Key Points
Question
Do injections of platelet-rich plasma improve the appearance of photoaged facial skin?
Findings
Based on this study of 27 participants in a randomized clinical trial who were masked to whether they received platelet-rich plasma or normal saline, the photoaged facial skin of those treated with platelet-rich plasma was found to be significantly less rough and wrinkled at 6 months after a single treatment.
Meaning
Platelet-rich plasma, as administered within the conditions of this randomized clinical trial, is effective for facial rejuvenation, particularly for textural improvement of photoaged skin.
This randomized clinical trial investigates whether platelet-rich plasma injection improves the visual appearance, including texture and color, of photodamaged facial skin in adults with bilateral cheek rhytids of Glogau class II or greater.
Abstract
Importance
There remains little experimental evidence and no randomized clinical trial to date to confirm the benefit of platelet-rich plasma (PRP) for facial rejuvenation.
Objective
To investigate whether PRP injection improves the visual appearance, including texture and color, of photodamaged facial skin.
Design, Setting, and Participants
In this randomized clinical trial, participants and raters were masked to groupings. The setting was an academic-based, urban outpatient dermatology practice in Chicago, Illinois. Participants were adults aged 18 to 70 years with bilateral cheek rhytids of Glogau class II or greater. The duration of the study was August 21, 2012, to February 16, 2016.
Interventions
Each participant received 3 mL intradermal injections of PRP to one cheek and sterile normal saline to the contralateral cheek.
Main Outcomes and Measures
Primary outcomes were photoaging scores (with subscores for fine lines, mottled pigmentation, roughness, and sallowness) as rated by 2 masked dermatologists. Secondary outcomes included participant self-assessment scores of improvement on a 5-point scale (worsening, no change, mild improvement, moderate improvement, or significant improvement), participant overall satisfaction scores on a 4-point scale (not satisfied, slightly satisfied, moderately satisfied, or very satisfied), and participant-reported or investigator-observed adverse events.
Results
Of 27 enrolled participants, 19 (mean [SD] age, 46.37 [10.88] years; 17 female) were analyzed. Reported adverse events, which were not associated with the study agent, included redness (n = 18), swelling (n = 16), bruising (n = 14), pruritus (n = 1), skin scaling (n = 1), and dryness of skin (n = 1). No participants reported any adverse events at 12 months. Mean (SD) photoaging scores rated by 2 dermatologists showed no significant difference between PRP and normal saline for fine lines (baseline, 1.00 [0.75] vs 1.05 [0.78]; 2 weeks, 0.95 [0.71] vs 0.95 [0.71]; 3 months, 0.95 [0.71] vs 0.95 [0.71]; 6 months, 0.95 [0.71] vs 0.95 [0.71]), mottled pigmentation (baseline, 1.21 [0.53] vs 1.21 [0.54]; 2 weeks, 1.16 [0.60] vs 1.16 [0.60]; 3 months, 1.00 [0.47] vs 1.11 [0.46]; 6 months, 1.16 [0.69] vs 1.16 [0.69]), skin roughness (baseline, 0.47 [0.61] vs 0.47 [0.61]; 2 weeks, 0.47 [0.61] vs 0.47 [0.61]; 3 months, 0.47 [0.61] vs 0.47 [0.61]; 6 months, 0.37 [0.60] vs 0.37 [0.68]), and skin sallowness (baseline, 1.11 [0.88] vs 1.11 [0.88]; 2 weeks, 0.95 [0.85] vs 0.95 [0.85]; 3 months, 0.58 [0.61] vs 0.58 [0.61]; 6 months, 0.37 [0.68] vs 0.37 [0.68]). At 6 months after a single treatment, participants rated the PRP-treated side as significantly more improved compared with normal saline for texture (mean [SD] self-assessment score, 2.00 [1.20] vs 1.21 [0.54]; P = .02) and wrinkles (mean [SD] self-assessment score, 1.74 [0.99] vs 1.21 [0.54]; P = .03).
