Abstract
OBJECTIVES:
Autoimmune pancreatitis (AIP) is increasingly recognized as a form of chronic pancreatitis. Systematic evaluation and management of AIP in the United States is reported only from one center. Our aim was to review the evaluation and management of AIP at a large tertiary center.
METHODS:
We retrospectively reviewed information on demographics, clinical presentation, laboratory and imaging findings, extrapancreatic involvement, treatment response, and recurrence in 26 patients with AIP treated at the University of Pittsburgh Medical Center from 1998 to 2007.
RESULTS:
The median age at presentation was 62.5 years (range: 23 – 86), 65 % were men, and 88 % were Caucasians. The most common presentation included new-onset mild abdominal pain (65%), jaundice (62%), and weight loss (42%). Pancreatic mass, enlargement, or prominence on imaging was present in 85% of the patients. Serum IgG4 (immunoglobulin-4) was elevated (>140 mg / dl) in 44 % (8 / 18) at presentation. The most common extrapancreatic finding was extrapancreatic/intrahepatic biliary strictures (35%). Peri-pancreatic vascular complications were noted in 23% of the patients. Six patients underwent partial or complete pancreatectomy. Partial or complete response was observed for initial steroid treatment in 19 patients and for methotrexate in 1 patient. Recurrences were common, especially in patients with extrapancreatic manifestations, and usually responded to a combination of steroids and azathioprine. Any one of the commonly used diagnostic criteria (Mayo Clinic’s HISORt criteria, the Japanese Pancreas Society criteria, Korean diagnostic criteria) was fulfilled in 85% of cases.
CONCLUSIONS:
In this second major US series, we confirm several findings previously reported in AIP. Our study highlights the presence of vascular complications in a subset of patients with AIP. The current diagnostic criteria may not identify all AIP patients.
INTRODUCTION
Autoimmune pancreatitis (AIP), also known as lymphoplasmacytic sclerosing pancreatitis (LPSP), is a rare disease of the pancreas that is now recognized to be part of a systemic fibroinflammatory syndrome complex known as immunoglobulin- 4 (IgG4)-related systemic disease (1–6). It is characterized by variably increased serum IgG4 levels and multi-organ IgG4-rich lymphoplasmacytic infiltration, and is highly responsive to steroid treatment (1–3,6,7). In addition to the pancreas, IgG4-related systemic disease may involve the biliary tree (IgG4-associated cholangitis), salivary glands (chronic sclerosing sialadenitis), retroperitoneum, lymph nodes, and kidneys (1,3–5,7,8). These extrapancreatic manifestations of the IgG4-related systemic disease can mimic other well-defined conditions, including primary sclerosing cholangitis, Sjogren’s syndrome, Riedel’s thyroiditis, and retroperitoneal fibrosis (1,3,5,6,9,10).
AIP predominantly affects men over the age of 50 years and presents most commonly as obstructive jaundice with mild or no abdominal pain (1,11–13). Other presentations include mild attacks of pancreatitis, recent onset of diabetes mellitus, weight loss, or other non-specific symptoms (11,12,14). The pancreatic gland may show focal or diffuse swelling, and the pancreatic duct may show diffuse or segmental narrowing on imaging (11,15,16). The unique histological pattern observed in AIP has been described as lymphoplasmacytic sclerosis, which involves a duct-centric lymphoplasmacytic infiltrate, storiform fibrosis concentrated around ducts and veins, and obliterative phlebitis–an infiltrate that preferentially affects venules (12). The presence of extrapancreatic manifestations is seen in approximately 40–50% of cases, which may precede the onset of AIP (1,12,17). As described initially, three major sets of diagnostic criteria for AIP have been proposed (12,18,19). However, it is increasingly recognized that many patients with suspected or proven AIP may not fulfill the established criteria (20).
Apart from the Mayo Clinic (12), reports on AIP from the United States have been limited to case reports (21–23). The aim of our study was to systematically review the evaluation and management in a large series of patients with AIP over a 10-year period at a tertiary care center in Pittsburgh (PA).
METHODS
Using the search terms, “autoimmune pancreatitis,” “lymphoplasmacytic sclerosing pancreatitis,” and “idiopathic rapidly progressive cholangitis,” we identified 29 patients with AIP in our medical records database who were evaluated in the Pancreatobiliary Clinic of the Digestive Disorders Center at the University of Pittsburgh Medical Center between 1998 and 2007. Three of these 29 patients, who had other malignancies (gastric cancer diagnosed during the evaluation of biliary strictures (IgG4 positive on biopsy) in one patient, steroid-responsive biliary strictures in parallel with the recurrence of gastric adenocarcinoma in one, and pancreatic adenocarcinoma diagnosed in one steroid-unresponsive patient who was initially diagnosed as AIP on pancreatic biopsy) were excluded from the study. Medical records of the remaining 26 patients were reviewed to obtain information on demographics, clinical presentation, laboratory data, radiological and histological features, response to treatment, and relapse on follow-up. We evaluated whether a patient fulfilled any of the three commonly used diagnostic criteria for AIP (Mayo Clinic’s HISORt criteria, the Japanese Pancreas Society criteria, and Korean diagnostic criteria) (12,18,19). The protocol was approved by the University of Pittsburgh Medical Center’s Quality Improvement Committee.
