Abstract
BioID has become an increasingly utilized tool for identifying candidate protein-protein interactions (PPIs) in living cells. This method utilizes a promiscuous biotin ligase, called BioID, fused to a protein-of-interest that when expressed in cells can be induced to biotinylate interacting and proximate proteins over a period of hours, thus generating a history of protein associations. These biotinylated proteins are subsequently purified and identified via mass spectrometry. Compared to other conventional methods typically used to screen strong PPIs, BioID allows for the detection of weak and transient interactions within a relevant biological setting over a defined period of time. Here we briefly review the scientific progress enabled by the BioID technology, detail an updated protocol for applying the method to proteins in living cells, and offer insights for troubleshooting commonly encountered setbacks.
Keywords: Protein-protein interactions, Proximity-dependent labeling, BioID, Biotinylation, Biotin Ligase
1. Introduction
Enzyme-mediated ligation methods can be used for several distinct purposes including building peptide/protein assemblies (1,2), making site-specific protein modifications (3,4), and elucidating protein-protein interactions (PPIs) (see (5) for review). Proteins regularly form complex and specific networks critical to the structural and functional integrity of the cell. PPIs play an integral role for maintaining cell integrity. They are characterized by proximity, affinity, and duration (6), and understanding PPIs helps reveal protein function, biological mechanisms, and corresponding roles in the progression of disease. BioID (proximity-dependent biotin identification) has become an established tool for identifying candidate proteins within a specific protein’s interactome, or to identify protein constituents of a specific cellular subcompartment. The BioID method conventionally utilizes a mutant of the Escherichia coli biotin protein ligase BirA (7). In its natural environment, wild-type BirA specifically binds and biotinylates the biotin carboxyl carrier protein subunit of acetyl-CoA carboxylase by forming an amide bond between a reactive biotin and a specific lysine residue on the protein surface. However, a mutated form of BirA (R118G), hereafter termed BioID, overcomes this specificity and behaves as a promiscuous biotin ligase (8–11). This mutation causes decreased affinity for and premature release of the reactive biotin-intermediate biotinoyl-adenylate (bioAMP) from the catalytic site allowing the promiscuous biotinylation of proximate proteins (7,10,11). The fusion of BioID to a protein-of-interest (POI) allows for the promiscuous biotinylation of proteins interacting with or proximate to the POI which can subsequently be isolated and identified via mass spectrometry (MS) (Figure 1) (7).
Figure 1.
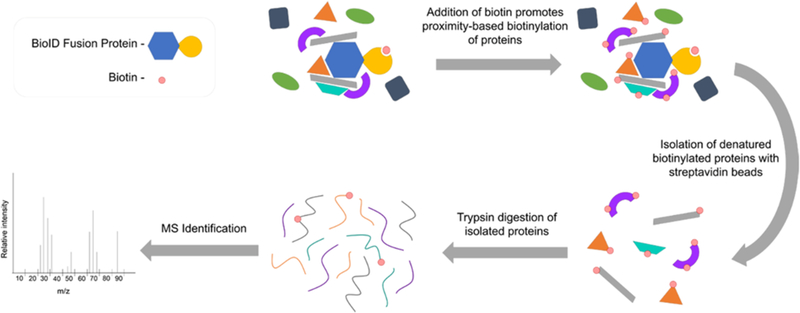
Overview of the BioID method. The BioID fusion protein induces biotinylation of proteins in a proximity-dependent manner. Biotinylated proteins are denatured followed by affinity-isolation using streptavidin-coupled beads. After subsequent tryptic digest, the isolated proteins are identified by MS analysis.
Since its development and initial application in 2012 (7), BioID has been cited and/or applied in over 200 PubMed-published articles and has been utilized in several unicellular organisms (see (12–15) for examples), mammalian cells (5), plants (16,17), and mice (18–20), including compartmental proteomics of a parasitic organism infecting mice (21). Novel or improved applications using BioID ligase have spurred numerous follow-up articles including a smaller version of BioID with improved sensitivity and localization (22), its use for identifying protein-RNA interactions (23), split-BioID studies (24,25), and faster versions of BioID (26). Several conventional approaches have been utilized to stably introduce BioID-fusion proteins to cells including transfection (7), viral infection (27), and more recently, CRISPR-Cas9 (28). A more sensitive approach to identify biotinylated peptides has also been developed using antibodies to isolate biotinylated peptides following tryptic digest of lysates after BioID labeling (29,30). This approach can identify the specific sites of biotinylation in order to inform on protein structure and proximity between proteins. BioID can also be used to map the protein constituents of individual subcellular compartments by utilizing either a minimal targeting motif or a full-length resident protein to screen a specific subcellular region (27). Due to its wide range of demonstrated applications and the ever-growing list of modifications and improvements for novel applications, BioID is an attractive method for identifying PPIs and mapping the constituency of subcellular compartments.