Conclusions and Relevance
Masked participants noted that both fine and coarse texture improved significantly more with a single treatment of PRP than with normal saline. Both participants and raters found PRP to be nominally but not significantly superior to normal saline.
Trial Registration
ClinicalTrials.gov Identifier: NCT01372566
Introduction
Platelet-rich plasma (PRP), a derivative of autologous whole blood that is thought to have particular wound-healing tissue regeneration properties, has entered the therapeutic armamentarium in dermatology.1,2 Preliminary work has investigated its effectiveness in the treatment of acne scars and androgenetic alopecia.3
Given the widespread patient interest to improve the aging face, rejuvenation of facial skin is another potential application for PRP. There are few studies on the effect of PRP on photoaged skin, and those that are published are cohort studies4,5,6,7 without controls. Regardless of the dearth of research, PRP has emerged as a popular clinical phenomenon, with practitioners offering it to patients (often in combination with microneedling) for a range of purported benefits, including facial rejuvenation. The objective of this randomized clinical trial was to assess, via a placebo-controlled design, the effects of PRP on the physical appearance of photoaged facial skin.
Methods
Trial Design
This study was a parallel, split-face, randomized clinical trial with 1:1 allocation in which participants and raters were masked to participant groupings. There were no substantive changes to the study methods after trial commencement. The study was reviewed and approved by the Northwestern University institutional review board. This trial was performed under Investigational Device Exemption 14587 from the US Food and Drug Administration and was registered at ClinicalTrials.gov (NCT01372566). Written informed consent was obtained from participants. The trial protocol is available in Supplement 1.
Participants
Eligible participants, both male and female, were adults aged 18 to 70 years with bilateral cheek rhytids of Glogau class II or greater (ie, wrinkles “in motion”). Exclusions included the following: pregnancy or lactation, blood or platelet disorders, genetic disorders affecting fibroblasts or collagen, facial surgery or semipermanent dermal fillers within 1 year, history of herpes simplex infection, active skin disease or infection in the treatment area, history of hypertrophic scars or keloids, immunosuppressive disorders or disorders treated with immunosuppressive agents (including corticosteroids), and history of skin cancer or actinic keratosis, as well as topical or oral tretinoin, chemical peeling, botulinum toxin injection, or laser and light treatment for facial rhytids or rejuvenation within the past 6 months or planned in the next 3 months. Data were collected in the clinical research section of the dermatology clinical outpatient service at the Northwestern University medical center in Chicago, Illinois.
Interventions
At screening, after eligibility assessment and written informed consent were obtained, participants’ demographic information and medical history were recorded, and laboratory testing (complete blood cell count, prothrombin time/partial thromboplastin time/international normalized ratio, liver function, and creatinine level) was performed. Those with abnormal laboratory values were not allowed to continue participation in the study.
At the first treatment visit, participants were instructed to avoid the use of any therapeutic agent for skin photoaging or rejuvenation and were provided sunscreen to apply to the whole face during the entire study period. Ten minutes after the face was washed at the study visit, baseline standardized digital photographs were obtained, and then inspection was conducted independently by 2 dermatologists (M.A. and K.P.), who assigned a photoaging score. Variations in scores were adjudicated by forced agreement. A topical anesthetic cream (EMLA; AstraZeneca) was applied to both cheeks approximately 1 hour before administration of the investigational product was to begin.
Venipuncture of a large peripheral vein was performed to collect blood from the participant. An autologous platelet concentrate procedure pack (SmartPrep 2 APC+; Harvest Technologies) and a blood processing centrifuge (SmartPrep 2 System; Harvest Technologies) were used to prepare the PRP sample. The blood sample was combined with acid citrate dextrose A, an anticoagulant, and spun in a centrifuge with 2 spins (a hard spin and a soft spin) to separate the PRP from the platelet-poor plasma. The remaining PRP was then injected into the cheek of the participant within the next 7 minutes. The complete method for venipuncture and preparation of PRP for injection is described in the eAppendix in Supplement 2.