Serum IgG4 levels were measured in 18 of 26 (69%) patients using automated nephelometry (IMMAGE, Beckman Coulter’s immunochemistry systems). Elevated serum IgG4 level was denoted by levels > 140 mg/dl. Levels of >280 mg/dl, which have recently been suggested to be highly specific for AIP, were noted (10).
Eight out of the ten pancreatic histology specimens, obtained during the management of these patients, were available for a microscopic review. These included six resection specimens, one exploratory wedge biopsy, and one endoscopic ultrasound (EUS)-guided trucut biopsy. One exploratory wedge biopsy and one computed tomography (CT)-guided pancreatic core biopsy were unavailable for review. Nine of the ten extrapancreatic histology specimens obtained during the management of these patients, were available for a microscopic review. These included five bile duct biopsies, one submandibular resection specimen, two ampullary biopsies, and one thyroid resection specimen. One submandibular resection was unavailable for review. All available specimens were reviewed by an expert pathologist (A.K.). Findings from the histology reports for the three specimens unavailable for review were noted.
Histological features used to support the diagnosis of AIP were similar to the study published from the Mayo clinic (12). These included the presence of a fibro-inflammatory process that was duct centric and contained numerous lymphocytes and plasma cells with variable neutrophils, obliterative venulitis, storiform fibrosis, and the presence of > 10 IgG4-positive plasma cells per high power field (12). Histological features used to support the diagnosis of extrapancreatic involvement included lymphoplasmacytic infiltration, accompanying fibrosis, and the presence of > 10 IgG4-positive plasma cells per high power field (4,9,12). IgG4 immunohistochemical staining was performed on all available pancreatic and extrapancreatic histological specimens using monoclonal anti-human IgG4 antibody (Zymed, San Francisco, CA) using a standard technique in a formalin-fixed paraffin-embedded tissue.
All pre-and post-treatment imaging studies were systematically reviewed. These included CT scans (in all 26 patients), Endoscopic Retrograde Cholangiograms (13 of 18), Endoscopic Retrograde Pancreatogram (13 of 14), magnetic resonance imaging (5 of 5), and positron emission tomogram (1 of 1) scans. EUS was successfully completed in 20 of 22 patients in whom it was attempted, and details of all studies were available for review.
A pancreatic mass was defined as the focal enlargement of the pancreas with a different density compared with that of the surrounding tissue. Pancreatic enlargement was defined as the enlargement of the pancreas in the absence of a discrete mass. Biliary strictures were defined as distal (intrapancreatic) or proximal (extrapancreatic common bile duct (CBD), hilar, or intrahepatic). Isolated intrapancreatic stricturing was considered as a part of AIP, whereas proximal (extrapancreatic CBD, hilar, or intrahepatic) strictures were considered as extrapancreatic manifestations.
The presence of extrapancreatic organ involvement, such as extrapancreatic biliary strictures, retroperitoneal fibrosis, sialadenitis, and sclerosing mesenteritis was noted. The presence of other extrapancreatic findings (e.g., vascular complications, splenic infarction, liver mass, enlarged lymph nodes, etc.) was recorded. In patients with diabetes mellitus, the timing of initial glucose intolerance and its relationship to the diagnosis of AIP was noted.
Information on the type of treatment (surgical or medical) was noted. In pat6ients who received medical treatment, indication, dosage, duration, and regimen of immunosuppressive therapy used was recorded. Response to therapy was defined as complete when symptomatic improvement and complete resolution of imaging abnormalities was present. In patients with a pancreatic mass, a complete resolution of imaging abnormalities was considered when the pancreas appeared normal or atrophic post treatment. In patients with biliary and/or pancreatic duct strictures, a complete resolution was defined by a return to normal caliber. An incomplete response to therapy was defined by partial symptomatic improvement and/or incomplete resolution of mass lesions or biliary strictures. In these cases, prolonged treatment with corticosteroids (>12 weeks) in conjunction with steroid-sparing agents (azathioprine) was attempted. In one patient, treatment was initiated with methotrexate.
RESULTS
Between 1998 and 2007, 26 patients with AIP were evaluated at the Pancreatobiliary Clinic of the Digestive Disorders Center at the University of Pittsburgh Medical Center. Any of the three commonly used diagnostic criteria for AIP were fulfilled in 22/26 of cases (85%) (HISORt in 16/26, diagnostic criteria 62%; the Japanese Pancreas Society criteria in 13/26, 50 %; and Korean diagnostic criteria in 16/26, 62 %).
Of the 16 patients who fulfilled the HISORt criteria, the diagnosis of AIP was established on pancreatic histology in 7 patients (total or partial pancreatic resection in 6 and CT-guided biopsy in 1), characteristic radiographic and serologic features in 8 patients, and by a combination of extrapancreatic involvement, serology, and response to treatment in 1 patient. In the 10 patients who did not fulfill the HISORt criteria, the diagnosis of AIP was established by a combination of characteristic imaging abnormality (pancreatic plus biliary in 7 and isolated pancreatic in 3 patients), elevated antinuclear antibody (ANA) (n = 3), resolution of radiographic abnormalities with immunosuppressive therapy, and exclusion of alternative diagnoses, such as pancreatic and biliary cancer. Data on demographics, clinical presentation, laboratory data, radiological findings, histological features, response to treatment, and relapse on follow-up in patients meeting the HISORt criteria (n = 16), and those not meeting the HISORt criteria for AIP (n = 10) (12) are presented in Tables 1 and 2, respectively.
Table 1.