Current methods for identifying PPIs include yeast two-hybrid, immunoprecipitation, BioID and APEX (engineered ascorbate peroxidase) (5). More recently, a new enzyme-mediated, proximity-based labeling method termed pupylation-based interaction tagging (PUP-IT) was developed (31). Each method has its own advantages and disadvantages, with one of the greatest benefits to BioID being the ability to detect weak and transient interactions between proteins over a period of time. However, due to the promiscuity of the ligase, it may not only biotinylate proteins interacting with the bait protein, but also proximate proteins which may not physically interact with the BioID-fused POI. The labeling radius of BioID has been measured to be approximately 10 nm (32), one of the strictest experimentally validated radii compared to other labeling methods (6). The use of a BioID-only control will help identify background proteins that have affinity to the ligase in addition to or instead of to the POI. Other benefits of the method include temporal control mediated by necessary biotin supplementation, lack of toxicity, and the ability to screen interactions in vivo or in a cellular environment of choice. Further, due to the inherent inducibility of the method, BioID permits comparative screens employing differentiation and/or drug treatment methods. Naturally, the appropriate targeting and functionality of the fusion protein is critical to the success of BioID, and certain tags and bulky fusions can interfere with crucial protein properties when expressed in cells (7). BioID is a 321 amino acid (aa) addition to the POI, slightly larger than the Green Fluorescent Protein (GFP), and has some potential to influence inappropriate localization or function of the fusion protein. To alleviate these potential complications, a smaller biotin ligase from Aquifex aeolicus (233 aa) was humanized and mutated (R40G) to create BioID2 (22). BioID2 was shown to improve localization and was more sensitive to a lower biotin concentration, potentially allowing for BioID to be introduced to new systems where biotinylation supplementation may not be easily accomplished. Unless specified, the use of the term BioID will refer to both forms of ligases when mentioned in this chapter.
Biotin supplementation, typically between 10–50 μM, is needed to induce significant biotinylation of proximate proteins allowing temporal control of the BioID labeling period (7). The optimal length of incubation with biotin supplementation ranges between 15–18 hours, after which total biotinylation plateaus (7). Due to the formation of a covalent bond between biotin and lysine residues on a protein, biotinylation appears to remain for the life of the protein (10) and can withstand stringent lysis and wash conditions, maximizing the purity of the isolated proteins that are sent for identification by MS (5). The identified proteins can then be investigated as candidate interactors with the POI.
Biotinylated proteins resulting from the use of BioID fall under three categories: (i) direct interactions (transient or stable), (ii) indirect interactions, or (iii) proximal proteins that do not interact directly or indirectly (7). Teasing out relevant identifications from the MS data can appear daunting, but the use of proper controls and the ability to perform rigorous statistical analysis when performing these experiments in triplicate can allow for the identification of even low-abundance and weak interactors (for example (33,34)). The following protocol details stable cell line validation and pulldown steps for BioID in living cells, along with troubleshooting tips, explanations, and recommendations for the successful execution and analysis of BioID experiments.
2. Materials
Prepare all solutions using ultrapure water (ddH2O; prepared by purifying deionized water, to attain a sensitivity of 18 MΩ-cm at 25 °C).
2.1. Fusion-Protein Validation in vitro
Target cells and appropriate medium (e.g. HeLa cells and DMEM +10% fetal bovine serum) (see Note 1).
General PCR reagents and equipment, e.g. thermocycler, polymerase, primers, template DNTPs.
Template for the expression of the POI, e.g. cDNA.
10 cm cell culture dishes.
6-well cell culture plates.
General transfection reagents, e.g. Lipofectamine 3000.
Coverslips.
Biotin Stock Solution (20x): 1 mM biotin in ddH2O. Dissolve 12.2 mg biotin in 50 mL serum-free cell culture medium. Vortex as needed in order to properly dissolve biotin. Filter sterilize solution through 0.22 µM filter unit and store at 4°C for up to 8 weeks.
Fixative solution: 3% para-formaldehyde (PFA) fixative in ddH2O (see Note 4).
Phosphate buffered saline (PBS; 1x).
PBST buffer solution: 1x PBS with 0.4% Triton X-100.
Primary antibody against appropriate tags (e.g. anti-myc/HA) or chicken anti-BioID (BID-CP-100, BioFront)/anti-BioID2 (BID2-CP-100, BioFront).
Appropriate secondary antibodies for detection via immunofluorescence (Alexa Fluors) and/or via western blot using horse-radish peroxidase (HRP).
Streptavidin-conjugated Alexa Fluors (Invitrogen) and HRP (AB7403, Abcam).
DNA labeling stain, e.g. 4ʹ,6-diamidino-2-phenylindole (DAPI) (Hoechst).
SDS-PAGE sample buffer: 50 mM Tris·HCl, 12% (w/v) sucrose, 2% (v/v) SDS, 0.004% (w/v) bromophenol blue, 20 mM dithiothreitol (DTT), pH 6.8. Prepare sample buffer freshly every day.