Each participant received intradermal injections of PRP to one cheek and sterile normal saline (equivalent fluid volume) to the contralateral cheek. The investigational product–filled syringe exterior was opacified with tape, and participants’ eyes were covered during injection of investigational product to maintain masking. Three milliliters of sterile normal saline was drawn from a single-use vial for injection to the allocated control side. Injections were performed 1 cm apart and at the level of the mid-dermis using a serial puncture technique with a 25-gauge needle. Aliquots of 0.02 mL per puncture were injected into the designated cheek from the zygomatic area to the mandibular area and from the nasolabial folds to the preauricular area, for a total of 3 mL per cheek. Immediately after injection, gentle pressure with gauze was applied for 5 minutes. The skin was then compressed with normal saline swabs for 15 minutes. For wound care, petrolatum-based emollients (Aquaphor Healing Ointment; Beiersdorf) were provided to each participant for daily home application for 7 days after injections.
At 2-week and 3-month follow-up study visits, digital photographs were again obtained as before, 2 dermatologists assigned photoaging scores based on these, each participant completed a self-assessment questionnaire, and adverse events were recorded. At the 6-month follow-up in addition to these procedures, participants completed an overall satisfaction questionnaire; in the event of an uneven contralateral outcome, participants were offered optional standard-of-care treatments for correction. At 12 months, a telephone follow-up was conducted with the participant to determine if he or she had any adverse events to report.
Outcomes
Primary outcome measures were photoaging scores for each cheek, as rated by 2 masked dermatologists at baseline, 2 weeks, 3 months, and 6 months, with consensus by forced agreement. Individual subscores were provided by the independent masked dermatologist assessors (M.A. and K.P.) for each time point as follows: fine lines (ranging from 0 [none] to 4 [many]), mottled pigmentation (ranging from 0 [even pigment] to 4 [marked hypopigmentation or hyperpigmentation]), roughness (ranging from 0 [smooth] to 4 [severe roughness]), and sallowness (ranging from 0 [pink] to 4 [most yellow]).
Secondary outcomes included participant self-assessment scores of each cheek at 2 weeks, 3 months, and 6 months, whereby change in irregular pigmentation, skin texture, and wrinkles was rated on a 5-point scale designed to rate response, with 0 indicating worsening, 1 indicating no change, 2 indicating mild improvement, 3 indicating moderate improvement, and 4 indicating significant improvement. Participant overall satisfaction scores were reported at 6 months for each cheek on a 4-point scale, with 1 indicating not satisfied, 2 indicating slightly satisfied, 3 indicating moderately satisfied, and 4 indicating very satisfied. Adverse events at any time point reported by participants or observed by investigators were also recorded.
Sample Size
For the primary end point of the difference between PRP and normal saline in change at 2 weeks for any of the 4 photoaging subscores, a sample of 30 sites (15 individuals) would provide 80% power to detect an effect size of 0.85 at a type I error rate of 1.2% (adjusted for multiple comparisons) using the Wilcoxon signed rank test. The same effect size would be detected in comparing change from 2 weeks to 3 months to 6 months to determine if any change was sustained.
Randomization
The randomization sequence was generated using a computerized random number generator. The allocation concealment mechanism was designed so that the person generating the random allocation sequence (E.P.) was contacted via telephone by the person who was conducting the study visit (R.H., A.C., or A.G.) before each assignment, at which point one of us (E.P.) accessed the computer to obtain and then convey the assignment.
Masking
In this randomized clinical trial, participants and those assessing outcomes (dermatologist raters M.A. and K.P.) were masked to assignments for study interventions. Care providers (including injector D.P.W.) were not masked. To ensure that masking was successful, the interventions were as similar as possible: similar volumes of PRP and normal saline were injected at similar depth and with similar lateral spacing into cheek areas of similar size using similar syringes and needles.
Statistical Analysis
Photoaging scores, participant self-assessment scores, and participant overall satisfaction scores were analyzed using the Wilcoxon signed rank test. The threshold of statistical significance was 2-sided P = .05.