Demographics, clinical presentation, workup and treatment of patients with autoimmune pancreatitis fulfilling the HISORt criteria seen at the University of Pittsburgh Medical Center from 1998 to 2007
| Patient number | Age at presentation (years)/gender | Presentation | Laboratory data | Serum lgG4 (mg/dl) | Imaging (CT, ERCP, EUS, MRI) | Histology | Associated findings/extra pancreatic manifestations | Response to treatment (initial treatment) | Follow-up (months) | Recurrence | HISORt group | Comments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipase | ALP | Direct bilirubin | Site | Finding | Rx | Clinical | Lab. | Imaging | ||||||||||
| 1 | 23/male | Abdominal pain | 1,980(↑) | 88 | <0.1 | 8 | Pancreatic tail mass, pancreatic duct stricture | Pancreas | LPSP | — | Sx | Yes | Yes | Yes | 8 | No | A | — |
| 2 | 29/female | Abdominal pain, jaundice, weight loss, steatorrhea | 465(↑) | 60 | 0.2 | 64 | Prominent hypoechoic pancreatic tail pancreatic duct stricture | Pancreas | LPSP | Retroperitoneal fibrosis | Sx | Yes | Yes | Yes | 10 | NO | A | ANA=l:320(↑) |
| 3 | 41/male | Jaundice, weight loss | 520s(↑) | 320(↑) | 1.6 | ND | Pancreatic head mass, distal CBD stricture | Pancreas | LPSP | — | Sx | Yes | Yes | Yes | 1 | Lost to follow up | A | — |
| 4 | 68/female | Jaundice, weight loss | 550(↑) | 250(↑) | ND | ND | Pancreatic head prominence, distal CBD stricture | Pancreas | LPSP | New-onset diabetes mellitus | Sx | Yes | Yes | Yes | 36 | No | A | ANA= 1:160(↑), CA 19–9=3,422(↑), CEA=1.4 |
| 5 | 72/male | Jaundice, weight loss | NA | 128 | NA | ND | Pancreatic head mass, distal CBD stricture | Pancreas | LPSP | — | Sx | Yes | — | Yes | 6 | No | A | CA 19–9=34, CEA=0.8 |
| 6 | 75/male | Abdominal pain, back pain | 48 | 77 | <0.1 | ND | Pancreatic body mass | Pancreas | LPSP | — | Sx | Yes | Yes | Yes | 3 | No follow data | A | — |
| 7 | 86/male | Abdominal pain, jaundice, weight loss, pruritus | 14 | 332(↑) | 2.4(↑) | 79 | Pancreatic head mass, pancreatic duct stricture | Pancreas, submandibular gland | LPSP, CSS (specimens not available to review) | IHBS, CSS | P | Yes | Yes | Yes | 5 | Yes | A | ANA<1: 40, serum lgG4=368(↑) (on recurrence) |
| 8 | 50/female | Abdominal pain, anorexia, bloating | 809(↑) | NA | NA | 178(↑) | Pancreatic tail mass, pancreatic duct stricture | — | — | — | P | Yes | Yes | Yes | 13 | Yes | B | ANA= 1:160(↑) |
| 9 | 43/male | Jaundice, steatorrhea, pruritus | 1,432(↑) | 240(↑) | 1.5(↑) | 199(↑) | Diffuse pancreatic enlargement, distal CBD stricture | — | — | New-onset diabetes mellitus, submandibular gland enlargement | P | Yes | Yes | Yes | 9 | Yes | B | ANA <1:40, CA 19–9=53 |
| 10 | 61/female | Abdominal pain, jaundice, weight loss, polyuria | 651(↑) | 71 | <0.1 | 148(↑) | Pancreatic head mass, pancreatic duct stricture | — | — | New-onset diabetes mellitus, submandibular gland enlargement | P | Yes | Yes | Yes | 36 | Yes | B | ANA= 1:640(↑) |
| 11 | 64/male | Weight loss, steatorrhea | 13 | 13 | <0.1 | 785(↑) | Pancreatic head mass, pancreatic duct stricture | Ampullary biopsy | Inconclusive | — | P | Yes | Yes | Yes | 7 | Yes | B | ANA= 1:80(↑), CA 19–9=10.6, CEA<0.5 |
| 12 | 68/female | Jaundice | 550(↑) | 250(↑) | NA | 825(↑) | Distal CBD stricture, pancreatic duct stricture, hypoechoic pancreas | — | — | — | P | Yes | Yes | Yes | 2 | Lost to follow up | B | ANA <1:40 |
| 13 | 69/male | Abdominal pain | 158 | 92 | <0.1 | 735(↑) | Multifocal pancreatic head and body mass, pancreatic duct stricture | — | — | — | P | Yes | Yes | Yes | 2 | On steroids taper at the end of study period | B | ANA< 1:80, CA 19–9=25.4 |
| 14 | 72/male | Abdominal pain, jaundice, weight loss, steatorrhea, pruritus | NA | 425(↑) | 1.3(↑) | 452(↑) | Pancreas head mass, pancreatic duct stricture | Bile duct | Inadequate sample | Hilar stricture, liver mass | P | Yes | Yes | Yes | 5 | No | B | ANA= 1:160(↑), CEA=1.5, |
| 15 | 78/male | Abdominal pain | 396(↑) | 71 | <0.1 | 286(↑) | Pancreatic tail prominence, pancreatic duct stricturing | — | — | — | P | Yes | Yes | Incomplete response | 3 | Steroids tapered and on azathiprine at end of study period | B | — |
| 16 | 70/male | Abdominal pain, jaundice, weight loss, pruritus | 44 | 664(↑) | 1.5(↑) | 57 | Pancreatic head prominence, irregular pancreatic duct | Sub-mandibular gland | CSS | New-onset diabetes mellitus, hilar stricture, CSS | P | Yes | Yes | Yes | 5 | Yes | C | ANA< 1:40, CA 19–9=27 |
CBD, common bile duct; CSS, chronic sclerosing sialadenitis (IgG>10/hpf); CT, computed tomography; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; IAC, immunoglobulin-4
(IgG4)-associated cholangitis; IHBS, intrahepatic biliary stricture; Lab., laboratory; LPSP, lymphoplasmacytic sclerosing pancreatitis (IgG>10 hpf); MRI, magnetic resonance imaging; NA, not available; ND, not done;
P, prednisone; PBDS, proximal common bile duct stricture; Rx, treatment; Sx, surgical resection.