Adult bovine serum (ABS) blocking buffer: 10% (v/v) ABS in 1% (w/v) Triton X-100 in PBS. Blocking buffer can be stored at 4°C for up to 4 weeks.
Tris buffer solution: 1 M Tris·HCl, pH 8.5.
30% hydrogen peroxide (H2O2) solution in ddH2O.
Enhanced chemiluminescence (ECL) reagent solution 1: in a 15 mL tube, add 1 mL 1 M Tris·HCl, pH 8.5, 45 μL coumaric acid, 100 μL luminol, and 8.9 mL H2O. Wrap in tin foil to protect from light. Solution can be stored at 4°C for up to 1 month.
ECL reagent solution 2: in 15 mL tube, add 1 mL 1 M Tris·HCl, pH 8.5, 6 μL 30% H2O2 solution and 9 mL H2O. Solution can be stored at 4°C for up to 1 month.
Sonicator, e.g. Branson Sonifier-250 or equivalent.
General reagents and equipment for western blot experiments, i.e. SDS-PAGE running apparatus, SDS-PAGE running buffer, SDS-PAGE transfer apparatus, SDS-PAGE transfer buffer, chemidoc imaging system.
2.2. BioID Pulldown
Two fully confluent 10 cm dishes of cells stably expressing each BioID construct (i.e. BioID-only control and BioID-POI – see Notes 5 & 6).
1 mM Biotin stock solution (recipe see above).
PBS (1x).
Lysis Buffer : 8 M urea in 50 mM Tris pH 7.4 with 1x Protease inhibitor (Halt Protease Inhibitor Cocktail, EDTA-Free, Thermo Scientific, 87785; 100 mM AEBSF·HCl (4-(2-Aminoethyl)benzenesulfonyl fluoride hydrochloride), 80 µM aprotinin, 5 mM bestatin, 1.5 mM E-64, 2 mM Leupeptin, 1 mM Pepstatin A) and 1 mM DTT. Prepare buffer freshly immediately before use – see Note 7).
Cell scrapers.
DNase/RNase-free tubes – 5 mL, 2 mL, and 1.5 mL.
Tube cap opener (see Note 8).
Pierce Universal Nuclease (Thermo, 88700).
Triton X-100 solution: 20% (v/v) Triton X-100 in ddH2O.
Gelatin-conjugated Sepharose 4B (GE Healthcare, 17095601).
Streptavidin Sepharose High Performance Beads (GE Healthcare, 17511301).
Sonicator (Branson Sonifier-250 or equivalent).
Wash Buffer: 8 M urea in 50 mM Tris pH 7.4. Prepare buffer fresh before use.
Bench-top rotator (e.g. Thermo Labquake).
SDS-PAGE sample buffer: 50 mM Tris·HCl, pH 6.8, 12% (w/v) sucrose, 2% (v/v) SDS, 0.004% (w/v) bromophenol blue, 20 mM DTT. Prepare sample buffer freshly every day.
SDS-PAGE sample buffer with 1 mM biotin: 50 mM Tris·HCl, pH 6.8, 12% (w/v) sucrose, 2% (v/v) SDS, 0.004% (w/v) bromophenol blue, 20 mM DTT, 1 mM biotin. Prepare sample buffer freshly every day.
Ammonium bicarbonate-biotin buffer: 50 mM ammonium bicarbonate with 1 mM biotin.
3. Methods
3.1. BioID Stable Cell Line Validation
Cells that stably express BioID fusion proteins need to be validated for fusion protein expression levels, subcellular localization, and capacity to biotinylate proximal proteins.
Use established cloning methods to fuse the POI in-frame with BioID in an appropriate expression vector (see Note 9).
Create stable cell lines expressing low-levels of BioID-only or BioID-POI (see Note 2) via desired method (e.g. viral transduction).
Plate two wells of a 6 well plate for each construct – one with a glass coverslip (for immunofluorescence) and one without (for western blot analysis).
-
To each well, add biotin to final concentration of 50 µM (from the biotin stock solution) and incubate cells overnight (16–18 h) under conditions optimal for the respective cell line (see Note 10). Always include a negative control (parental cell line) and a positive control (BioID-only).
Confirm expression and localization of BioID-POI, along with properly localized promiscuous biotinylation by immunofluorescence (Figure 2A).
Fix and permeabilize cells on coverslips. Briefly wash cells with PBS. Add fixative solution and allow cells to fix for 10 mins at room temperature (RT).
Remove fixative solution, add PBST and allow cells to incubate and permeabilize for 15 mins at RT.
Probe cells with anti-BioID/BioID2 antibody (or appropriate tag antibody) for 20 min.
-
Wash 3 times with PBS. Probe with corresponding secondary Alexa Fluor, streptavidin-conjugated Alexa Fluor, and DNA labeling stain (e.g. Hoechst, DAPI). Mount coverslips onto microscope slides for viewing.
Confirm expression of BioID-POI along with promiscuous biotinylation by Western Blot analysis (Figure 2B).
