Abstract
There have been a number of developments in screw design and implantation techniques over recent years, including proposal of an alternative trajectory for screw fixation aimed at increasing purchase of pedicle screws in higher density bone. Cortical bone trajectory (CBT) screw insertion follows a lateral path in the transverse plane and caudocephalad path in the sagittal plane. This technique has been advocated because it is reportedly less invasive, improves screw−bone purchase and reduces neurovascular injury; however, these claims have not been supported by robust clinical evidence. The available evidence was therefore reviewed to assess the relative merits of CBT and highlight areas for further research. To this end, a search of relevant published studies reporting biomechanical, morphometric or clinical outcomes after use of CBT screws in patients with spinal pathologies was performed via six electronic databases.
Keywords: Cortical bone trajectory, Cortical screw, Medio‐lateral superior trajectory, Pedicle fixation, Pedicle screw
Introduction
Pedicle screw fixation has been the mainstay technique for lumbar spine stabilization for several decades, its superior biomechanical strength and properties surpassing alternative forms of fixation1. Pedicle screw fixation offers multiple advantages, allowing superior correction of spinal deformities, and reduced rates of loss of fixation and non‐union2, 3. Therefore, this technique has been used in the treatment of a number of lumbar disorders such as treatment of fractures, tumors and degenerative disease and so on.
The traditional insertion pathway for pedicle screws involves a transpedicular lateral to medial trajectory with the initial insertion point at the junction of the transverse process and lateral wall of the facet4, 5. Several complications are associated with conventional pedicle screw fixation. Screw misplacement rates for pedicle fixation reportedly range from 21%–40% despite the use of navigation techniques6, 7, 8. Screw loosening and loss of surgical construct stability may occur, particularly in patients with osteopenia or osteoporosis9. Additional drawbacks include the significant muscle dissection required for pedicle screw insertion because of its lateral to medial trajectory10, 11 and increased risk of neurovascular injury documented by multiple reports of incorrect placement of pedicle screws12, 13.
Over recent years, there have been a number of developments in screw design and implantation techniques, including a proposal for an alternative trajectory for screw fixation aimed at increasing purchase of the pedicle screw in higher density bone. Santoni et al. were the first one to report the cortical bone trajectory (CBT), in which screws follow a lateral path in the axial plane and caudocephalad path in the sagittal plane. In contrast to conventional pedicle screw fixation, CBT screws do not penetrate the vertebral body trabecular space14. Although several morphometric and biochemical studies have supported the use of the CBT approach5, 12, 14, 15, 16, 17, 18, 19, 20, there have been few clinical studies investigating outcomes of this technique in patients with lumbar spine pathologies5, 12, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29. The trajectory of the CBT screw is demonstrated in Fig. 1. We will here review the history, development, and biomechanical and clinical evidence for CBT as an alternative technique for pedicle screw insertion.
Figure 1.
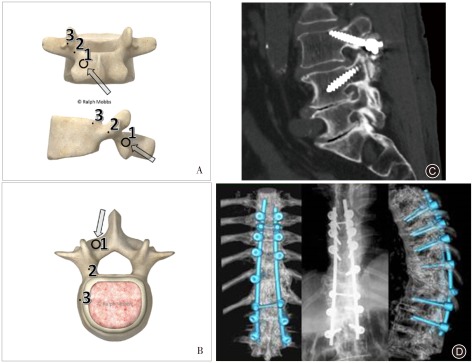
Medio‐lateral superior trajectory (MLST) for cortical bone trajectory screws. (A) Model showing the starting point for the MLST technique (Point 1). Points 2 and 3 demonstrate the trajectories that the surgeon can use during lateral or anteroposterior radiography, respectively. (B) Model showing the axial trajectory for an MLST screw (arrow). The screw follows a medial to lateral path, thus avoiding lateral dissection of the paraspinal musculature. (C) Lateral radiograph showing the trajectory of an MLST screw in L 3, starting at the pars with the screw angled towards the lateral aspect of the endplate. Note the L 4 pedicle screw is angled in a superior‐inferior direction, the opposite of the MLST screw. Image adapted with permission from Mobbs et al.30. (D) Three‐dimensional CT demonstrating CBT/MLST screw insertion.
Methods
A search of relevant published reports was performed via six electronic databases; namely, Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, American College of Physicians Journal Club and Database of Abstracts of Review of Effectiveness31 from their dates of inception to March 2015. To maximize the sensitivity of the search strategy, the terms: “cortical bone”, “cortical bone trajectory”, “CBT”, “medial‐lateral superior trajectory”, “spine”, and “pedicle screw” were combined as either key words or MeSH terms. The reference lists of all retrieved articles were reviewed to identify additional potentially relevant studies. Biomechanical, morphometric or clinical studies that reported complications, technique, efficacy, anatomy or animal or cadaveric studies on CBT screw fixation for spinal pathologies were included. Full text articles and abstracts were included in the present review; however, editorial and commentary texts were excluded.