All but 3 patients (1, 3, 10) were Caucasians. Abdominal pain was mild in all patients, not requiring narcotics. Normal laboratory values: lipase ≤ 200 IU/l, alkaline phosphatase (ALP) = 40–125 IU/l, direct bilirubin = 0.1–0.4 mg/dl, serum IgG4 ≤ 140 mg/dl, Carcinoembryonic antigen (CEA)≤5 ng/ml, Cancer associated antigen 19–9 (CA 19–9) ≤ 38 U/ml, Anit-nuclear antibody (ANA) ≤ 1:80.
Table 2.
Demographics, clinical presentation, workup, and treatment of patients with autoimmune pancreatitis not fulfi lling the HISORt criteria seen at the University of Pittsburgh Medical Center from 1998 to 2007
| Patient number | Age at presentation (years)/gender | Presentation | Laboratory data | Serum lgG4 (mg/dl) | Imaging (CT, ERCP, EUS, MRI) | Histology | Associated findings/extra pancreatic manifestations | Response to treatment (initial treatment) | Follow-up (months) | Recurrence | Comments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipase | ALP | Direct bilirubin | Site | Finding | Rx | Clinical | Laboratory tests | Imaging | |||||||||
| 17 | 30/female | Abdominal pain | 420(↑) | 86 | 0.1 | 30 | Diffuse pancreatic enlargement, pancreatic duct stricture | Pancreas (was on steroids for UP) | LPSP (lgG4 negative) | — | P | Yes | Yes | Incomplete response | 10 | No | ANA= 1:80(↑), CA19–9 = 21.6, CEA<0.5 |
| 18 | 33/male | Abdominal pain, jaundice, steator-rhea | 30 | 467(↑) | 0.6(↑) | 34.7 | Diffuse pancreatic enlargement, pancreatic duct stricture, distal CBD stricture | Bile duct | Inadequate sample | — | P | Yes | Yes | Yes | 5 | No | ANA>l:80(↑), CA 19–9=43.7(↑), CEA= 1.9 |
| 19 | 34/female | Abdominal pain, jaundice | NA | 799(↑) | 1.4(↑) | ND | Pancreatic tail mass, distal CBD stricture, pancreatic duct stricture | Pancreas Thyroid | LPSP (lgG4 negative) Riedel’s thyroiditis (inconclusive for AlP) | Hilar stricture, PBDS, Riedel’s thyroiditis, sclerosing mesenteritis | P | Yes | Yes | Yes | 24 | Yes | CA 19–9 = 13.7, CEA<0.5 |
| 20 | 37/female | Abdominal pain | 2,136(↑) | 67 | <0.1 | 36 | Hypoechoic pancreatic head and pancreatic duct stricture | — | — | — | P | Yes | Yes | Yes | 20 | Yes | ANA<l: 40, CA 19–9 = 20.1 |
| 21 | 42/female | Weight loss, neck mass | NA | NA | NA | <8 | Pancreatic tail mass | — | — | PBDS, biopsy-proven Riedel’s thyroiditis | P | Yes | Yes | Yes | 6 | No | ANA<1:40 |
| 22 | 55/male | Abdominal pain, weight loss, pruritus | 385(↑) | 240(↑) | ND | 38 | Pancreatic head mass | — | — | — | P | Yes | Yes | Yes | 5 | No | ANAcl: 40, CA19–9 = 25, CEA<0.5, |
| 23 | 67/male | Abdominal pain, jaundice | 344(↑) | 189(↑) | 4.0 | 80 | Pancreatic head mass, pancreatic duct stricture | Ampullary biopsy | Inconclusive | Hilar stricture | P | Yes | Yes | Yes | 4 | Yes | ANA=l:l:80(↑), CA 19–9=9.5, CEA<0.5 |
| 24 | 60/male | Jaundice, pruritus | 30 | 298(↑) | 1.2(↑) | ND | Pancreatic head mass | Bile duct | Inadequate sample | IHBS, hilar stricture | MTx | Yes | Yes | Yes | 84 | Yes | ANA< 1:40, CA19–9 = 13.5, CEA<0.5 |
| 25 | 66/male | Jaundice | 630(↑) | 339(↑) | 1.5(↑) | ND | Diffuse pancreatic enlargement, distal CBD stricture | Bile duct | Inadequate sample | IHBS, retroperitoneal fibrosis | P | Yes | Yes | Incomplete response | 12 | Yes | ANA >1:1,280(↑), CA19–9 = 116.5(↑), CEA = 1.1 |
| 26 | 72/male | Abdominal pain, jaundice, pruritus | 38 | 240(↑) | 1.1(↑) | ND | Pancreatic tail mass, pancreatic duct stricture | Pancreatic Bile duct | Chronic pancreatitis (specimen not available) Inconclusive | PBDS, IHBS | P | Yes | Yes | Incomplete response | 12 | Yes | AN A< 1:40, CA19–9 = 35.5, CEA<0.5 |
AIP, autoimmune pancreatitis; CBD, common bile duct; CT, computed tomography; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; IHBS, intrahepatic biliary stricture; ITP, idiopathic thrombocytic purpura; LPSP, lymphoplasmacytic sclerosing pancreatitis; MRI, magnetic resonance imaging; Mtx, methotrexate; NA, not available; ND, not done; P, prednisone; PBDS, proximal CBD stricture;Rx, treatment.