Briefly wash cells in other 6 well plate with PBS. For adherent cells, add 1 mL PBS to well and aspirate. For suspension cells, spin cells down in a 15 mL conical tube at 250 x g for 5 minutes, resuspend cells in 1 mL PBS, and spin cells down again at 250 x g for 5 minutes.
Add SDS-PAGE sample buffer for lysis. Use 200 µL for one million cells, or adjust accordingly. Lysate should be viscous.
Collect lysate in an 1.5 mL Eppendorf tube and heat to 98°C for 5 min to denature proteins.
Sonicate each sample to shear DNA using a Branson Sonifier 250 (or equivalent) for 30 seconds at 30% duty cycle and Output Level 3.
Run lysate on SDS-PAGE gel to separate proteins. Recommended settings of 120 V for 60 minutes.
Transfer proteins onto a PVDF/nitrocellulose membrane. Standard conditions for tank blotting are 100 V for 45 mins.
Probe membrane with Streptavidin-HRP (1:10000, AB7403) in PBST for one hour at room temperature.
Wash membrane twice with 5mL PBS, at least 5 minutes per wash.
Incubate membrane in ABS blocking buffer for 5 minutes to soak up residual streptavidin-HRP.
Wash membrane once with PBS.
Aliquot and combine enhanced chemiluminescence solutions 1 and 2 (1:1, v/v), then immediately add to blot membrane prior to exposure to film or ChemiDoc imaging to capture western blot image observe biotinylated proteins (Figure 2B) (see Note 11).
Quench HRP activity of the chemiluminescence solution by incubating blot membrane in 30% H2O2 for 20 minutes at room temperature (see Note 12). Rinse residual H2O2 away with two subsequent PBS washes and block membrane in ABS blocking buffer for 30 minutes.
Probe membrane with appropriate antibody to visualize BioID-POI expression and migration (Figure 2B). Compared to a control lane there should be a clear band of the expected MW for the BioID-fusion protein.
Figure 2.
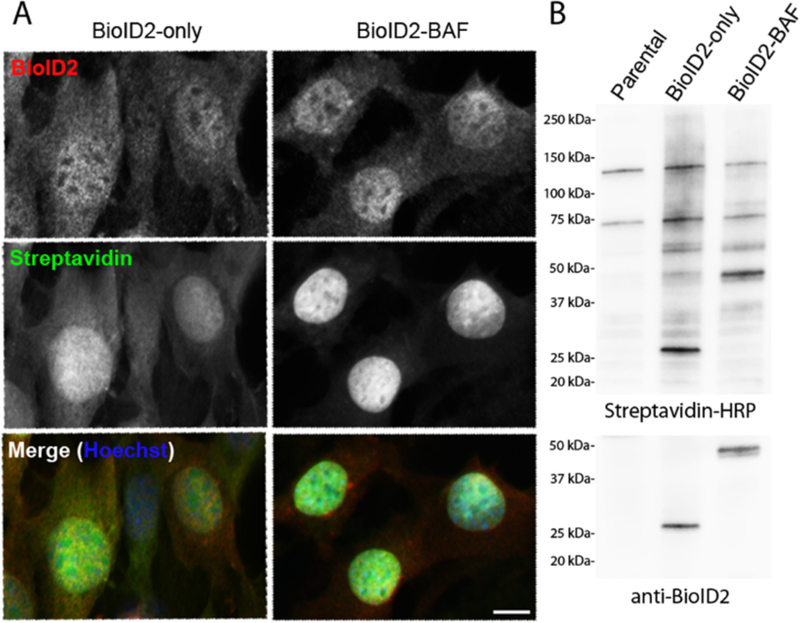
Representation of stable BioID-fusion protein expression in cells. NIH3T3 cells stably expressing BioID2-only or a BioID-fusion protein of interest (BioID2-BAF) were analyzed for fusion protein expression, localization and biotinylation by fluorescence microscopy and immunoblot. A) Using florescent microscopy, fusion proteins were detected by anti-BioID2 antibody (red) and biotinylation was detected by streptavidin-488 Alexa Fluor (green). Scale bar = 10 µm. B) Biotinylation efficiency and fusion protein expression in whole cell lysate were also assessed by western blot, using streptavidin-HRP and anti-BioID2 antibody.
3.2. BioID Pulldown
In order to identify proteins by MS that are proximate to the BioID fusion proteins the biotinylated proteins must be isolated from the cells.
3.2.1. Cell Lysis and Protein Denaturation
In order to isolate biotinylated proteins, the cells must first be lysed and the proteins denatured.
For each condition (i.e. BioID2-only control and BioID2-POI), plate cells on at least two 10 cm plates. When performing BioID in triplicate, plate six 10 cm plates per construct (see Note 13).
When cells are ~80% confluent, replace media and add biotin to a final concentration of 50 µM from the biotin stock solution. Incubate overnight (16–18 h) under conditions optimal for the respective cell line.