Results
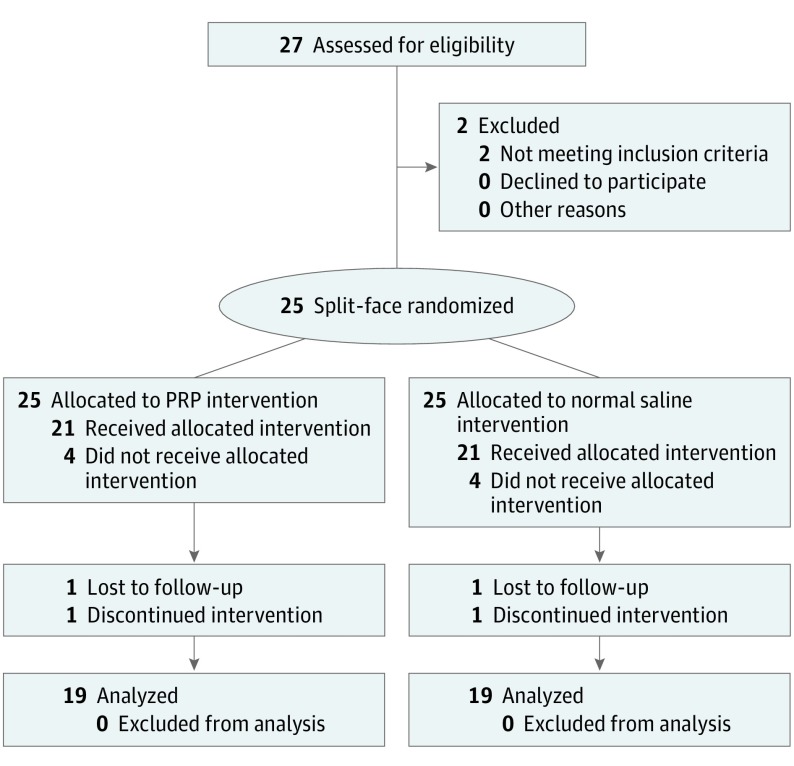
The duration of the study spanned August 21, 2012, to February 16, 2016. Of 27 participants enrolled in the study, 8 were discontinued from study participation (4 were lost to follow-up after screening and enrollment, 2 failed screening because of ≥1 abnormal laboratory result, 1 was lost to follow-up after study injections, and 1 withdrew because of intolerance to injections), and 19 completed the study per protocol and were analyzed for primary and secondary outcome measures (Figure). Reported adverse events were not significantly associated with either PRP or normal saline and included localized injection site reactions (redness [n = 18], swelling [n = 16], bruising [n = 14], pruritus [n = 1], skin scaling [n = 1], and dryness of skin [n = 1]), which all resolved in less than 2 weeks after study injections. No participants reported any adverse events at the 12-month follow-up telephone call. Participant demographics are listed in Table 1. Photoaging scores rated by 2 masked dermatologist photograph raters are listed in (Table 2). Participant self-assessment scores are listed in Table 3. The mean (SD) participant overall satisfaction scores at 6 months were 2.00 (0.88) for PRP and 1.47 (0.77) for normal saline (mean difference, 0.53; P = .06).
Figure. CONSORT Diagram of Participant Recruitment and Flow.
CONSORT indicates Consolidated Standards of Reporting Trials; PRP, platelet-rich plasma.
Table 1. Participant Demographics.
| Variable | Value |
|---|---|
| Total participants | 19 |
| Age, mean (SD), y | 46.37 (10.88) |
| Sex, No. (%) | |
| Male | 2 (11) |
| Female | 17 (89) |
| Race, No. (%) | |
| White | 16 (84) |
| Black | 1 (5) |
| Other | 2 (11) |
| Ethnicity, No. (%) | |
| Not Hispanic or Latino | 15 (79) |
| Other | 4 (21) |
Table 2. Photoaging Scores as Rated by 2 Masked Dermatologist Photograph Raters Comparing Injections of PRP With Injections of Normal Saline Over Time.