All patients were Caucasians. Abdominal pain was mild in all patients, not requiring narcotics. Normal laboratory values: lipase≤200 IU/l, alkaline phosphatase (ALP) = 40–125 IU/l, direct bilirubin = 0.1–0.4 mg/dl, serum
IgG4≤140 mg/dl, carcinoembryonic antigen (CEA)≤5 ng/ml, cancer associated antigen 19–9 (CA 19–9)≤38 U/ml, antinuclear antibody (ANA)≤1:40.
Clinical presentation
The median age of patients at presentation was 62.5 years (range: 23–86). Two-thirds of the patients were ≥ 50 years of age, two-thirds were men, and all but three (88%) patients were Caucasians. The most common presenting symptoms included new-onset abdominal pain in 17 (65%), obstructive jaundice in 16 (62%), weight loss in 11 (42%), and steatorrhea in 5 (19%) patients. Pain was mild in 16 of the 17 patients with new-onset abdominal pain that did not need narcotics for pain control. Two additional patients were on narcotics for chronic abdominal pain of unclear etiology, which was unrelated to the diagnosis of AIP. Overall, 6 of 26 patients (23%) had diabetes mellitus at the time of evaluation. Four of these patients had developed diabetes within 2 years preceding the diagnosis of AIP. The other two patients had long-standing diabetes but reported worsening of glycemic control in the months preceding the diagnosis of AIP. Glycemic control improved in five out of six patients with diabetes after treatment with corticosteroids. One patient was lost to follow-up.
Serology
An increase in serum IgG4 level to > 140 and > 280 mg/dl was observed in 8 (44.4%) and 5 (27.8%) of the 18 patients, respectively, in whom it was measured. Interestingly, one patient with normal IgG4 levels at presentation had increased serum IgG4 during recurrence of the disease. Antinuclear antibody titer was increased to ≥1:80 in 10 of 20 (50%) cases in whom it was measured. Cancer associated antigen 19–9 (CA 19–9) was measured in 15 patients and was increased (>38 U/ml) in 4 (27%) patients.
Imaging studies
On pre-treatment CT scans (n = 26), 15 patients had a pancreatic mass (head in 8, tail in 5, body in 1, and multifocal in 1), 4 had diffuse pancreatic enlargement, 3 had localized prominence of the pancreas without a distinct mass, and 4 patients had a normal-appearing pancreas. The findings on ERCP (endoscopic retrograde cholangiopancreatography) showed pancreatic duct stricture in 13 patients (diffuse in 7, focal in 6), distal (intrapancreatic) CBD stricture in 8, proximal CBD stricture in 3, hilar stricture in 5, and intrahepatic biliary stricture in 4 patients. Three of the patients with extensive hilar stricturing were initially diagnosed as cholangiocarcinoma. EUS findings in the 20 patients with a completed examination included a hypoechoic pancreatic mass in 13, diffuse hypoechoic and/or heterogeneous pancreatic parenchymal changes in 13, the main pancreatic duct stricture with upstream dilatation in 4, and features of chronic pancreatitis in 2 patients. EUS findings in the four patients with a normal-appearing pancreas on CT scan included a hypoechoic mass in the head of the pancreas in one, hypoechoic changes and pancreatic duct narrowing in the tail of the pancreas in one, diffuse hypoechoic changes in the head and body of the pancreas in one, and pancreatic duct narrowing with hypoechoic changes in the head of the pancreas in one. EUS-guided fine needle aspiration cytology of the pancreas was performed in 19 patients. EUS-guided core biopsy was performed in one patient showing features of LPSP, but the IgG4 staining was negative.