Results
Biomechanical and Morphometric Evidence
Since the seminal biomechanical study of Santoni et al.14, several biomechanical and morphometric studies comparing the properties of CBT screw fixation with those of traditional pedicle screws have been performed. Our review of published reports identified nine relevant studies, these are summarized in Table 1.
Table 1.
Overview of biomechanical studies investigating CBT for screw fixation
| First author | Country | Study design | Number of subjects | Methods | Results |
|---|---|---|---|---|---|
| Radcliff et al.19, 2014 | USA | In vivo biomechanical study using fresh‐frozen cadaveric lumbar spines | 8 | Intact range of motion data was collected using Optotrak Certus software during flexibility testing. Measurements done before and after CBT or pedicle screws | Average relative motion between L3‐L5 increased slightly from the PreFat values in most loading modes, the largest being flexion/extension (FE) in the pedicle group (ΔFE: 3.68 % ± 3.15% Intact); only lateral bending decreased |
| Matsukawa et al.5, 2014 | Japan | Insertional torque measurements in vivo | 48 | Torque measurement device | CBT screws showed 2.01 times higher torque. CBT with hybrid technique had 1.7 times higher torque than traditional screws. Positive linear correlations between maximum insertional torque and bone mineral density. |
| Matsukawa et al.12, 2014 (2) | Japan | CT scans of 50 adults used for morphometric measurement of CBT trajectory | 50 healthy subjects (morphometric study), 19 patients with lumbosacral CBT fixation | CT scans of 50 adults used for morphometric measurement of CBT trajectory, 19 patients with CBT and torque measurements | CT analysis revealed that the penetrating S1 endplate technique did not cause any neurovascular injury anteriorly in any case. The new technique demonstrated an average of 141% higher insertional torque than the traditional monocortical technique. |
| Matsukawa et al.20, 2014 (3) | Japan | Morphometric and cadaveric biomechanic study | 50 CTs of healthy subjects, 24 cadavers with thoracic CBT screws | 50 healthy patients for morphometric measurement of thoracic CBT. Insertional torque of pedicle screws was compared with traditional technique on 24 cadaveric thoracic vertebrae. | All morphometric parameters of thoracic CBT increased from T9 to T12. The new technique demonstrated average 53.8% higher torque than the traditional technique (P < 0.01). |
| Calvert et al.16, 2014 | USA | In vitro biochemical study | 10 fresh frozen human lumbar spines, instrumented at L3−L4, five with CBT, five with pedicle screws | Construct stiffness was recorded in flexion/extension, lateral bending, and axial rotation. L3 screw pullout strength was tested to failure, mechanical stiffness recorded | CBT rescue screws provided stiffness in flexion/extension and axial rotation similar to that provided by the initial pedicle screw construct prior to failure. In pullout testing, cortical rescue screws retained 60% of the original pedicle screw pullout strength |
| Baluch et al.17, 2014 | USA | Human cadaveric biochemical study | Seventeen vertebral levels (T11–L5) from human cadavers | Quantitative CT was performed at 17 vertebral levels (T11–L5). Each vertebra had CBT and traditional screw inserted on contralateral sides. Cyclic craniocaudal toggling was performed. | The force necessary to displace the screws was also significantly greater for the cortical versus the traditional screws. There was no difference in axial pullout strength |
| Perez‐Orribo et al.18, 2013 | USA | Cadaveric lumbar specimens | Four groups of seven | Non‐destructive flexibility tests were conducted on 28 cadaveric specimens. | Stability was equivalent after CBT or traditional pedicle screws. Traditional screws were stiffer during axial rotation. Outcomes were not affected by transforaminal lateral interbody fixation (TLIF) versus direct lateral interbody fixation (DLIF), or presence of interbody. |
| Matsukawa et al.15, 2013 | Japan | Morphometric study, CBT for lumbar pedicle screw using CT | 100 CTs of adults | CT was performed on 100 adult patients and pedicle morphology parameters recorded and assessed | The mean diameter gradually increased from L1 to L5. The mean length from L1 to L5, the lateral angle from L1 to L5, and the cephalad angle from L1 to L5, were measured successfully. |
| Santoni et al.14, 2009 | USA | Human cadaveric biomechanical study | 24 tested lumbar vertebral bodies | CBT and traditional screws were inserted into vertebral model on contralateral sides. Pullout and toggle testing was conducted. | CBT demonstrated a 30% increase in uniaxial yield pullout load relative to the traditional pedicle screws. No significant difference in construct stiffness was noted. Positive correlations were demonstrated between trajectory and bone density scans (qCT) and pullout force for both pedicle screws. |
In 2013, Matsukawa et al. performed a morphometric study in which they analyzed 100 CT scans of adult patients who had undergone spinal imaging, providing the first detailed measurements of the new cortical trajectory15. Subsequently, Perez‐Orribo et al. carried out non‐destructive flexibility tests on cadaveric lumbar spines18. No differences in mean range of motion or lax zone were found between CBT and traditional pedicle screws during flexion or extension tests. However, it was noted that CBT allowed a greater range of “stiff zone” in axial rotation tests than did traditional pedicle screws. In another study evaluating similar variables, Calvert et al. demonstrated that CBT screws provided similar stiffness in flexion, extension, lateral bending and axial rotation tests as traditional pedicle screws in cases of rescue screw constructs16. This study provides evidence for widening the applicability of CBT screws as an alternative to traditional pedicle screws in the repair of failed pedicle screw constructs. A recent study also compared the long‐term stability of CBT and traditional screws following simulated in vivo spine wear19. Pre‐fatigue and post‐fatigue were statistically equivalent for both CBT and traditional techniques during flexion‐extension, lateral bending and axial rotation flexibility tests. Thus, there is increasing evidence suggesting that CBT achieves at least comparable stability to traditional pedicle screws.