Wash cells twice with 2 mL PBS to remove excess biotin. After the second wash, tilt plates and allow excess PBS to accumulate at the bottom of the plate for one minute. Aspirate excess PBS.
At RT, lyse cells by adding 540 µL lysis buffer to each plate. Using a cell scraper, collect cells into a 15 mL conical tube. Combine the lysed cells from the two 10 cm plates.
Add 0.1 µL Pierce Universal Nuclease to each sample, vortex briefly, and incubate for 10 minutes at RT.
Add 60 µL 20% Triton X-100 solution (final concentration 1%).
From this point forward, keep cells on ice. Sonicate each sample for 30 seconds at 30% duty cycle and Output Level 3.
Pause for one minute, allow the lysate to cool on ice, then repeat step 5 (see Note 14).
Repeat steps 7 and 8, for a total of three rounds of sonications for 30 seconds each.
Add 1260 µL lysis buffer and sonicate for one minute.
Evenly distribute the sample into two 2 mL Eppendorf tubes (~1200 µL each)
Centrifuge samples at 16500 x g for 10 minutes at 4°C.
Transfer the supernatant to a fresh 5 mL tube (see Note 15).
3.2.2. Pre-Clearing Samples
A pre-clearing step is performed to help remove proteins that will non-specifically stick to other proteins or sepharose beads.
Wash 200 µL of gelatin sepharose beads in 500 µL lysis buffer in a 1.5 mL Eppendorf tube.
Centrifuge beads at 800 x g for 3 minutes.
Remove supernatant and wash the beads again with 500 µL lysis buffer.
Repeat step 2 and discard the supernatant.
Resuspend beads with 1 mL sample and transfer bead/sample mixture to sample tube.
Incubate on a rotator for 2 hours at 4°C.
Centrifuge at 800 x g for 5 minutes at 4°C.
Avoid disturbing the gelatin beads and transfer pre-cleared supernatant to a new 5 mL tube.
3.2.3. Streptavidin Incubation
To isolate biotinylated proteins the cell lysate is incubated with streptavidin-conjugated sepharose beads.
3.2.4. Bead-Washing
To remove proteins that are not tightly bound to the streptavidin sepharose the beads are washed.
Centrifuge at 800 x g for 5 minutes at 4°C.
Remove and discard supernatant.
Resuspend beads in 1 mL wash buffer and transfer to new 1.5 mL Eppendorf tube.
Wash the beads on a rotator at room temperature for 8 minutes.
Centrifuge at 800 x g for 3 minutes.
Discard the supernatant.
Repeat wash steps 3–6 three more times - transferring beads to a fresh tube for each wash - for a total of four washes.
Resuspend the beads in 1 mL wash buffer.
Transfer 100 µL to a new 1.5 mL Eppendorf tube for further analysis by western blot.
For western blot analysis: centrifuge at 800 x g for 3 minutes. Discard supernatant and resuspend beads with 100 µL SDS-PAGE Sample Buffer containing 1 mM biotin. Continue with 3.2.5 step 1.
Centrifuge the tube with the remaining 900 µL of beads in wash buffer at 800 x g for 3 minutes.
Discard supernatant and resuspend beads in 50 µL 50 mM ammonium bicarbonate containing 1 mM biotin (Ammonium bicarbonate-biotin buffer) (see Note 17).
Flash freeze in liquid nitrogen and store at −80°C until ready to ship for MS analysis.
3.2.5. Post-Pulldown Western Blot Analysis
Analyze isolated biotinylated proteins via western blotting by loading 10–15 µL of each sample, following standard western blot running/transfer protocol, and probing the membrane with streptavidin-HRP as described above in 3.1 step 12–19.
If substantial biotinylation is detected, samples may be sent for MS analysis (see Note 18). Generally, there should be considerable levels of biotin labeling in lanes expressing BioID fusion proteins compared to control cells those cells that do not express BioID fusion proteins.
3.2.6. Analysis of BioID Mass Spectrometry Data
To identify proteins that have been isolated by the BioID pulldown, protein MS is utilized.
Generally, biotinylated proteins are cleaved off of the streptavidin beads by tryptic digestion prior to MS analysis (See Note 19).
Use BioID-only control data to remove background proteins from candidate list by comparing label-free quantification (LFQ) intensities and removing known contaminant proteins (see Note 20).
4. Notes
For optimal BioID conditions, cells should be grown at 37°C in cell culture media that does not contain biotin supplementation in excess of what is normally present in 10% serum. Culturing BioID cell lines in cell culture media with biotin (e.g. RPMI 1640) can result in constant biotinylation.
BioID/BioID2 expression vectors are available through the non-profit plasmid repository Addgene at www.addgene.com/Kyle_Roux.
Fusion-protein validation can be performed using transient expression of an expression vector with a strong promoter (e.g. CMV), if desired. However, stable low-level expression avoids mislocalization of the fusion proteins, which is optimal for reliable BioID pulldown results. Preferably, cells should not vary widely in BioID expression levels.
