Abstract
Importance
Retinopathy of prematurity (ROP) is a leading cause of childhood blindness worldwide. Optical coherence tomography (OCT) has improved the care of adults with vitreoretinal disease, and OCT angiography (OCTA) is demonstrating promise as a technique to visualize the retinal vasculature with lower risk and cost than fluorescein angiography. However, to date, there are no commercially available devices able to obtain ultra–wide-field OCT or OCTA images in neonates.
Objective
To obtain ultra–wide-field OCT and OCTA images in neonates with ROP using a prototype handheld OCT and OCTA device.
Design, Setting, and Participants
This observational case series was conducted from March 1 to April 1, 2017, in an academic medical center among 4 neonates with ROP in the neonatal intensive care unit and in the operating room.
Main Outcomes and Measures
Acquisition of wide-field OCT and OCTA images using a handheld prototype OCTA and ultra–wide-field OCT device.
Results
Images were obtained from 4 neonates (1 girl and 3 boys; mean age, 38 weeks’ postmenstrual age [range, 34-43 weeks]) with various stages of ROP: 3 in the neonatal intensive care unit and 1 in the operating room. The system can obtain noncontact en face OCT images and horizontal line scans with an approximately 40° field of view and up to 100° (ultra–wide-field) using a contact lens–based approach in a single 2-second scan. In addition, 20° × 20° (approximately 4 × 4-mm) OCTA scans were obtained in patients with ROP in a single 2-second scan.
Conclusions and Relevance
Optical coherence tomography and OCTA are gaining popularity in pediatric retinal imaging. This study reports on OCTA and ultra–wide-field OCT images in 4 neonates with various stages of ROP that were obtained using a prototype handheld device. Additional studies will be needed to prove the clinical value of this technology.
This case series describes the use of a prototype handheld device to obtain ultra–wide-field optical coherence tomography and optical coherence tomography angiography images in neonates with retinopathy of prematurity.
Key Points
Question
Is it possible to perform optical coherence tomography angiography in neonates with retinopathy of prematurity using a handheld device?
Finding
In this case series of 4 neonates with retinopathy of prematurity (3 in the neonatal intensive care unit and 1 under anesthesia in the operating room), handheld optical coherence tomography angiography was able to demonstrate retinal and choroidal vascular changes following laser treatment.
Meaning
Handheld optical coherence tomography angiography was able to noninvasively visualize microvascular changes in retinopathy of prematurity, suggesting that future development and clinical validation of this technology in retinopathy of prematurity may be feasible.
Introduction
Retinopathy of prematurity, one of the leading causes of childhood blindness in the world,1 develops when prematurely born infants with immature retinal vasculature are introduced to a relatively hyperoxic environment at birth, which can lead to abnormal vascular development.2 The international classification of retinopathy of prematurity defines the following 3 characteristics of ROP: zone (how much of the retina is vascularized), stage (how abnormal the vessels and vitreoretinal interface are at the border of avascular retina), and plus disease (how dilated and tortuous the arteries and veins are in the posterior pole).3 The criterion standard for diagnosing ROP has historically been indirect ophthalmoscopy performed by an experienced examiner, although image-based diagnosis with telemedicine interpretation of color fundus images has recently demonstrated promise, especially for implementation in regions where experienced examiners may not be available.4 In both cases, however, interobserver agreement on zone, stage, and plus disease has been shown to be variable and subjective.5
Optical coherence tomography (OCT) has improved the care of adult patients with vitreoretinal diseases, and there is early evidence that OCT may aid in the diagnosis of some aspects of ROP.6,7 Optical coherence tomography angiography (OCTA) detects intrinsic motion within the layers of the retina to depict blood flow and is increasingly being used in adult retinal diseases such as age-related macular degeneration and diabetic retinopathy to directly visualize normal and abnormal retinal blood flow in vivo.8 There are currently no commercially available portable devices capable of performing OCTA in neonates with ROP. There has been a single report of performance of imaging on an outpatient infant with ROP using a commercially available OCTA device manufactured by Optovue,9 and Chen et al10 recently reported on a microscope-mounted device capable of obtaining OCTA images in the operating room. Here, we report our initial results using a prototype handheld OCT and OCTA device in the neonatal intensive care unit and operating room in neonates with ROP.