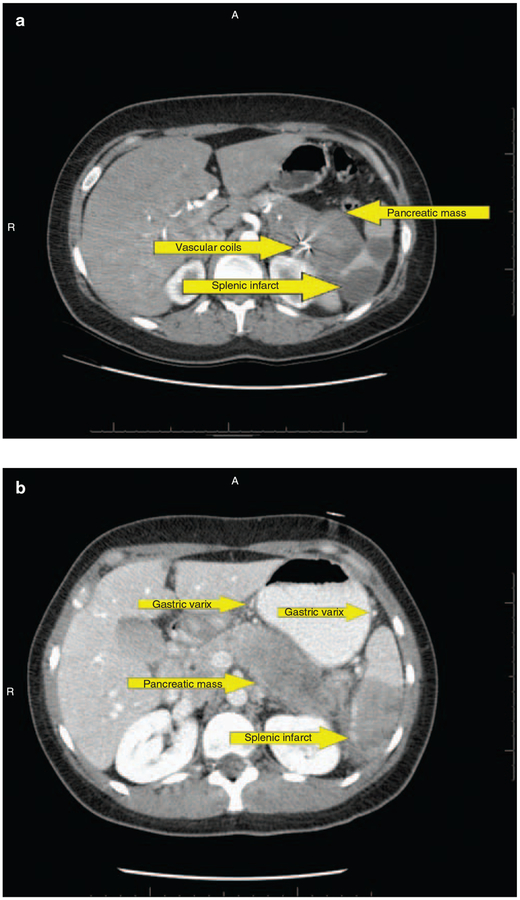
At diagnosis, 6 of the 26 (23%) patients were found to have involvement of major peri-pancreatic vasculature, including encasement and thrombosis of splenic vessels, splenic infarction secondary to splenic vascular involvement, and occlusion of the confluence between superior mesenteric, portal, and splenic veins (Table 3 and Figure 1). Hypercoagulable workup in all of these patients was negative. Vascular findings were more common in patients who had a mass in the pancreatic tail or diffuse pancreatic enlargement. The clinical course of one patient with splenic vein thrombosis was complicated by hematemesis secondary to gastric variceal bleeding that necessitated the placement of vascular coils to control bleeding (Figure 1a). In another patient, portal hypertensive gastropathy was observed during ERCP. None of these patients were treated with anticoagulants.
Table 3.
Clinically significant or impending vascular complications seen in a series of 26 autoimmune pancreatitis patients at the University of Pittsburgh
| Patient number | Pancreas phenotype | Vascular complication |
|---|---|---|
| 1 | Pancreatic tail mass | Splenic vein encasement Gastric varices |
| 19 | Pancreatic tail mass | Splenic vein thrombosis Splenic infarct Gastric varices |
| 21 | Pancreatic tail mass | Splenic vein obliterated Splenic varices Spontaneous splenorenal shunt |
| 26 | Pancreatic tail mass | Splenic vein thrombosis Gastric varices |
| 25 | Diffuse pancreatic enlargement | Splenic vein occlusion Gastric varices |
| 17 | Diffuse pancreatic enlargement | Splenic artery occlusion Splenic vein occlusion Splenic infarct |
Figure 1.
Contrast-enhanced computed tomography scan in (a) a patient with autoimmune pancreatitis showing a pancreatic mass, splenic infarct, vascular coil in splenic artery, and perigastric varices. (b) Another patient showing pancreatic mass, splenic infarct, and perigastric varices; 337 ×264mm (96×96 d.p.i.).
Histology and cytology
Of the 10 patients who underwent pancreatic histology, histological examination and IgG4 staining in 7 patients supported the diagnosis of AIP. One patient whose biopsy specimens did not fulfill all histological criteria for AIP had received prednisone for 4 weeks before obtaining biopsy specimens. One patient was diagnosed as chronic pancreatitis at an outside facility and his specimen was unavailable for review.
Histological examination of extrapancreatic specimens supported the diagnosis of chronic sclerosing sialadenitis with positive IgG4 staining in two patients. Of the remaining eight extrapancreatic histology specimens (five bile duct, two ampullary, and one thyroid), four were inadequate and four were inconclusive (Tables 1 and 2). An ampullary biopsy obtained in two patients was inconclusive.
EUS-guided fine needle aspiration was performed on 19 patients. There was no evidence of malignancy in 18 patients; epithelial features suspicious for malignancy were noted in one patient, which led to a pancreatic resection. Four of our 19 patients had either lymphocytes or stromal fragments in the absence of malignancy (but not both), and these subtle features were insufficient to render a diagnosis of AIP.
Extrapancreatic manifestations
Extrapancreatic involvement was observed in nine (35%) patients at presentation. This included biliary strictures (proximal CBD, hilar, or intrahepatic) in seven patients, and biliary strictures plus chronic sclerosing sialadenitis in two patients. Biliary dilatation and stenting was performed in all of these patients at initial presentation, and stents were removed after the resolution of strictures with immunosuppressive therapy. During follow-up, some patients required repeated dilatation and stenting. One of the patients with biliary strictures also had histologically confirmed Riedel’s thyroiditis and sclerosing mesenteritis. Two patients had submandibular gland swelling and two others had retroperitoneal fibrosis, but a histological evaluation was not performed in them.
Abnormally enlarged lymph nodes (>1 cm in long axis) in the upper abdomen (peri-pancreatic, porta hepatis, celiac, aortocaval) were seen on imaging studies in nine patients. EUS-guided fine needle aspiration cytology was performed in five of these patients and was negative for malignancy.
Treatment and follow-up
Six patients underwent partial or complete pancreatectomy for suspected pancreatic adenocarcinoma. One of these six patients had been treated empirically with prednisone for 4 weeks with no clinical and radiological improvement and underwent pancreatic tail resection. This patient and three other patients were followed-up after surgery for 6, 8, 10, and 36 months, respectively, without recurrence of disease. The remaining two patients were lost to follow-up.
One patient seen in the initial study period was treated with methotrexate. He responded completely within 6 weeks of initiating the therapy and remission was maintained with methotrexate 15 mg orally every week. Eight months later, he developed a recurrent biliary stricture treated with corticosteroid, balloon dilatation, and stenting, and azathioprine was added for long-term immunosuppression. A second recurrence was documented 5 years later, which was treated with corticosteroid, balloon dilatation, and stenting. This patient was on chronic immunosuppression with azathioprine at the end of the study period.