Methanol fixation intensifies detection of endogenously biotinylated proteins in the mitochondria of the cell. Paraformaldehyde is the recommended fixative for these immunofluorescence studies.
Cells should preferably be stably expressing low levels of BioID-only or BioID-POI to avoid mislocalization due to overexpression. BioID-only controls are required to properly analyze subsequent MS data.
Two 10 cm plates is the recommended minimum for a given BioID pulldown, based on common cell types such as HEK-293, HeLa, NIH3T3, BJ-5ta, and U2OS. Fewer cells will generally yield detection of fewer candidates. Adjust the number of 10 cm plates utilized for each condition based on cell size and allowable confluency.
Urea solutions are unstable, especially when heated, and can cause carbamylation of proteins. Urea solutions should always be made fresh immediately prior to use to avoid these artifacts and the resulting mass change that could complicate MS data.
Keratins and other protein contaminants can potentially mask real signals in the MS data. Keep hair tied back, avoid talking and use a face shield, gloves, DNase/RNase-free tubes, and tube cap openers to minimize risk of contamination (see (35) for more information on common contaminants identified in MS data).
The BioID fusion protein should localize and function normally. It may be necessary to test and compare BioID fusion to both the N- and C-terminus before moving forward with one or both for stable cell line creation and subsequent pulldown. A flexible linker, such as a GGGGS repeat, can be fused between the BioID and POI to increase the labeling radius of the ligase if appropriate (22) or to mitigate any other concerns of steric hindrance between the ligase and POI. Further, if the POI is of high molecular weight and the desire is to map PPIs along the entire protein, a flexible linker of defined length could be useful.
Endogenous biotin levels and the low levels of biotin that may or may not be present in culture medium is generally insufficient to promote substantial biotinylation. Biotin supplementation is typically required to initiate the promiscuous biotinylation of proximate proteins.
There is often a large number of bands of variable intensity, sometimes resembling a smear representing the myriad of proteins biotinylated by BioID.
The streptavidin-biotin interaction is too strong to efficiently interrupt via conventional stripping methods; therefore, HRP activity can be quenched with H2O2 in order to probe and image the blot further with other antibodies.
Performing BioID experiments in triplicate greatly reduces the risk of false positive identifications by allowing for the use of stricter data analysis methods including statistical bioinformatics and improved data visualization methods (e.g. volcano plots).
This step prevents the sample from overheating during sonication.
If necessary, the lysate can be flash-frozen at this point. All samples in a single BioID pulldown should undergo pre-clearing and all future steps together, in parallel. If other samples are not yet ready, flash-freeze and store at −80°C.
The amount of biotinylated proteins recovered for MS following pulldown plateaus after incubation with streptavidin beads for 4 hours (May and Roux, unpublished data). Prolonged incubation with streptavidin could potentially introduce background proteins via non-specific binding.
Beads should be resuspended in ammonium bicarbonate with excess biotin to aid the recovery of biotinylated peptides following on-bead trypsin digestion prior to MS analysis.
An analysis via western blot should be performed prior to sending the samples for MS analysis as the quality of the western blot often reflects the quality of MS data that can be expected.
Due to the extremely strong affinity of streptavidin to biotin and the resistance of biotin-bound streptavidin to trypsin, the majority of biotinylated peptides are not identified as they likely remain bound to the beads.
In order for a protein to be considered a “candidate interactor,” it should be enriched (e.g. more than 3-fold) in the BioID-POI samples compared to the BioID-only control samples. Certain proteins tend to be abundant in all BioID samples, including AHNAK, FLNA, PARP1, EEF1A1, PRKDC, TOP1, and PKM. Endogenous carboxylases, histones, and ribosomes also tend to be enriched in all BioID pulldowns, regardless of the POI, and should generally be suspected of being background.
Conflict of Interest Statement: Sanford Research has licensed BioID reagents developed by KJR to BioFront Technologies.