| Variable | Mean (SD) Photoaging Score | |||||
|---|---|---|---|---|---|---|
| Baseline | 2 wk | 3 mo | 6 mo | Baseline vs 6 mo | ||
| Mean Difference | P Value | |||||
| Fine Lines | ||||||
| PRP | 1.00 (0.75) | 0.95 (0.71) | 0.95 (0.71) | 0.95 (0.71) | 0.05 | >.99 |
| Normal saline | 1.05 (0.78) | 0.95 (0.71) | 0.95 (0.71) | 0.95 (0.71) | 0.11 | .50 |
| PRP vs normal saline | ||||||
| Mean difference | −0.05 | 0 | 0 | 0 | NA | NA |
| P value | >.99 | NA | NA | NA | NA | NA |
| Mottled Pigmentation | ||||||
| PRP | 1.21 (0.53) | 1.16 (0.60) | 1.00 (0.47) | 1.16 (0.69) | 0.05 | >.99 |
| Normal saline | 1.21 (0.54) | 1.16 (0.60) | 1.11 (0.46) | 1.16 (0.69) | 0.05 | >.99 |
| PRP vs normal saline | ||||||
| Mean difference | 0 | 0 | −0.11 | 0 | NA | NA |
| P value | NA | NA | >.99 | NA | NA | NA |
| Roughness | ||||||
| PRP | 0.47 (0.61) | 0.47 (0.61) | 0.47 (0.61) | 0.37 (0.60) | 0.11 | .50 |
| Normal saline | 0.47 (0.61) | 0.47 (0.61) | 0.47 (0.61) | 0.37 (0.68) | 0.11 | .50 |
| PRP vs normal saline | ||||||
| Mean difference | 0 | 0 | 0 | 0 | NA | NA |
| P value | NA | NA | NA | NA | NA | NA |
| Sallowness | ||||||
| PRP | 1.11 (0.88) | 0.95 (0.85) | 0.58 (0.61) | 0.37 (0.68) | 0.74 | .002 |
| Normal saline | 1.11 (0.88) | 0.95 (0.85) | 0.58 (0.61) | 0.37 (0.68) | 0.74 | .002 |
| PRP vs normal saline | ||||||
| Mean difference | 0 | 0 | 0 | 0 | NA | NA |
| P value | NA | NA | NA | NA | NA | NA |
Abbreviations: PRP, platelet-rich plasma; NA, not applicable.
Table 3. Participant Self-assessment Scores as Rated by Participants Comparing Injections of Platelet-Rich Plasma (PRP) With Injections of Normal Saline Over Time.
| Variable | Mean (SD) Participant Self-assessment Score | ||
|---|---|---|---|
| 2 wk | 3 mo | 6 mo | |
| Pigmentation | |||
| PRP | 1.47 (0.84) | 1.68 (1.16) | 1.84 (1.12) |
| Normal saline | 1.31 (0.67) | 1.47 (0.84) | 1.21 (0.63) |
| PRP vs normal saline | |||
| Mean difference | 0.16 | 0.21 | 0.63 |
| P value | .75 | .53 | .07 |
| Texture | |||
| PRP | 1.79 (0.92) | 1.95 (1.08) | 2.00 (1.20) |
| Normal saline | 1.58 (0.77) | 1.68 (0.89) | 1.21 (0.54) |
| PRP vs normal saline | |||
| Mean difference | 0.21 | 0.26 | 0.79 |
| P value | .50 | .33 | .02 |
| Wrinkles | |||
| PRP | 1.53 (0.70) | 1.63 (0.96) | 1.74 (0.99) |
| Normal saline | 1.58 (0.84) | 1.37 (0.83) | 1.21 (0.54) |
| PRP vs normal saline | |||
| Mean difference | −0.05 | 0.26 | 0.53 |
| P value | >.99 | .38 | .03 |
| Telangiectasias | |||
| PRP | 1.42 (0.77) | 1.53 (1.07) | 1.63 (0.96) |
| Normal saline | 1.21 (0.54) | 1.47 (1.02) | 1.11 (0.46) |
| PRP vs normal saline | |||
| Mean difference | 0.21 | 0.05 | 0.53 |
| P value | .53 | .97 | .06 |
Mean (SD) photoaging scores, independently rated by the 2 dermatologists, showed no significant difference between PRP and normal saline for all clinical variables, including subscores for fine lines (baseline, 1.00 [0.75] vs 1.05 [0.78]; 2 weeks, 0.95 [0.71] vs 0.95 [0.71]; 3 months, 0.95 [0.71] vs 0.95 [0.71]; 6 months, 0.95 [0.71] vs 0.95 [0.71]), mottled pigmentation (baseline, 1.21 [0.53] vs 1.21 [0.54]; 2 weeks, 1.16 [0.60] vs 1.16 [0.60]; 3 months, 1.00 [0.47] vs 1.11 [0.46]; 6 months, 1.16 [0.69] vs 1.16 [0.69]), roughness (baseline, 0.47 [0.61] vs 0.47 [0.61]; 2 weeks, 0.47 [0.61] vs 0.47 [0.61]; 3 months, 0.47 [0.61] vs 0.47 [0.61]; 6 months, 0.37 [0.60] vs 0.37 [0.68]), and sallowness (baseline, 1.11 [0.88] vs 1.11 [0.88]; 2 weeks, 0.95 [0.85] vs 0.95 [0.85]; 3 months, 0.58 [0.61] vs 0.58 [0.61]; 6 months, 0.37 [0.68] vs 0.37 [0.68]) (Table 2).