Methods
We developed a handheld OCT and OCTA system using a 100-kHz tunable laser (Axsun Technologies), with a lateral resolution of 17 μm and axial resolution of 5 μm.11 We developed separate scanning protocols ranging from 2 to 4 seconds using both noncontact-based and contact lens–based approaches. The noncontact method provided a field of view of approximately 40°and was obtained by holding the probe directly above the surface of the eye. A real-time en face OCT display helped align the scanning region, which was controlled by a second operator on the computer. Using a pediatric wide-angle lens and a contact lens–based approach, we were able to obtain both ultra–wide-field (UWF) (approximately 100° × 100°) OCT and 20° × 20° (approximately 4 × 4-mm) OCTA scans in 2 seconds. We obtained images from March 1 to April 1, 2017. The protocol was approved by the Oregon Health & Science University Institutional Review Board and followed the tenets of the Declaration of Helsinki.12 Parents provided written informed consent.
Results
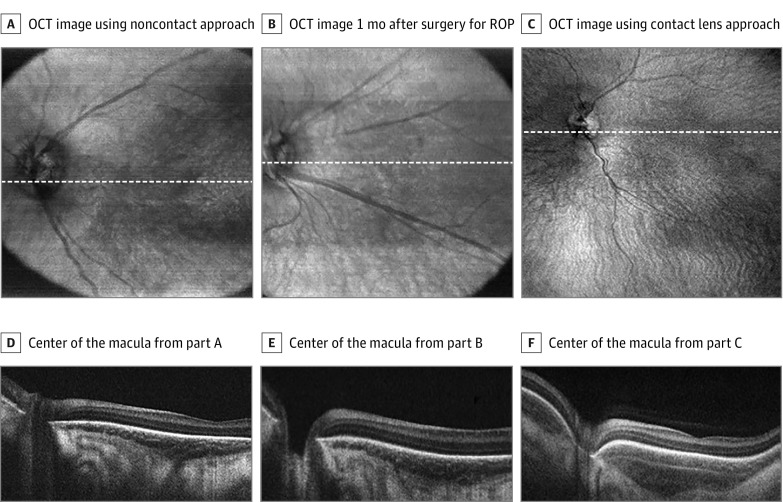
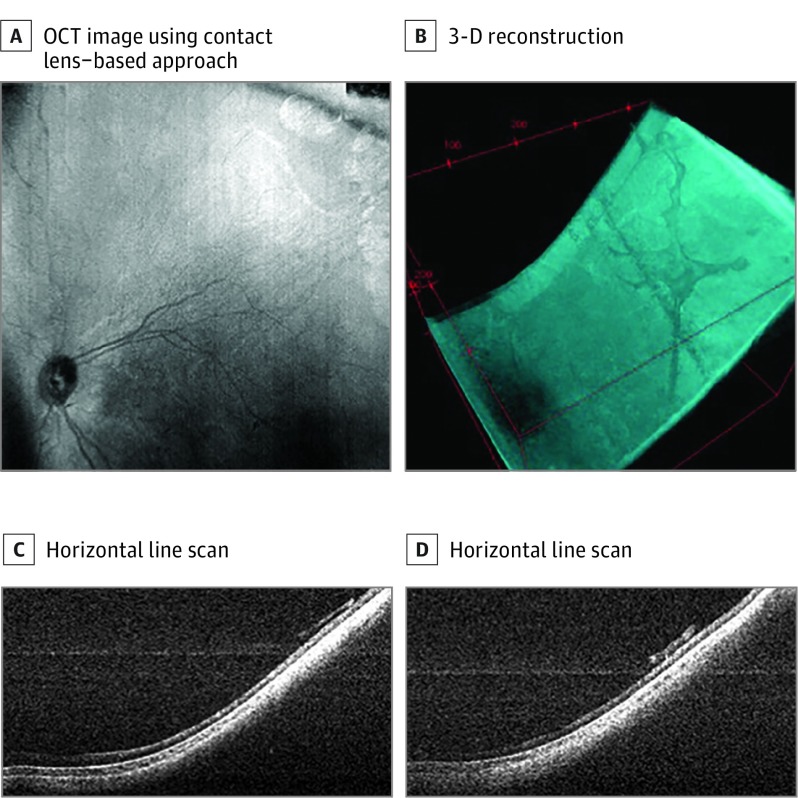
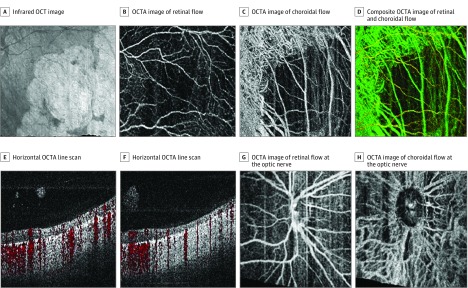
A total of 4 neonates were included in this series and ranged in age from 34 to 43 (mean, 38) weeks’ postmenstrual age. Three were male, 3 were non-Hispanic white, and 1 was Hispanic. We obtained images using both contact lens–based and noncontact-based approaches. Figure 1 shows 3 representative sets of images from 3 neonates who underwent imaging during routine ROP screening. The noncontact scans demonstrate an approximately 40° field of view with en face infrared images that are displayed on the monitor in real time, and several representative line scans. Figure 1C was obtained using a contact lens–based approach, demonstrating a larger field of view. The OCT scans demonstrate various degrees of foveal development and choroidal thickness. In addition, in 1 neonate who required surgery for a retinal detachment, we obtained contact UWF OCT images (Figure 2) and OCTA images (Figure 3) during examination while the patient was under anesthesia. Figure 2 demonstrates the 100° field of view we were able to capture and the 3-dimensional reconstruction of these scans demonstrating the organization of preretinal hyperreflective material on UWF OCT imaging that clinically corresponded to whitish preretinal fibrotic membranes in regions of previous extraretinal neovascularization. The OCTA images in Figure 3 demonstrate attenuated retinal flow in regions of previous laser treatment, loss of choriocapillaris with sparing of the larger choroidal vessels, and the absence of flow in the preretinal membranes.
