as follows: (i) preoperative MRI showing low intensity signals on T1WI or high intensity signals on T2WI in the cervical spinal cord with no definite evidence of an injured disc; (ii) one or two level pre‐existing compression to the cord that meets the indications for anterior surgery; and (iii) recent deterioration in neurological function after trauma to the cervical spine.
Exclusion criteria were as follows: (i) preoperative X‐ray, CT or MRI showing obvious cervical fracture or dislocation; (ii) intolerance to anesthesia or surgery because of comorbidities; and (iii) the pathologies for which an anterior approach would be unsuitable, such as significant cervical canal stenosis or continuous OPLL.
From January 2007 to December 2011, 16 cases of cervical SCIWORA were enrolled in this study. None of them had definite evidence of injured discs on MRI. They comprised 12 men and 4 women aged 35–71 years (mean 46.5 years). Relevant traumatic events were traffic accidents in six and accidental falls in ten cases. All patients had numbness, tingling and weakness in the upper limbs and half had the same symptoms in the lower limbs. The average preoperative Japanese Orthopaedic Association (JOA) score was 9.1 ± 1.8. Although preoperative X‐ray, CT and MRI showed no obvious fracture or dislocation in the cervical spine, pre‐existing pathologies, including cervical disc herniation in ten and OPLL in six cases, were detected. There were high signals in the spinal cord on MRI T2WI in all cases, swollen soft tissue anterior to the cervical spine in nine and cervical disc degeneration in all 16. All patients underwent anterior cervical surgeries for preexisting spinal cord compression and IODCI was performed on the suspicious discs after exposure had been achieved. Contrast leakage through the anterior annulus fibrosus was defined as definite disc injury and through the posterior annulus fibrosus as suspicious. Patients with only one ruptured disc underwent anterior cervical discectomy and fusion, whereas those with additional disc herniations or OPLL underwent anterior cervical corpectomy and fusion with fixation of the ruptured segment. The time between injury and surgery was 3 to 12 days (mean, 7 days). JOA scores, X‐rays and CT scans were checked 2 weeks, 3 months, 6 months, 1 year and 2 years postoperatively (Table 1).
Table 1.
Patients' characteristics and details of variables evaluated
| No. | Age/sex | Injured disc | JOA score | Surgery time (min) | Blood loss (mL) | |||
|---|---|---|---|---|---|---|---|---|
| Preoperative | 2 weeks* | 3 months* | Final follow‐up | |||||
| 1 | 35/M | C3–4, C4–5 | 9 | 14 | 15 | 16 | 140 | 200 |
| 2 | 44/F | C5–6 | 10 | 15 | 16 | 17 | 95 | 50 |
| 3 | 40/M | C6–7 | 8 | 12 | 14 | 14 | 95 | 80 |
| 4 | 43/M | C3–4 | 7 | 14 | 15 | 15 | 100 | 50 |
| 5 | 65/F | C6–7 | 10 | 11 | 12 | 13 | 80 | 80 |
| 6 | 46/M | C4–5 | 11 | 15 | 16 | 16 | 120 | 140 |
| 7 | 71/M | C4–5, C5–6 | 9 | 13 | 14 | 15 | 155 | 300 |
| 8 | 67/M | C6–7 | 9 | 13 | 14 | 15 | 120 | 100 |
| 9 | 37/M | C5–6 | 11 | 14 | 14 | 15 | 100 | 110 |
| 10 | 39/M | C5–6 | 7 | 11 | 13 | 14 | 115 | 120 |
| 11 | 41/M | C5–6 | 8 | 11 | 13 | 14 | 125 | 130 |
| 12 | 54/M | C5–6, C6–7 | 8 | 14 | 16 | 16 | 160 | 330 |
| 13 | 37/M | C5–6 | 9 | 14 | 15 | 15 | 85 | 60 |
| 14 | 45/F | C4–5 | 10 | 14 | 15 | 16 | 90 | 50 |
| 15 | 39/M | C6–7 | 10 | 13 | 15 | 16 | 80 | 60 |
| 16 | 41/F | C5–6 | 9 | 14 | 15 | 15 | 95 | 60 |
Note: *Postoperative; F, female; M, male.