Overall, participant self-assessment scores of improvement for pigmentation, texture, wrinkles, and telangiectasias were higher for PRP compared with normal saline at 6 months after injections, when participants rated the PRP-treated side as significantly more improved compared with the normal saline side for texture (mean [SD] self-assessment score, 2.00 [1.20] vs 1.21 [0.54]; P = .02) and wrinkles (mean [SD] self-assessment score, 1.74 [0.99] vs 1.21 [0.54]; P = .03). The PRP treatments for pigmentation and telangiectasias were both nominally but not significantly improved compared with normal saline at 6 months (Table 3).
Discussion
Although this randomized clinical trial did not result in significant improvement through assessment of standardized photographs of photoaged cheek skin treated with injections of PRP compared with normal saline, the participants themselves rated the PRP-treated side to be more improved, with significant improvement in both texture and wrinkles and a suggestion toward significant improvement in both pigmentation and telangiectasias. Participant overall satisfaction was also greater for PRP compared with normal saline. Participants, like the photograph raters, were also masked to minimize bias. While this was not designed to be a safety study, no serious adverse events were observed, and recorded skin effects were localized to the injection sites, were transient, and were fully remitting in the short term.
The results of this study suggest that PRP for facial rejuvenation may at least temporarily improve the visual appearance of photoaged skin. Improvements may be subtle and difficult to detect for external raters relying on photographs, which may contain less information than live viewing. Participants may have been able to see differences more clearly because they knew their faces intimately and in great detail and had time to scrutinize their appearance at length and in close-up view (eg, by using magnification mirrors).
Methodologically, this randomized clinical trial was designed to minimize confounders. A randomized controlled design was used; in addition, the sham control was designed to be similar to the active treatment. Because of this design, it was possible to mask both participants and raters. Participant-reported outcomes, which have increasingly displaced more paternalistic investigator ratings,8 were thus valuable in this study because they were untainted by knowledge of which treatment had been received. The study was also adequately powered by using a split-face design to further limit interindividual differences. Finally, the study looked at outcomes as far out as 6 months to ensure that detected beneficial effects were not merely the result of possible confounding issues, such as transient wound healing and posttreatment edema obscuring wrinkles, smoothing texture, and concealing pigmentary abnormalities.
Limitations
A limitation to this study is that several participants terminated early, likely because of the follow-up visits required after a single treatment session visit. Furthermore, it could be argued that, instead of a single session only, multiple sessions of PRP injections to the targeted skin sites might have produced a greater and even cumulative benefit. The split-face design, while helpful in allowing patients to self-compare treatment with control, may theoretically have decreased the difference between treatment and control to the extent that PRP had a remote effect even to the contralateral side of the face. There is no evidence for this last limitation because participants were clearly able to detect and report differences between both sides.