Figure 1. Optical Coherence Tomography (OCT) in Children in the Neonatal Intensive Care Unit With Retinopathy of Prematurity (ROP) Using Handheld Prototype Swept-Source OCT.
A, En face OCT image of a neonate obtained in 2 seconds during routine screening using a noncontact approach. B, En face OCT image of a neonate obtained in 2 seconds during routine screening using a noncontact approach 1 month after laser surgery for type 1 ROP. C, En face OCT image of a neonate obtained obtained in 2 seconds during routine screening using contact lens approach. D, Horizontal line scan through the center of the macula of the neonate from part A. E, Horizontal line scan through the center of the macula of the neonate from part B. F, Horizontal line scan through the center of the macula of the neonate from part C. White dashed lines in A represent corresponding line scans in D, white dashed lines in B represent corresponding line scans in E, and white dashed lines in C represent corresponding line scans in F.
Figure 2. Ultra–Wide-Field Optical Coherence Tomography (UWF OCT) of a Child After Laser Surgery for Type 1 Retinopathy of Prematurity.
A, En face OCT image obtained in 2 seconds during examination while the patient was under anesthesia in the operating room using a contact lens–based approach. B, Reprocessed 3-dimensional reconstruction demonstrating organization of preretinal hyperreflective material. C, Representative horizontal line scan from the UWF OCT scans demonstrating attached retina with evidence of overlying hyperreflective membranes that corresponded clinically to whitish preretinal fibrotic membranes in regions of previous extraretinal neovascularization. D, Representative horizontal line scan from the UWF OCT scans demonstrating attached retina with evidence of overlying hyperreflective membranes that corresponded clinically to whitish preretinal fibrotic membranes in regions of previous extraretinal neovascularization.
Figure 3. Contact Wide-Field Optical Coherence Tomography Angiography (OCTA) of a Child After Laser Surgery for Type 1 Retinopathy of Prematurity.
A, Infrared en face OCT image of area imaged in parts A-D. B, OCTA image of retinal flow. C, OCTA image of choroidal flow demonstrating loss of choriocapillaris but persistence of larger choroidal vessels. D, Color composite OCTA image of retinal and choroidal flow. E, Horizontal OCTA line scan with OCTA image demonstrating increased signal penetration in areas of previous laser treatment, attenuated flow in atrophic retina (flow indicated in red), and no flow in overlying fibrous proliferation. F, Horizontal OCTA line scan with OCTA image demonstrating increased signal penetration in areas of previous laser treatment, attenuated flow in atrophic retina (flow indicated in red), and no flow in overlying fibrous proliferation. G, OCTA image of retinal flow at the optic nerve, and demonstrating some motion artifact (vertical lines). H, OCTA image of choroidal flow at the optic nerve. All images obtained in 4 × 4-mm OCTA scans during examination while the patient was under anesthesia in the operating room.
Discussion
We present data from a prototype, handheld OCT device that can obtain UWF OCT and OCTA images in the neonatal intensive care unit and operating room in patients with ROP. There is good rationale to believe that objective anatomical evaluation of patients with ROP using OCT and OCTA could improve our understanding of and ability to manage disease in patients with ROP. Currently available portable OCT technology has identified subclinical changes in ROP, such as cystoid macular edema, incomplete foveal development, and choroidal thickness changes as well as vitreoretinal traction, retinoschisis, and retinal detachments, and early studies have suggested that the 3-dimensional architecture of the retinal vasculature may correlate with the diagnosis of plus disease.7,13 Optical coherence tomography is ideal for evaluating the structural changes in the retina and vitreous in ROP, and the use of UWF OCT may improve our understanding of peripheral vitreoretinal interface changes in ROP that precede and can lead to retinal detachment. As shown in Figure 2, we are able to visualize preretinal membranes at the border of laser treatment by using UWF OCT in a patient with a history of type 1 ROP.