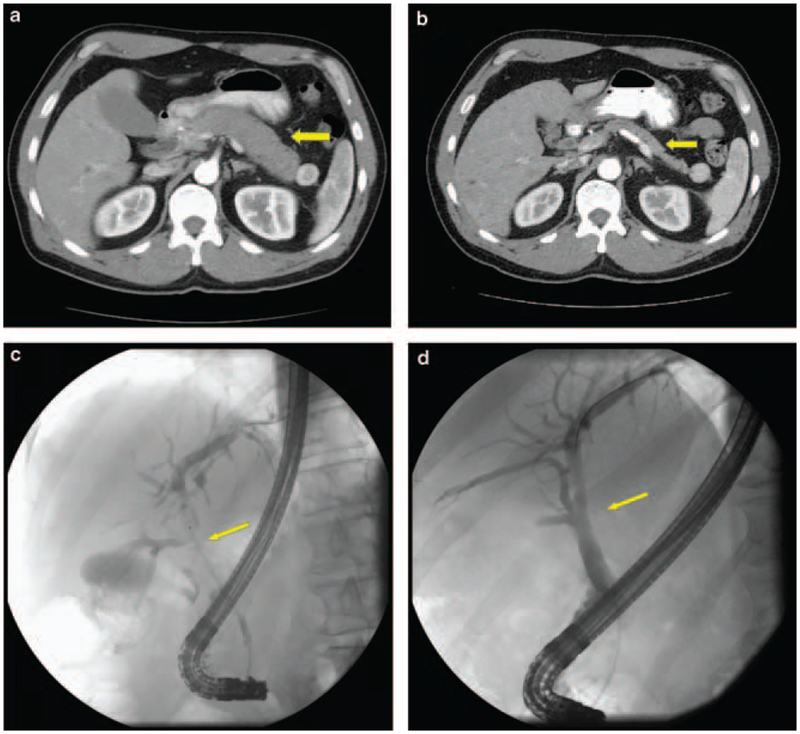
The remaining 19 patients received corticosteroids as the initial treatment. The usual regimen was oral prednisone 40 mg/day for 4 weeks followed by a taper of 5 mg/week. Within 12 weeks of initiating the therapy, a complete response (Figure 2) was observed in 15 (79%) and incomplete response in 4 (21%) patients. Among the 15 patients with a complete response, 9 had a recurrence within 8–12 weeks of steroid withdrawal presenting as recurrent biliary strictures in 4, recurrent pancreatic swelling in 3, and as recurrent stricturing of the main pancreatic duct in 2 patients. Recurrences were treated with corticosteroids during the acute flare-up and azathioprine was added for longterm immunosuppression. All nine patients responded and are being maintained on long-term azathioprine. Four of the 15 patients who responded completely with the initial therapy had no evidence of recurrence after steroid withdrawal during a follow-up of 5, 5, 5, and 6 months, respectively. One of the complete responders was lost to follow-up and one was being tapered off steroids at the end of the study period.
Figure 2.
Contrast-enhanced computed tomography in a patient with autoimmune pancreatitis (a) before initiating steroid treatment showing diffuse (sausage-shaped) pancreatic enlargement and (b) after 6 weeks of steroid treatment showing a decrease in size of the pancreas. Cholangiogram in a patient showing (c) extensive hilar stricturing before treatment and (d) after treatment showing resolution of strictures; 165×152 mm (96 × 96 d.p.i.).
Among the four patients with incomplete response to steroids, two had persistent pancreatic enlargement (complicated in one by the development of a cyst in the body and the tail), whereas two patients had partial resolution of biliary strictures. Of these four patients, three (including both patients with resistant biliary strictures) eventually responded to a combination of prednisone and azathioprine for 8, 4, and 4 months, respectively, followed by azathioprine alone. Attempts for withdrawing azathioprine in the two patients with biliary strictures were unsuccessful because of recurrent stricturing. The remaining one patient with incomplete response to steroids was started on azathioprine a few weeks before the end of the study period.
A subset analysis of treatment response in nine patients with extrapancreatic biliary strictures showed recurrence in 78% (7/9) after the withdrawal of immunosuppressive therapy. Four of the six patients who had achieved complete remission with initial prednisone therapy relapsed within 6–8 weeks of treatment withdrawal. Two patients with incomplete response to steroids, who had achieved complete remission with prolonged prednisone and azathioprine therapy, experienced relapse within 6–8 weeks of treatment withdrawal. One patient who was initially managed with methotrexate experienced a relapse while on treatment. Recurrences in all of these patients were similar to recurrent hilar and/or intrahepatic biliary strictures. After the first relapse, all patients were treated with prednisone and azathioprine, and long-term remission was achieved in six patients (median follow-up: 6, range: 5–84 months). Three patients experienced more than one relapse, requiring a high dose of corticosteroids during disease flares. One patient developed atrophy of the left lobe of the liver secondary to recurrent stricturing of intrahepatic biliary ducts.
One patient with pancreatic tail mass who underwent pancreatic tail resection and splenectomy had no evidence of splenic vascular compromise on repeat studies at 1 year. Five other patients who had vascular complications received immunosuppression for AIP. During follow-up, chronic splenic occlusion was observed in three of these five patients at 6, 8, and 14 months, respectively; stable splenic infarcts were seen in two and persistent gastric varices were observed in two patients.