Compared with prior work reported for PRP and facial rejuvenation, this study has reduced bias by design. Earlier work had predominantly looked only at the skin of those treated with PRP, with no comparator or control.5 Also, measurements have often not been obtained by masked participants or investigators. Moreover, there has been a reliance on intermediate outcome measures, such as biochemical markers, histologic features, skin elasticity, and other clinical or physiological features detected by devices, rather than the visual appearance of skin features as rated by humans.4 The present study was designed specifically to correct for these deficiencies. As has been suggested,9 a criterion standard for aesthetic procedures is visible change noticeable by the patient.
Conclusions
This is, to our knowledge, the first study to date of participants in a randomized clinical trial who did not know if they received PRP or normal saline, and the findings suggest that PRP may have benefit for reducing the visible signs of photoaging, particularly because they were detectable by the participants themselves 6 months after a single treatment session. It remains to be seen how long the benefit of such treatment may last and to what extent repeat sessions of PRP administration can maximize the extent and/or persistence of effect. Larger studies are warranted to also enable subgroup analyses that show which particular types of patients may see the best results. In the meantime, it is reassuring that some of the optimism reported for earlier open-label studies has now been borne out in a controlled investigation.
Trial Protocol
eAppendix. Supplementary Appendix
Data Sharing Statement
References
- 1.Kim H, Gallo J. Evaluation of the effect of platelet-rich plasma on recovery after ablative fractional photothermolysis. JAMA Facial Plast Surg. 2015;17(2):97-102. doi: 10.1001/jamafacial.2014.1085 [DOI] [PubMed] [Google Scholar]
- 2.de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149. doi: 10.1001/jama.2009.1986 [DOI] [PubMed] [Google Scholar]
- 3.Kramer ME, Keaney TC. Systematic review of platelet-rich plasma (PRP) preparation and composition for the treatment of androgenetic alopecia [published online May 22, 2018]. J Cosmet Dermatol. [DOI] [PubMed] [Google Scholar]
- 4.Gawdat HI, Tawdy AM, Hegazy RA, Zakaria MM, Allam RS. Autologous platelet-rich plasma versus readymade growth factors in skin rejuvenation: a split face study. J Cosmet Dermatol. 2017;16(2):258-264. doi: 10.1111/jocd.12341 [DOI] [PubMed] [Google Scholar]
- 5.Charles-de-Sá L, Gontijo-de-Amorim NF, Takiya CM, et al. Effect of use of platelet-rich plasma (PRP) in skin with intrinsic aging process. Aesthet Surg J. 2018;38(3):321-328. doi: 10.1093/asj/sjx137 [DOI] [PubMed] [Google Scholar]
- 6.Cabrera-Ramírez JO, Puebla-Mora AG, González-Ojeda A, et al. Platelet-rich plasma for the treatment of photodamage of the skin of the hands [in Spanish]. Actas Dermosifiliogr. 2017;108(8):746-751. doi: 10.1016/j.ad.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 7.Cameli N, Mariano M, Cordone I, Abril E, Masi S, Foddai ML. Autologous pure platelet-rich plasma dermal injections for facial skin rejuvenation: clinical, instrumental, and flow cytometry assessment. Dermatol Surg. 2017;43(6):826-835. doi: 10.1097/DSS.0000000000001083 [DOI] [PubMed] [Google Scholar]
- 8.Reid EE, Haley AC, Borovicka JH, et al. Clinical severity does not reliably predict quality of life in women with alopecia areata, telogen effluvium, or androgenic alopecia. J Am Acad Dermatol. 2012;66(3):e97-e102. doi: 10.1016/j.jaad.2010.11.042 [DOI] [PubMed] [Google Scholar]
- 9.Klassen AF, Cano SJ, Schwitzer JA, et al. Development and psychometric validation of the FACE-Q skin, lips, and facial rhytids appearance scales and adverse effects checklists for cosmetic procedures. JAMA Dermatol. 2016;152(4):443-451. doi: 10.1001/jamadermatol.2016.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Supplementary Appendix
Data Sharing Statement