To evaluate the fixation strength of cortical screws under physiological loads, Baluch et al. performed a human cadaveric study in which they inserted CBT screws on one side and traditional pedicle screws on the contralateral side on 17 vertebral levels (T11−L5) and then performed quantitative CT scans17. After cycle craniocaudal toggling under increasing physiological loads, they demonstrated that CBT screws were associated with improved resistance to toggle testing (184 cycles vs. 102 cycles) and resistance force (398 N vs. 300 N) compared with traditional trajectory screws. Thus, these researchers demonstrated that CBT screws achieved superior stability to traditional screws. Matsukawa et al. measured the insertional torque of CBT and traditional screws intra‐operatively in 48 patients and showed that insertional torque of a pedicle screw strongly predicts pullout strength5, 32. They also demonstrated that the insertional torque of CBT screws was 1.71 times greater than that of the traditional technique, suggesting an advantage for CBT screws. However, it should be noted that this favorable difference cannot be attributed to the cortical trajectory alone: the shorter screw length and smaller screw diameter in CBT may also contribute to the pullout force and screw stability, respectively. The biomechanical evidence for CBT screws is summarized in Table 1.
Clinical Evidence
There is a lack of comprehensive and robust clinical evidence for CBT screws; to the best of our knowledge, no systematic review of such studies has yet been published. In our review of published reports, we identified 10 relevant studies reporting clinical outcomes for CBT pedicle screws, these are summarized in Table 2.
Table 2.
Overview of clinical studies investigating CBT screw fixation
| First author | Country | Study design/Quality of evidence | Number of patients | Indication | Technique | Outcomes | Complications |
|---|---|---|---|---|---|---|---|
| Takata et al.29, 2014 | Japan | R, OS/very poor | 6 | Degenerative spondylolisthesis | Hybrid CBT‐pedicle screw technique | Mean operation time 175.8 min (150–200 min). Blood loss 70–200 mL. No patients required transfusions | One patient had a mild infection of unknown origin 6 weeks after the surgery. The mean percent slippage before surgery was 19.8%, and this was reduced to 3.9% after surgery and maintained for 3 months |
| Rodriguez et al.28, 2014 | USA | R, OS/very poor | 5 | Symptomatic adjacent‐segment lumbar disease. All patients had previously undergone lumbar fusion with traditional pedicle fixation | CT (O‐arm) image‐guided navigation for hybrid contralateral CBT and traditional screw insertion | The average operative duration was 218 min (range, 175–315 min). Estimated blood loss was less than 500 mL for all but one subject. Cortical screws were 5.5 mm in diameter for all cases. Patients discharged after an average 2.8 days hospitalization | There were no postoperative complications. All patients in the cohort endorsed improved symptoms at their last clinical visit. Three of five patients had a decrease in the number of medications taken for symptom relief |
| Okudaira et al.27, 2014 | Japan | R, OS/poor | 16 CBT versus 19 open PLIF | Various lumbar pathologies (surgical indication unspecified) | Lumbar interbody fusion with CBT versus conventional open PLIF | CBT operation time shorter (148 min vs. 184 min). Less blood loss (132 g vs. 184 g). Fewer days needed to return to normal temp (4.6 vs. 7.8 days). Comparable pain and functional outcomes | None for CBT. One case of deep wound infection and concomitant permanent neural damage occurred in PLIF group |
| Mizuno et al.26, 2014 | Japan | R, OS/poor | 12 | Single‐level lumbar spondylolisthesis | PLIF or TLIF MI surgery using CBT screws | Recovery rate was 66.1% | One intraoperative complication, which was a cortical bone fracture at the screw compression. At 20‐month follow‐up, there were no findings of screw loosing. Four screws (8.3%) were judged to have perforated the wall of pedicles and vertebral bodies |
| Matsukawa et al.12, 2014 | Japan | R, OS/poor | 19 | Degenerative disorders requiring lumbosacral CBT fixation, lumbar foraminal stenosis | Five patients underwent hybrid CBT, the rest conventional CBT | Postoperative CT scans showed all pedicle screws had been implanted in the correct position | Not reported |
| Iwatsuki et al.25, 2014 | Japan | R, OS/very poor | Eight (four with modified isthmus‐guided CBT) | Lumbar degenerative spondylolisthesis | Isthmus guided CBT screw fixation | Not reported |
No complications. 4 screw misplacements in traditional CBT group, 1 screw misplacement in the isthmus‐guided CBT group |