The use of wide-angle fluorescein angiography when incorporating angiography into the evaluation of patients with ROP has demonstrated some utility in improving diagnostic agreement for zone and stage and for evaluating atypical patterns of retinal neovascularization.14 Optical coherence tomography angiography can provide similar information without fluorescein dye injection, but does not provide the same field of view. In theory, however, OCTA could be used to visualize the developing retinal and choroidal vasculature and has the potential to improve our understanding of both normally developing immature retinal vessels and the development of abnormal shunt vessels and neovascularization in ways not possible with fluorescein angiography. Figure 3 demonstrates in vivo retinal and choroidal vascular changes using a handheld OCTA device in an infant after laser treatment.
Limitations
The limitations of this study include its small sample size, lack of comparison with color fundus photography and fluorescein angiography, and lack of healthy controls. In these images, the OCTA image resolution is less than what can be obtained in commercially available devices in cooperative patients.
Conclusions
To our knowledge, there is no commercially available handheld device capable of obtaining UWF OCT and/or OCTA images and there are added technical challenges when trying to develop and validate this technology for neonates. In particular, the quality of OCTA images is sensitive to motion artifact (relative motion between the camera and patient). Using our 100-kHZ swept-source prototype, we observed some motion artifact even in sedated neonates (Figure 3). The use of handheld OCTA in awake adult patients by using this device in a noncontact fashion with minimal motion artifact has previously been reported11; however, the use of a contact lens was found to be helpful for image stabilization and OCTA acquisition in neonates. Postprocessing image registration and the development of active tracking may improve acquisition of OCTA images in neonates in the future. Nonetheless, these data demonstrate proof of principle that it is feasible to obtain high-quality UWF OCT and OCTA images using a handheld device, and there is potential for future development and clinical validation of this technology in patients with ROP.
References
- 1.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020—the right to sight. Bull World Health Organ. 2001;79(3):227-232. [PMC free article] [PubMed] [Google Scholar]
- 2.Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367(26):2515-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Committee for the Classification of Retinopathy of Prematurity The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123(7):991-999. [DOI] [PubMed] [Google Scholar]
- 4.Quinn GE, Ying G-S, Daniel E, et al. ; e-ROP Cooperative Group . Validity of a telemedicine system for the evaluation of acute-phase retinopathy of prematurity. JAMA Ophthalmol. 2014;132(10):1178-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang MF, Jiang L, Gelman R, Du YE, Flynn JT. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol. 2007;125(7):875-880. [DOI] [PubMed] [Google Scholar]
- 6.Lee AC, Maldonado RS, Sarin N, et al. . Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina. 2011;31(8):1470-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinekar A, Mangalesh S, Jayadev C, Maldonado RS, Bauer N, Toth CA. Retinal imaging of infants on spectral domain optical coherence tomography. Biomed Res Int. 2015;2015:782420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagiel A, Sadda SR, Sarraf D. A Promising future for optical coherence tomography angiography. JAMA Ophthalmol. 2015;133(6):629-630. [DOI] [PubMed] [Google Scholar]
- 9.Vinekar A, Chidambara L, Jayadev C, Sivakumar M, Webers CAB, Shetty B. Monitoring neovascularization in aggressive posterior retinopathy of prematurity using optical coherence tomography angiography. J AAPOS. 2016;20(3):271-274. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Viehland C, Carrasco-Zevallos OM, et al. . Microscope-integrated optical coherence tomography angiography in the operating room in young children with retinal vascular disease. JAMA Ophthalmol. 2017;135(5):483-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Liu L, Campbell JP, Huang D, Liu G. Handheld optical coherence tomography angiography. Biomed Opt Express. 2017;8(4):2287-2300. doi: 10.1364/BOE.8.002287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 13.Lee H, Proudlock FA, Gottlob I. Pediatric optical coherence tomography in clinical practice—recent progress. Invest Ophthalmol Vis Sci. 2016;57(9):OCT69-OCT79. [DOI] [PubMed] [Google Scholar]
- 14.Klufas MA, Patel SN, Ryan MC, et al. . Influence of fluorescein angiography on the diagnosis and management of retinopathy of prematurity. Ophthalmology. 2015;122(8):1601-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]