IODCI and Surgical Technique
After general anesthesia had been induced, the patients were placed in the conventional supine position and the cervical spine exposed via a Smith–Robinson approach. Suspicious discs according to the location of high signals in the spinal cord on preoperative MRI were selected for IODCI. Needles were inserted into the center of the nucleus pulposus of the target disc under C‐arm monitoring. The contrast, iohexol, was injected steadily into the target disc, keeping the injection pressure under 300 kPa. Meanwhile, the integrity of the disc and leakage of contrast observed. After determining the responsible disc, the anterior longitudinal ligament (ALL) was opened to explore the integrity of the anterior annulus fibrosus (AAF). The disc and cartilage of the end plate were then removed completely. A cage filled with allograft bone was inserted into the disc space, after which the segment was fixed with a plate. For patients with additional disc herniation or OPLL, corpectomy, mesh graft and plate fixation were performed.
Evaluation
Radiological Assessment
Radiological assessment comprised (i) antero‐posterior (AP) and lateral X‐ray films to assess cervical fracture or dislocation; (ii) CT to assess decompression, fixation and fusion; (iii) MRI to assess signal changes in disc, spinal cord or soft tissue compared with preoperatively; and (iv) IODCI to assess cervical disc rupture and contrast leakage. Similar to the Adams Discography Classification5, the morphology of the injected discs was classified as cotton ball, lobular, irregular, fissured or ruptured. Cotton ball and lobular types are regarded as normal, irregular type denotes degeneration and fissured and ruptured types denote injury, especially when contrast has leaked into the spinal canal. In addition, rupture of the ALL or AAF is a definite sign of disc injury.
Clinical Evaluation
Clinical evaluation was based on JOA scores (i) JOA score (maximum 17 points); and (ii) JOA recovery rate: (postoperative JOA score − preoperative JOA score)/(17 − preoperative JOA score) × 100%.
Surgical Time, Blood Loss and Complications
Surgical time and blood loss were noted. Complications comprised deep wound infection, deterioration in neurological function, failure of fixation, injury to vertebral arteries, cerebrospinal fluid leakage, axial neck pain and hematoma.
Follow‐Up Time Points
All patients were followed up 2 weeks, 3 months, 6 months, 1 year and 2 years postoperatively. AP, lateral and dynamic radiographs of the cervical spine were taken at each time point and CT scans were performed 2 weeks and 6 months after surgery.
Statistical Analysis
An SPSS 10.0 statistical package was used for data analysis, measurement data were compared with one‐way anova. The level of significance was P < 0.05 in all analyses.
Results
Clinical Outcomes
No rupture of ALL was detected during surgery in this group. When the AAF had ruptured, there was a sense of emptiness on palpation of the surface of the ALL. After incision of the ALL, an irregular transverse fissure could be seen on the AAF. All patients were followed up for an average of 24.4 months (range 6 months to 4 years). The JOA scores at 2 weeks, 3 months and the last postoperative follow‐up were 13.3 ± 1.5, 14.5 ± 1.6, and 15.1 ± 1.5 respectively and the recovery rates 53.2%, 68.3%, and 75.9%, respectively. The differences between postoperative JOA scores at each time point and preoperative scores were all statistically significant (all P < 0.05). The operative time averaged 110 min and blood loss 120 mL (see Table 1).
Radiological Results
Thirty‐two discs were suspected of being injured preoperatively; 19 of these were identified by IODIC as ruptured (see Table 1). AAF rupture was identified in 11 patients whereas the ALL remained intact in all cases (Figs 1, 2, 3, 4, 5).
Figure 1.
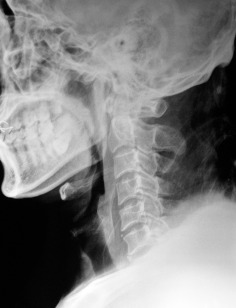
Preoperative X‐ray showed neither fracture nor dislocation of the cervical spine.
Figure 2.
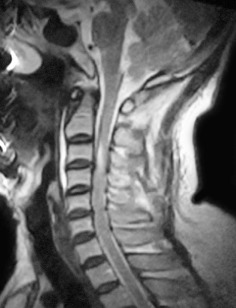
Preoperative MRI showed high intensity signals in the cervical spinal cord at the level of C 3 on T2WI; there is no obvious evidence of disc injury.
Figure 3.
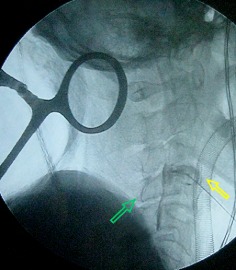
Intraoperative disc contrast injection showing both anterior and posterior contrast leakage from the C 3–4 disc. The green arrow indicates contrast entering the canal and flowing caudally. The yellow arrow indicated the contrast breaking through the anterior annulus fibrosis.