| Gonchar et al.23, 2014 | Japan | P, OS/poor | 30 CBT versus 30 PLIF + PPS | L3−L5 spondylolisthesis | Minimally invasive PLIF using either CBT or PPS techniques | Both CBT and PPS were effective in improving JOABPEQ, ODI and VAS scores. However, better improvement in VAS seen in CBT group. Less intraoperative blood loss in CBT group (118 mL vs. 280 mL). Lower operation time (120 min vs. 137 min) | Screw loosening more frequent with PPS (6 cases vs. 1 case). There were 3 cases of radiologic non‐union in PPS and none in CBT group. Percentage slip loss of correction was significantly higher in PPS (2.3% vs. 10.4%). Slip angle loss of correction was significantly higher in PPS (2.3° vs. 5.2°) |
| Gonchar et al.24, 2014 | Japan | R, OS/poor | 100 CBT versus 63 PS | Spine deformity, degenerative disease, osteoporotic vertebral collapse, trauma | CBT or traditional PS. Procedures included MIS‐PLIF or TLIF and deformity correction techniques | Mean operation time was 162 mins for CBT and 177 mins for PS. Mean intraoperative blood loss was 177 mL for CBT and 334 ml for PS. Mean pre‐operative ODI score was 43% for CBT and 45% for PS | There was only one case of CBT loosening (1%) and 16 cases of PS loosening (25%). There was one pseudarthrosis in CBT (fusion rate 99%) and 6 pseudarthrosis in PS (fusion rate 90%). There were 2 cases of CBT screw breakage, however, both led to successful fusion |
| Ueno et al.22, 2013 | Japan | Case report/very poor | 1 | Degenerative lumbar scoliosis, osteoporosis | Double‐trajectory technique: cortical bone technique + traditional technique | Complete correction achieved, Cobb angle of 0°, lumbar lordosis of 46°. No impediment to daily activities | No complications at 14‐month follow‐up |
| Steel et al.21, 2004 | Australia | R, OS/poor | 18 | Thoracolumbar burst fracture (T10−L2) | Single‐level pedicle screw fixation, “medial‐lateral superior trajectory” | All patients mobilized within 10 days of surgery. All patients with stable fusion at 6‐week follow‐up. Only one patient reported significant back pain | Minor wound infection (1 case) |
MIS, minimally invasive; OS, observational study; PLIF, posterior lumbar interbody fusion; PPS, percutaneous pedicle screw; PS, pedicle screw; R, retrospective; TLIF, transforaminal lumbar interbody fusion.
In 2004, Steel et al. reported using a medio‐lateral superior trajectory for CBT screw placement in 18 patients with thoracolumbar burst fractures21. All patients were mobilized within 10 days of surgery and had stable fusions at 6‐month follow‐up. One patient reported back pain at 12‐month follow‐up and was found to have non‐union. As to complications, there was one case of wound infection, but no cases of neurological deficit, delayed kyphotic deformities or instrument failure. The authors concluded that the CBT approach is safe and effective for fixation and stabilization of fractured segments.
In 2014, Gonchar et al. reported a retrospective comparative study of 100 CBT and 63 traditional pedicle screw patients who had spinal deformity, degenerative disease, osteoporotic vertebral collapse or trauma24. The durations of surgery were similar in the two groups (162 min vs. 177 min, respectively). There was significantly less blood loss in the CBT group (177 mL vs. 334 mL) but similar improvement in Visual Analogue Scale (VAS), Oswestry Disability Index (ODI) and Japanese Association Back Pain Evaluation Questionnaire (JOABPEQ) pain scores in the two groups. Most notably, there was one case (1%) of screw loosening in the CBT group versus 16 cases (25%) in the traditional pedicle group. Gonchar and colleagues subsequently reported results of a prospective comparative study of 30 CBT versus 30 traditional pedicle screws in patients undergoing posterior lumbar interbody fusion surgery23. Indications included L3−L5 spondylolisthesis. While both screw fixation approaches significantly improved pain scores, greater improvement was noted in the CBT group. Screw loosening occurred more frequently in the traditional pedicle fixation group (six cases vs. one case); additionally, there were three cases of non‐union in the pedicle group versus none in the CBT group. Slip loss correction also occurred less frequently in the CBT group. The authors concluded that single level minimally invasive surgery with CBT is associated with lower rates of screw loosening and less frequent loss of correction and is less invasive than traditional approaches.
Iwatsuki et al. performed isthmus‐guided CBT pedicle screw fixation on eight patients25. This technique involves using the lateral margin of the isthmus and superior margin of the intervertebral foramen as reference points for screw insertion during lateral fluoroscopy, thus solving the problem of using a degenerated inferior articular process as a reference point (as in the original CBT technique) or an incorrect reference point of screw insertion resulting from lateral slippage. No complications such as dural injury, nerve root disorders or fractures were noted. Only one screw was misplaced in patients treated using the modified CBT technique, demonstrating excellent accuracy of screw insertion with this approach.