References
- 1.Fierer JO, Veggiani G, Howarth M (2014) SpyLigase peptide-peptide ligation polymerizes affibodies to enhance magnetic cancer cell capture. Proc Natl Acad Sci U S A 111 (13):E1176–1181. doi: 10.1073/pnas.1315776111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu CF, Tam JP (2001) Subtilisin-catalyzed synthesis of amino acid and peptide esters. Application in a two-step enzymatic ligation strategy. Org Lett 3 (26):4157–4159 [DOI] [PubMed] [Google Scholar]
- 3.Strijbis K, Spooner E, Ploegh HL (2012) Protein ligation in living cells using sortase. Traffic 13 (6):780–789. doi: 10.1111/j.1600-0854.2012.01345.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liebscher S, Kornberger P, Fink G, Trost-Gross EM, Hoss E, Skerra A, Bordusa F (2014) Derivatization of antibody Fab fragments: a designer enzyme for native protein modification. Chembiochem 15 (8):1096–1100. doi: 10.1002/cbic.201400059 [DOI] [PubMed] [Google Scholar]
- 5.Kim DI, Roux KJ (2016) Filling the Void: Proximity-Based Labeling of Proteins in Living Cells. Trends Cell Biol 26 (11):804–817. doi: 10.1016/j.tcb.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rees JS, Li XW, Perrett S, Lilley KS, Jackson AP (2015) Protein Neighbors and Proximity Proteomics. Mol Cell Proteomics 14 (11):2848–2856. doi: 10.1074/mcp.R115.052902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roux KJ, Kim DI, Raida M, Burke B (2012) A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol 196 (6):801–810. doi: 10.1083/jcb.201112098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon K, Streaker ED, Ruparelia S, Beckett D (2000) Multiple disordered loops function in corepressor-induced dimerization of the biotin repressor. J Mol Biol 304 (5):821–833. doi: 10.1006/jmbi.2000.4249 [DOI] [PubMed] [Google Scholar]
- 9.Kwon K, Beckett D (2000) Function of a conserved sequence motif in biotin holoenzyme synthetases. Protein Sci 9 (8):1530–1539. doi: 10.1110/ps.9.8.1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi-Rhee E, Schulman H, Cronan JE (2004) Promiscuous protein biotinylation by Escherichia coli biotin protein ligase. Protein Sci 13 (11):3043–3050. doi: 10.1110/ps.04911804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cronan JE (2005) Targeted and proximity-dependent promiscuous protein biotinylation by a mutant Escherichia coli biotin protein ligase. J Nutr Biochem 16 (7):416–418. doi: 10.1016/j.jnutbio.2005.03.017 [DOI] [PubMed] [Google Scholar]
- 12.Friedman WJ, Dreyfus CF, McEwen B, Black IB (1988) Substance K (NKA) increases tyrosine hydroxylase mRNA in cultured substantia nigra. Brain Res 427 (2):203–205 [DOI] [PubMed] [Google Scholar]
- 13.Morriswood B, Havlicek K, Demmel L, Yavuz S, Sealey-Cardona M, Vidilaseris K, Anrather D, Kostan J, Djinovic-Carugo K, Roux KJ, Warren G (2013) Novel bilobe components in Trypanosoma brucei identified using proximity-dependent biotinylation. Eukaryot Cell 12 (2):356–367. doi: 10.1128/EC.00326-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen AL, Kim EW, Toh JY, Vashisht AA, Rashoff AQ, Van C, Huang AS, Moon AS, Bell HN, Bentolila LA, Wohlschlegel JA, Bradley PJ (2015) Novel components of the Toxoplasma inner membrane complex revealed by BioID. MBio 6 (1):e02357–02314. doi: 10.1128/mBio.02357-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batsios P, Meyer I, Graf R (2016) Proximity-Dependent Biotin Identification (BioID) in Dictyostelium Amoebae. Methods Enzymol 569:23–42. doi: 10.1016/bs.mie.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 16.Khan M, Youn JY, Gingras AC, Subramaniam R, Desveaux D (2018) In planta proximity dependent biotin identification (BioID). Sci Rep 8 (1):9212. doi: 10.1038/s41598-018-27500-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Q, Zhou Z, Luo W, Fang M, Li M, Li H (2017) Screening of Proximal and Interacting Proteins in Rice Protoplasts by Proximity-Dependent Biotinylation. Front Plant Sci 8:749. doi: 10.3389/fpls.2017.00749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brudvig JJ, Cain JT, Schmidt-Grimminger GG, Stumpo DJ, Roux KJ, Blackshear PJ, Weimer JM (2018) MARCKS Is Necessary for Netrin-DCC Signaling and Corpus Callosum Formation. Mol Neurobiol 55 (11):8388–8402. doi: 10.1007/s12035-018-0990-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan PK, Srikumar T, Dingar D, Kalkat M, Penn LZ, Raught B (2014) BioID data of c-MYC interacting protein partners in cultured cells and xenograft tumors. Data Brief 1:76–78. doi: 10.1016/j.dib.