Figure 4.
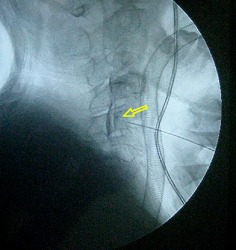
IODCI in C 4–5 disc showed contrast breaking through the posterior annulus fibrosus (yellow arrow); the annulus fibrosus (AAF) appears intact.
Figure 5.
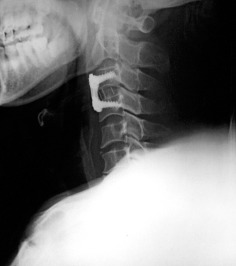
Postoperative X‐ray film after C 3–4 discectomy, cage graft and plate fixation.
Complications
There was one case of hoarseness and three of trapezius muscle pain in this group, all resolved within 2 weeks with conservative treatment. No serious complications such as deep infection, deterioration of neurological function, vertebral artery injury or internal fixation failure occurred.
Discussion
Importance of Early Surgery for Acute Spinal Cord Injury
It is well known that pathological changes in the cervical spinal cord comprise hemorrhage, edema and necrosis in the acute phase; macrophage accumulation and capillary proliferation in the subacute phase; and spinal cord atrophy, myelomalacia, gliosis and traumatic syringomyelia in the chronic phase6. If a timely decompression of the cervical spinal cord is performed in the acute phase, the pathological changes can be completely reversed7. In contrast, myelomalacia and necrosis rarely resolve even if late surgery is performed8. Fehlings et al. performed a multicenter, international, prospective cohort study of 313 patients with acute cervical SCI. Of these, 182 underwent early surgery, the remaining 131 having late surgery. Of the 222 patients with follow‐up data available at 6 months post injury, 19.8% of those who had undergone early surgery showed a two or more grade improvement in Abbreviated Injury Scale scores compared with 8.8% of those who underwent late decompression9. Thus, the importance of early surgical intervention to the injured spinal cord cannot be overestimated.
Determining the Segment Responsible for Acute Spinal Cord Injury
A ruptured disc can undergo a transient dislocation at the moment of traumatic event, causing acute spinal cord injury. Identifying the responsible disc is undoubtedly a precondition for early surgical intervention. Thus far, MRI is the most effective means of diagnosing spinal cord injury because of its unique advantages in evaluating pathological changes in spinal cord and predicting the prognosis. Spinal cord edema appears as slightly low or normal intensity signals on T1WI MRI and homogenous high intensity signals on T2WI MRI. High intensity signals of the cervical spinal cord often appear close to the corresponding injured segments. Unfortunately, in some cases there are high signal zones across multiple segments, making it difficult to identify the injured disc merely by high signal zones in the cord. Moreover, the diagnostic sensitivity of MRI for rupture of the annulus fibrosus, PLL or ALL is not as good as for identifying spinal cord pathology.
Cervical IODCI is superior to CT, MRI and CT myelography (CTM) for revealing pathology within the disc. Takahiko et al. compared the diagnostic accuracy of MRI, CTM and CT IODCI in 15 cases of foraminal cervical disc herniation. MRI was positive in two, suspicious in six and negative in seven; CTM was positive in seven and negative in eight; and CT IODCI was positive in all 15 cases10. Given the extremely low efficacy of MRI for identifying disc injury, IODCI is recommended for reliably identifying the segments responsible for SCIWORA.
Guo et al. analyzed 203 cases of SCIWORA and concluded that hyperextension is the most frequent mechanism. According to MRI findings, PLL rupture is the most common type of injury, accounting for 43.8%, followed by ALL rupture in 25.1%11. The cervical PLL is comparatively narrow and thin, and strongly adherent to the posterior annulus fibrosus. In patients with non‐traumatic disc herniation, it is not infrequent for the nucleus pulposus to break through the PLL and posterior annulus fibrosus. IODIC can rarely distinguish whether rupture of the PLL is traumatic or not. In this study, PLL rupture was considered to indicate injury and was detected in all patients. Because the ALL is much thicker and wider than the PLL, the former is seldom ruptured in non‐traumatic disc herniation. When the mechanism of injury is hyperextension, the ALL is the most likely structure to be ruptured. Accordingly, rupture of the ALL should be considered relatively definite evidence of disc injury. In contrast to the finding of previous studies, ALL rupture was not identified in any of our study subjects, which may be attributable to our exclusion criteria, one of which was presence of cervical fracture and dislocation on preoperative X‐ray, CT or MRI. When both ALL and AAF rupture have occurred, MRI is much more likely to detect instability of the cervical spine. Additional IODIC would be unnecessary in such cases. In this group, we detected 11 cases of AAF rupture with intact ALLs, the ruptured AAF being identified only after incision of the ALL. IODIC is of significant value in this type of potential disc injury.