Mizuno et al. reported results from a retrospective analysis of 12 patients with single‐level lumbar spondylolisthesis who underwent posterior or transforaminal lumbar interbody fusion surgery using CBT screws26. One intraoperative complication was noted, namely a cortical bone fracture at the site of screw compression. There were no cases of surgery‐related spinal nerve injury or neurological deficits. At 20‐month follow‐up of five patients, successful fusion was noted with no failure of hardware or fracture of screw rods. No loose screws were detected. However, four screws (8.3%) were judged to have perforated the walls of the pedicles and vertebral bodies; however, no nerve injury had resulted from this.
In a retrospective comparative study of 16 CBT versus 19 traditional screws in open posterior lumbar interbody fusions for single‐level lumbar spondylolisthesis, Okudaira et al. demonstrated that CBT screws were associated with shorter duration of surgery (148 min vs. 184 min), less blood loss (132 g vs. 184 g), and similar pain and functional outcomes compared with traditional screws27. No complications were noted in the CBT group; however, there was one case of deep infection and permanent neural damage in the traditional pedicle screw group. Overall, CBT was less invasive, required less exposure and resulted in faster recovery with fewer complications.
Rodriguez et al. reported outcomes of CBT screw fixation in five patients28. No postoperative complications were observed and all patients were discharged after an average hospital stay of 2.8 days. All patients reported improvements in symptoms at follow‐up visits, three reporting a reduction in analgesic dosage. Thus, this small study demonstrated good clinical outcomes for CT‐guided CBT pedicle screw placement.
Takata et al. reported a mean operation duration of 175.8 mins, with intraoperative blood loss ranging from 70 mL to 200 mL in six patients with degenerative spondylolisthesis29. No patients required blood transfusions. There were no surgical complications, with the exception of a mild infection of unidentified origin at 6‐week follow‐up. After the 3‐month follow‐up, the mean slippage rate decreased from 19.8% to 3.9% at.
Discussion
Previous Studies
Prior to the introduction of CBT by Santoni et al.14, several studies had proposed alternative pedicle screw trajectories that did not follow the anatomical axis of the pedicle. In 1976 and 1992, Roy‐Camille et al. described a vertical screw insertion trajectory that crossed the axis of the pedicle, which contacts a greater proportion of cortical bone at its endpoint than with traditional insertion techniques33, 34. However, it was only in 2007 that Sterba and colleagues formally assessed the biomechanical properties of vertical straight screws, and demonstrated that they are associated with superior stability and pullout strength compared with conventionally angled pedicle screw pathways35.
There have been reports of the application of cortical trajectory for pedicle fixation in clinical practice for over 10 years21, 30. The CBT approach, which was initially termed the “medio‐latero‐superior trajectory” (MLST) technique (Fig. 1), was initially used for monosegmental screw fixation of thoracolumbar burst fractures. Satisfactory outcomes were obtained, with no cases of neurological deficit or instrumentation failure. These early studies laid down the groundwork for the development of alternative trajectories for pedicle screws.
Recent Developments
Since its initial description in 2009, several advances and variations in the CBT approach for pedicle fixation have been reported. In many patients with interbody fusion, the inferior articular process cannot be used as an accurate marker for CBT screw insertion because it has degenerated. Iwatsuki et al. described an isthmus‐guided CBT for pedicle screw insertion25. This modification provides alternative markers for accurate insertion of CBT screws and incorporates intraoperative fluoroscopy imaging confirmation to avoid screw malpositioning.
Ueno et al. recently reported a single case in which the double‐trajectory technique was used; this involves simultaneous insertion of both CBT and traditional pedicle screws into the same pedicles22. The cortical screw trajectories converge, which is expected to increase pullout strength and grip force on the vertebral bodies.
Matsukawa et al. have extended the use of CBT screws to sacral pedicle screw trajectories, which penetrate the S1 superior endplate through a more medial entry point than with traditional approaches20. The insertion point is located at the junction of the S1 superior articular process and the inferior border of the L5 inferior articular process; this technique achieves a 141% increase in insertional torque over traditional techniques.
The use of CT‐guided navigation during placement of CBT screws for adjacent‐segment lumbar disease has recently been described by Rodriguez et al.28. The main advantage offered by this novel approach is that it eliminates the need to expose, remove or connect pre‐existing hardware. Whether this technique is suitable for multi‐level lumbar fusion has not yet been determines.
Mizuno et al. recently reported a novel approach termed midline lumbar fusion that aims at simultaneously performing microsurgical laminectomy and CBT screw fixation via a posterior midline approach26. The advantage offered by this approach is that both decompression and fusion can be performed using a single access, thus avoiding the damage that results from using multiple accesses in separate operations. These authors advocate this approach in patients with lumbar spondylolisthesis requiring concomitant midline decompression.
Advantages and Disadvantages
The novel CBT technique for pedicle screw fixation has several advantages over traditional approaches.
The cortical trajectory is considered less invasive than the traditional screw trajectory. The initial insertion point is located medial on the pars interarticularis, which translates into smaller initial incisions and less muscle dissection and retraction. This is particularly pertinent in obese or morbidly obese patients, in whom incision, exposure and access to the surgical site is especially challenging. Perioperatively, this advantage theoretically translates into a reduction in intraoperative and postoperative blood loss, postoperative pain, duration of hospitalization and an enhancement of postoperative recovery.