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uezu A, Kanak DJ, Bradshaw TW, Soderblom EJ, Catavero CM, Burette AC, Weinberg RJ, Soderling SH (2016) Identification of an elaborate complex mediating postsynaptic inhibition. Science 353 (6304):1123–1129. doi: 10.1126/science.aag0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kehrer J, Frischknecht F, Mair GR (2016) Proteomic Analysis of the Plasmodium berghei Gametocyte Egressome and Vesicular bioID of Osmiophilic Body Proteins Identifies Merozoite TRAP-like Protein (MTRAP) as an Essential Factor for Parasite Transmission. Mol Cell Proteomics 15 (9):2852–2862. doi: 10.1074/mcp.M116.058263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DI, Jensen SC, Noble KA, Kc B, Roux KH, Motamedchaboki K, Roux KJ (2016) An improved smaller biotin ligase for BioID proximity labeling. Mol Biol Cell 27 (8):1188–1196. doi: 10.1091/mbc.E15-12-0844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramanathan M, Majzoub K, Rao DS, Neela PH, Zarnegar BJ, Mondal S, Roth JG, Gai H, Kovalski JR, Siprashvili Z, Palmer TD, Carette JE, Khavari PA (2018) RNA-protein interaction detection in living cells. Nat Methods 15 (3):207–212. doi: 10.1038/nmeth.4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schopp IM, Amaya Ramirez CC, Debeljak J, Kreibich E, Skribbe M, Wild K, Bethune J (2017) Split-BioID a conditional proteomics approach to monitor the composition of spatiotemporally defined protein complexes. Nat Commun 8:15690. doi: 10.1038/ncomms15690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Munter S, Gornemann J, Derua R, Lesage B, Qian J, Heroes E, Waelkens E, Van Eynde A, Beullens M, Bollen M (2017) Split-BioID: a proximity biotinylation assay for dimerization-dependent protein interactions. FEBS Lett 591 (2):415–424. doi: 10.1002/1873-3468.12548 [DOI] [PubMed] [Google Scholar]
- 26.Branon TC, Bosch JA, Sanchez AD, Udeshi ND, Svinkina T, Carr SA, Feldman JL, Perrimon N, Ting AY (2018) Efficient proximity labeling in living cells and organisms with TurboID. Nat Biotechnol 36 (9):880–887. doi: 10.1038/nbt.4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birendra K, May DG, Benson BV, Kim DI, Shivega WG, Ali MH, Faustino RS, Campos AR, Roux KJ (2017) VRK2A is an A-type lamin-dependent nuclear envelope kinase that phosphorylates BAF. Mol Biol Cell 28 (17):2241–2250. doi: 10.1091/mbc.E17-03-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long S, Brown KM, Sibley LD (2018) CRISPR-mediated Tagging with BirA Allows Proximity Labeling in Toxoplasma gondii. Bio Protoc 8 (6). doi: 10.21769/BioProtoc.2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DI, Cutler JA, Na CH, Reckel S, Renuse S, Madugundu AK, Tahir R, Goldschmidt HL, Reddy KL, Huganir RL, Wu X, Zachara NE, Hantschel O, Pandey A (2018) BioSITe: A Method for Direct Detection and Quantitation of Site-Specific Biotinylation. J Proteome Res 17 (2):759–769. doi: 10.1021/acs.jproteome.7b00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Udeshi ND, Pedram K, Svinkina T, Fereshetian S, Myers SA, Aygun O, Krug K, Clauser K, Ryan D, Ast T, Mootha VK, Ting AY, Carr SA (2017) Antibodies to biotin enable large-scale detection of biotinylation sites on proteins. Nat Methods 14 (12):1167–1170. doi: 10.1038/nmeth.4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Q, Zheng J, Sun W, Huo Y, Zhang L, Hao P, Wang H, Zhuang M (2018) A proximity-tagging system to identify membrane protein-protein interactions. Nat Methods 15 (9):715–722. doi: 10.1038/s41592-018-0100-5 [DOI] [PubMed] [Google Scholar]
- 32.Kim DI, Kc B, Zhu W, Motamedchaboki K, Doye V, Roux KJ (2014) Probing nuclear pore complex architecture with proximity-dependent biotinylation. Proceedings of the National Academy of Sciences of the United States of America 111 (24):E2453–E2461. doi: 10.1073/pnas.1406459111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fredriksson K, Van Itallie CM, Aponte A, Gucek M, Tietgens AJ, Anderson JM (2015) Proteomic analysis of proteins surrounding occludin and claudin-4 reveals their proximity to signaling and trafficking networks. PLoS One 10 (3):e0117074. doi: 10.1371/journal.pone.0117074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacquet K, Banerjee SL, Chartier FJM, Elowe S, Bisson N (2018) Proteomic analysis of NCK½ adaptors uncovers paralog-specific interactions that reveal a new role for NCK2 in cell abscission during cytokinesis. Mol Cell Proteomics doi: 10.1074/mcp.RA118.000689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellacheruvu D, Wright Z, Couzens AL, Lambert JP, St-Denis NA, Li T, Miteva YV, Hauri S, Sardiu ME, Low TY, Halim VA, Bagshaw RD, Hubner NC, Al-Hakim A, Bouchard A, Faubert D, Fermin D, Dunham WH, Goudreault M, Lin ZY, Badillo BG, Pawson T, Durocher D, Coulombe B, Aebersold R, Superti-Furga G, Colinge J, Heck AJ, Choi H, Gstaiger M, Mohammed S, Cristea IM, Bennett KL, Washburn MP, Raught B, Ewing RM, Gingras AC, Nesvizhskii AI (2013) The CRAPome: a contaminant repository for affinity purification-mass spectrometry data. Nat Methods 10 (8):730–736. doi: 10.1038/nmeth.2557 [DOI] [PMC free article] [PubMed] [Google Scholar]