Advantages and Drawbacks of IODIC
We confirmed the following advantages of IODIC in this study: (i) it can reveal ruptured structures within discs, thus identifying the responsible segment; (ii) compared with percutaneous disc injection, intraoperative disc injection avoids the risk of injury to the great vessels, trachea and esophagus during needle puncture; and (iii) because the cervical spine is adequately exposed, the needle can be positioned to guarantee reaching the center of the disc, which is an important factor in the accuracy of IODIC. However, this procedure also has the following drawbacks: (i) more intraoperative maneuvers and consequent prolongation of surgical time; (ii) frequently use of fluoroscopy increases X‐ray exposure of the patients; and (iii) excessive injection pressure can lead to further extrusion of the nucleus pulposus and aggravate neurological symptoms, which is why we kept the injection pressure under 300 kPa12.
Key Points
Rupture of the ALL is definite evidence of cervical disc injury.
IODIC can detect cervical disc rupture that is rarely detected by other radiological methods.
IODIC can be of great value in determining the segment responsible for SCIWORA.
Disclosure: The manuscript submitted does not contain information about medical device(s)/drug(s). No funds were received in support of this work. No relevant financial activities outside the submitted work.
References
- 1. Burke DC. Traumatic spinal paralysis in children. Paraplegia, 1974, 11: 268–276. [DOI] [PubMed] [Google Scholar]
- 2. Pang D, Wilberger JE Jr. Spinal cord injury without radiographic abnormalities in children. J Neurosurg, 1982, 57: 114–129. [DOI] [PubMed] [Google Scholar]
- 3. Maeda T, Ueta T, Mori E, et al Soft‐tissue damage and segmental instability in adult patients with cervical spinal cord injury without major bone injury. Spine (Phila Pa 1976), 2012, 37: E1560–E1566. [DOI] [PubMed] [Google Scholar]
- 4. Zhang L, Zeitoun D, Rangel A, Lazennec JY, Catonné Y, Pascal‐Moussellard H. Preoperative evaluation of the cervical spondylotic myelopathy with flexion‐extension magnetic resonance imaging: about a prospective study of fifty patients. Spine (Phila Pa 1976), 2011, 36: E1134–E1139. [DOI] [PubMed] [Google Scholar]
- 5. Adams MA, Dolan P, Hutton WC. The stages of disc degeneration as revealed by discograms. J Bone Joint Surg Br, 1986, 68: 36–41. [DOI] [PubMed] [Google Scholar]
- 6. Holmes JF, Mirvis SE, Panacek EA, et al Variability in computed tomography and magnetic resonance imaging in patients with cervical spine injuries. J Trauma, 2002, 53: 524–530. [DOI] [PubMed] [Google Scholar]
- 7. Fehlings MG, Perrin RG. The role and timing of early decompression for cervical spinal cord injury: update with a review of recent clinical evidence. Injury, 2005, 36 (Suppl. 2): B13–B26. [DOI] [PubMed] [Google Scholar]
- 8. Green C, Butler J, Eustace S, Poynton A, O'Byrne JM. Imaging modalities for cervical spondylotic stenosis and myelopathy. Adv Orthop, 2012, 2012: 908324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fehlings MG, Vaccaro A, Wilson JR, et al Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS ONE, 2012, 7: e32037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamasaki T, Baba I, Tanaka S, et al Clinical characterizations and radiologic findings of pure foraminal‐type cervical disc herniation: CT discography as a useful adjuvant in its precise diagnosis. Spine (Phila Pa 1976), 2005, 30: E591–E596. [DOI] [PubMed] [Google Scholar]
- 11. Guo H, Liu J, Qi X, et al Epidemiological characteristics of adult SCIWORA in Tianjin, China: a preliminary study. Eur Spine J, 2012, 21: 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang JD, Xia Q, Hu YC, et al The relationship between discography pressure and outcome of anterior lumbar interbody fusion for discogenic low back pain. Zhonghua Gu Ke Za Zhi, 2009, 29: 123–127 (in Chinese). [Google Scholar]