The CBT approach may reduce injury or trauma to neurovascular structures in close proximity to the pedicle. Given that the trajectory is laterally directed in the transverse plane and caudocephalad in the sagittal plane, the screw follows a path away from the nerve roots, dural sac and anterior vascular structures. Therefore, there is a reduced risk of neurovascular damage, which means a safer profile for insertion of CBT screws.
CBT may also avoid injury to the medial branch nerves, which arise from the dorsal rami of each lumbar spinal nerve. Normally, the medial branch nerve is susceptible to injury by traditional pedicle screws, especially ones near the mammillary process15, 36. However, in the case of CBT, reduced risk of injury to the medial branch nerve may translate into reduced risk of postoperative radiculitis15.
Several drawbacks and complications are associated with the CBT approach to screw fixation. There is an increased risk of pedicle fracture if an inappropriately sized screw diameter is utilized. Incorrect depth of screw penetration can also increase the risk of upper nerve root injury26, 37, 38. These potential complications highlight the need for thorough comprehension of the anatomy both pre‐ and intra‐operatively15. Failure of the pedicle screws to line up in the sagittal plane may increase the challenge and difficulty of rod placement. In patients with partially or fully destructed articular joints, particularly in those with intervertebral fusion, landmarks for CBT screw insertion may no longer be available25. The novel isthmus‐guided CBT approach by Iwatsuki et al. may help solve this problem. Like other surgical approaches requiring intraoperative imaging, there is potentially increased radiation exposure in CBT procedures, particularly for operators early in their learning curve and with longer operation times25. Hybrid CBT approaches may also extend the surgical procedure and increase exposure times, which may lead to increased infection rates, blood loss and associated expenses. It must be noted that most biomechanical and clinical studies thus far have been heterogeneous in terms of their CBT techniques, screw size and diameter; this should be kept in mind when planning such procedures.
Limitations of Clinical Evidence
Overall, the currently available clinical evidence provides promising preliminary data demonstrating that the efficacy and safety of CBT screws is at least comparable to that of traditional pedicle screws. However, these clinical studies are constrained by the following limitations:
Because the reported studies are small, there may be lack of adequate statistical power to detect complications associated with CBT for pedicle screw fixation.
The non‐randomized study designs mean that potential selection bias may undermine the reported results and trends.
Because there is heterogeneity amongst the studies in terms of CBT surgical technique, screw length and diameter, navigation assistance techniques and follow‐up durations, their results may not be directly comparable.
Consequently, the data published to date are too few and short to allow for definitive conclusions and formal recommendations.
Several other limitations should be considered. Although biomechanical studies suggest that CBT screws have favorable pull‐out strength, the average screw core diameter is usually smaller than with the traditional technique. There are insufficient data addressing the screw breakage rate and frequency of delayed union. Additionally, because many surgeons use a free‐hand technique when inserting traditional pedicle screws, the time‐per‐screw insertion is expected to be shorter than with CBT screws, because no imaging guidance is necessary.
Indications and Contraindications
Future large prospective studies with standardized surgical technique and outcome reporting are required to provide robust clinical evidence for CBT pedicle fixation31, 39. Based on current available evidence, the indications for CBT screw insertion include: (i) posterior fixation during lumbar fusion; (ii) decompressive laminectomy if fusion is required; (iii) transforaminal lumbar interbody fusion requiring instrumentation; (iv) obese patients who would benefit from an instrumentation approach that requires less anatomical exposure; and (v) patients with osteopenia or osteoporosis who would benefit from the increased screw‐bone purchase and stability of CBT screws. The CBT technique may be useful in conditions such as osteogenesis imperfecta or neuromuscular scoliosis with significant osteoporosis; however, this possibility is yet to be supported by clinical data.
Relative contraindications for CBT pedicle screws include: (i) longer constructs of greater than three levels or multi‐level scoliosis; and (ii) narrow or medialized pars, and congenitally small pedicles. Absolute contraindications include (iii) congenital pars defects and lack of cortical bone at the pars.
Conclusions
In summary, the recently introduced CBT/MLST for pedicle screws offers several advantages over traditional pedicle screws. Biomechanical studies have confirmed the advantages of the former, including improved bone‐screw purchase and stability that are at least comparable to those of traditional trajectories. However, there is still a lack of robust clinical data for CBT in lumbar surgery. Further clinical studies with long‐term follow‐up are required to investigate the long‐term outcomes of CBT pedicle screws for stabilization in various lumbar spine pathologies.
Disclosure: The authors declare that they have no conflict of interests regarding this review. No funds were received in support of this work.
References
- 1. Roy‐Camille R, Saillant G, Mazel C. Plating of thoracic, thoracolumbar, and lumbar injuries with pedicle screw plates. Orthop Clin North Am, 1986, 17: 147–159. [PubMed] [Google Scholar]
- 2. Suk SI, Lee CK, Min HJ, Cho KH, Oh JH. Comparison of Cotrel−Dubousset pedicle screws and hooks in the treatment of idiopathic scoliosis. Int Orthop, 1994, 18: 341–346. [DOI] [PubMed] [Google Scholar]
- 3. Hart RA, Hansen BL, Shea M, Hsu F, Anderson GJ. Pedicle screw placement in the thoracic spine: a comparison of image‐guided and manual techniques in cadavers. Spine (Phila Pa 1976), 2005, 30: E326–E331. [DOI] [PubMed] [Google Scholar]
- 4. Inceoğlu S, Montgomery WH Jr, St Clair S, McLain RF. Pedicle screw insertion angle and pullout strength: comparison of 2 proposed strategies. J Neurosurg Spine, 2011, 14: 670–676. [DOI] [PubMed] [Google Scholar]
- 5. Matsukawa K, Yato Y, Kato T, Imabayashi H, Asazuma T, Nemoto K. In vivo analysis of insertional torque during pedicle screwing using cortical bone trajectory technique. Spine (Phila Pa 1976), 2014, 39: E240–E245. [DOI] [PubMed] [Google Scholar]
- 6. Weinstein JN, Spratt KF, Spengler D, Brick C, Reid S. Spinal pedicle fixation: reliability and validity of roentgenogram‐based assessment and surgical factors on successful screw placement. Spine (Phila Pa 1976), 1988, 13: 1012–1018. [DOI] [PubMed] [Google Scholar]
- 7. Laine T, Lund T, Ylikoski M, Lohikoski J, Schlenzka D. Accuracy of pedicle screw insertion with and without computer assistance: a randomised controlled clinical study in 100 consecutive patients. Eur Spine J, 2000, 9: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gertzbein SD, Robbins SE. Accuracy of pedicular screw placement in vivo . Spine (Phila Pa 1976), 1990, 15: 11–14. [DOI] [PubMed] [Google Scholar]
- 9. Wittenberg RH, Shea M, Swartz DE, Lee KS, White AA 3rd, Hayes WC. Importance of bone mineral density in instrumented spine fusions. Spine (Phila Pa 1976), 1991, 16: 647–652. [DOI] [PubMed] [Google Scholar]
- 10. Mobbs RJ, Sivabalan P, Li J. Technique, challenges and indications for percutaneous pedicle screw fixation. J Clin Neurosci, 2011, 18: 741–749. [DOI] [PubMed] [Google Scholar]
- 11. Rao PJ, Maharaj MM, Phan K, Lakshan Abeygunasekara M, Mobbs RJ. Indirect foraminal decompression following anterior lumbar interbody fusion (ALIF): a prospective radiographic study using a new pedicle to pedicle (P‐P) technique. Spine J, 2015, 15: 817–824. [DOI] [PubMed] [Google Scholar]
- 12. Matsukawa K, Yato Y, Hynes RA, et al Cortical bone trajectory for thoracic pedicle screws: a technical note. J Spinal Disord Tech, 2014. Jul 29. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13. Luther N, Iorgulescu JB, Geannette C, et al Comparison of navigated versus non‐navigated pedicle screw placement in 260 patients and 1434 screws: screw accuracy, screw size, and the complexity of surgery. J Spinal Disord Tech, 2015, 28: E298–E303. [DOI] [PubMed] [Google Scholar]
- 14. Santoni BG, Hynes RA, McGilvray KC, et al Cortical bone trajectory for lumbar pedicle screws. Spine J, 2009, 9: 366–373. [DOI] [PubMed] [Google Scholar]
- 15. Matsukawa K, Yato Y, Nemoto O, Imabayashi H, Asazuma T, Nemoto K. Morphometric measurement of cortical bone trajectory for lumbar pedicle screw insertion using computed tomography. J Spinal Disord Tech, 2013, 26: E248–E253. [DOI] [PubMed] [Google Scholar]
- 16. Calvert GC, Lawrence BD, Abtahi AM, Bachus KN, Brodke DS. Cortical screws used to rescue failed lumbar pedicle screw construct: a biomechanical analysis. J Neurosurg Spine, 2015, 22: 166–172. [DOI] [PubMed] [Google Scholar]
- 17. Baluch DA, Patel AA, Lullo B, et al Effect of physiological loads on cortical and traditional pedicle screw fixation. Spine (Phila Pa 1976), 2014, 39: E1297–E1302. [DOI] [PubMed] [Google Scholar]
- 18. Perez‐Orribo L, Kalb S, Reyes PM, Chang SW, Crawford NR. Biomechanics of lumbar cortical screw‐rod fixation versus pedicle screw‐rod fixation with and without interbody support. Spine (Phila Pa 1976), 2013, 38: 635–641. [DOI] [PubMed] [Google Scholar]
- 19. Radcliff K, Klocke N, Harris J, et al Simulating the long‐term biomechanical performance of cortical screws: can a more MIS screw option provide equivalent stability as traditional pedicle screws? Proceeding of SMISS Global Forum, 2014.
- 20. Matsukawa K, Yato Y, Kato T, Imabayashi H, Asazuma T, Nemoto K. Cortical bone trajectory for lumbosacral fixation: penetrating S‐1 endplate screw technique: technical note. J Neurosurg Spine, 2014, 21: 203–209. [DOI] [PubMed] [Google Scholar]
- 21. Steel TR, Rust TM, Fairhall JM, Mobbs RJ. Monosegmental pedicle screw fixation for thoraco‐lumbar burst fracture. J Bone Joint Surg Br, 2004, 86 (Suppl. IV): 458. [Google Scholar]
- 22. Ueno M, Imura T, Inoue G, Takaso M. Posterior corrective fusion using a double‐trajectory technique (cortical bone trajectory combined with traditional trajectory) for degenerative lumbar scoliosis with osteoporosis: technical note. J Neurosurg Spine, 2013, 19: 600–607. [DOI] [PubMed] [Google Scholar]
- 23. Gonchar I, Kotani Y, Matsumoto Y. Cortical bone trajectory versus percutaneous pedicle screw in minimally invasive posterior lumbar fusion. Spine J, 2014, 14: S114–S115. [Google Scholar]
- 24. Gonchar I, Kotani Y, Matsui Y, Miyazaki T, Kasemura T, Masuko T. Experience of 100 consecutive spine reconstructions using cortical bone trajectory (CBT) screws vs traditional pedicle screws. Proceeding of SMISS Global Forum, 2014.
- 25. Iwatsuki K, Yoshimine T, Ohnishi Y, Ninomiya K, Ohkawa T. Isthmus‐guided cortical bone trajectory for pedicle screw insertion. Orthop Surg, 2014, 6: 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mizuno M, Kuraishi K, Umeda Y, Sano T, Tsuji M, Suzuki H. Midline lumbar fusion with cortical bone trajectory screw. Neurol Med Chir (Tokyo), 2014, 54: 716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okudaira T, Konishi H, Baba H, Hiura K, Yamashita K, Yamada S. Comparison study of lumbar interbody fusion with cortical bone trajectory screws versus conventional open posterior lumbar interbody fusion. Proceeding of SMISS Global Forum, 2014.
- 28. Rodriguez A, Neal MT, Liu A, Somasundaram A, Hsu W, Branch CL Jr. Novel placement of cortical bone trajectory screws in previously instrumented pedicles for adjacent‐segment lumbar disease using CT image‐guided navigation. Neurosurg Focus, 2014, 36: E9. [DOI] [PubMed] [Google Scholar]
- 29. Takata Y, Matsuura T, Higashino K, et al Hybrid technique of cortical bone trajectory and pedicle screwing for minimally invasive spine reconstruction surgery: a technical note. J Med Invest, 2014, 61: 388–392. [DOI] [PubMed] [Google Scholar]
- 30. Mobbs RJ. The “medio‐latero‐superior trajectory technique”: an alternative cortical trajectory for pedicle fixation. Orthop Surg, 2013, 5: 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phan K, Tian DH, Cao C, Black D, Yan TD. Systematic review and meta‐analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg, 2015, 4: 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zdeblick TA, Kunz DN, Cooke ME, McCabe R. Pedicle screw pullout strength. Correlation with insertional torque. Spine (Phila Pa 1976), 1993, 18: 1673–1676. [DOI] [PubMed] [Google Scholar]
- 33. Roy‐Camille R, Saillant G, Berteaux D, Salgado V. Osteosynthesis of thoraco‐lumbar spine fractures with metal plates screwed through the vertebral pedicles. Reconstr Surg Traumatol, 1976, 15: 2–16. [PubMed] [Google Scholar]
- 34. Roy‐Camille R. Posterior screw plate fixation in thoracolumbar injuries. Instr Course Lect, 1992, 41: 157–163. [PubMed] [Google Scholar]
- 35. Sterba W, Kim DG, Fyhrie DP, Yeni YN, Vaidya R. Biomechanical analysis of differing pedicle screw insertion angles. Clin Biomech (Bristol, Avon), 2007, 22: 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bogduk N, Long DM. The anatomy of the so‐called “articular nerves” and their relationship to facet denervation in the treatment of low‐back pain. J Neurosurg, 1979, 51: 172–177. [DOI] [PubMed] [Google Scholar]
- 37. Lonstein JE, Denis F, Perra JH, Pinto MR, Smith MD, Winter RB. Complications associated with pedicle screws. J Bone Joint Surg Am, 1999, 81: 1519–1528. [DOI] [PubMed] [Google Scholar]
- 38. Schulze CJ, Munzinger E, Weber U. Clinical relevance of accuracy of pedicle screw placement. A computed tomographic‐supported analysis. Spine (Phila Pa 1976), 1998, 23: 2215–2220, discussion 20−21. [DOI] [PubMed] [Google Scholar]
- 39. Phan K, Mobbs RJ. Systematic reviews and meta‐analyses in spine surgery, neurosurgery and orthopedics: guidelines for the surgeon scientist. J Spine Surg, 2015, doi: 10.3978/jss.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]