DISCUSSION
Our report is the second major series on AIP patients from the United States. We confirm several of the findings on clinical presentation, imaging, and treatment response reported in previous studies on AIP (1,12–14,19,24). The lack of sensitivity of the currently established clinical criteria for diagnosis observed by us indicates that our knowledge of the complete spectrum of this disease is still evolving. Our study highlights the presence of vascular complications in a subset of AIP patients.
At presentation, none of the three commonly used diagnostic criteria (HISORt, the Japanese Pancreas Society, and Korean) were fulfilled in 15% of the patients (12,19). A lower sensitivity can be explained by several factors. Serum IgG4 was not measured in 8/26 patients. Four of these eight patients were evaluated before 2002 when the association between IgG4 and LPSP was not well known (13). These four patients had unexplained pancreatic disease (pancreatic mass plus biliary strictures) with a negative workup for known etiologies, including cancer. A complete resolution of pancreatic and/or extrapancreatic manifestations with immunosuppressive therapy (12 weeks or long term) was noted in all four patients. An increased serum IgG4 would have satisfied the HISORt B criteria in these patients.
Another four patients seen after 2002 underwent pancreatic resections for presumed pancreatic cancer without determination of IgG4 (all had LPSP and positive IgG4 staining). Of the two patients whose pancreatic biopsies did not fulfill all histological features of AIP, one had received an empirical steroid trial after pancreatic cancer was ruled out by EUS. Eight extrapancreatic histological specimens were either inconclusive or inadequate for evaluation. ERCP was not performed in all patients to delineate the presence of the irregular, non-dilated pancreatic duct, and finally, an excellent response to treatment with steroids was observed. None of the patients were diagnosed with pancreas or biliary cancer (the most common differential diagnosis). The clinical course and response to treatment indicates accuracy of AIP diagnosis in a majority of our patients. Pursuing additional workup (biopsies, ERCP, surgery) to enable fulfillment of the established criteria could have resulted in a higher sensitivity.
Several of the findings on clinical presentation (age, gender, prevalence of jaundice, diabetes, steatorrhea, and prevalence of extrapancreatic manifestations) in our series were similar to those reported from the United States, Japan, and other centers (11–14). Although the proportion of patients reporting abdominal pain in our series was higher than previously reported (12,14), it is important to note that the pain was mild in most patients and did not require narcotics for pain control. Two patients had chronic abdominal pain of unclear etiology and were on narcotics. They did not report a history of chronic alcoholism, and their imaging studies did not show features of chronic pancreatitis. Abdominal pain in both patients persisted despite resolution of pancreatic/extrapancreatic manifestations with steroids.
Serum IgG4 measurements are now performed routinely in patients with suspected AIP. The proportion of patients who had an increase in IgG4 (>140 mg/dl in 44% and >280 mg/dl in 27.8%) was lower than reported by Chari and colleagues (10) (>280 mg/dl in 53%). Although the exact reason for this difference is unclear, it emphasizes that a lack of IgG4 increase does not exclude the presence of AIP. At the same time, it is important to recognize that mild increases are oft en seen in patients with pancreatic cancer (10,25), which is the most important differential diagnosis of AIP.
EUS-guided fine needle aspiration was performed on 19 patients primarily to evaluate for malignancy, which is the most important differential diagnosis in patients with suspected AIP. In 18 out of 19 patients, a diagnosis of pancreatic cancer could be ruled out with confidence. In one patient, epithelial features suspicious of malignancy were noted leading to pancreatic resection. A recent study reported that clinical and radiological findings in conjunction with stromal fragments containing a lymphoid infiltrate on fine needle aspiration cytology could support a diagnosis of AIP and exclude carcinoma (26,27). Four of our 19 patients had either lymphocytes or stromal fragments in the absence of malignancy (but not both), and these subtle features were insufficient to render a diagnosis of AIP. Recent reports have indicated that a core specimen obtained by trucut biopsy during EUS can be used to histologically diagnose AIP (26,28). However, this procedure can be performed only at expert centers by endosonographers with considerable experience. As the risk associated with EUS-guided fine needle aspiration is low, we believe that the primary role of fine needle aspiration during the evaluation of a patient with suspected AIP will be to rule out malignancy, rather than to rule in a diagnosis of AIP.
An important observation in our study was the presence of peri-pancreatic vascular complications in 23% (6/26) of the patients. These findings varied from compression of vasculature to frank encasement and thrombosis of splenic, mesenteric vessels, and splenic infarction secondary to vascular compromise. Vascular complications were found to be closely related to the site of pancreatic inflammation and mass formation. Although the exact cause of this finding is unclear, we speculate that pancreatic and peri-pancreatic inflammation could have resulted in stasis and inflammation of the vascular wall predisposing the vessel to develop thrombosis.
Similar to previous reports, an excellent response was seen in an initial course of steroids (7,29–31). However, recurrences were common and necessitated the concomitant use of azathioprine to maintain remission. Recurrences were seen more frequently in patients who had extrapancreatic biliary strictures. IgG4-associated cholangitis is a newly described entity representing biliary manifestations of the IgG4-related systemic disease (2,32,33). It is defined as biliary strictures that respond to or improve with steroid therapy (2,32,33) and can oft en be confused with primary sclerosing cholangitis (2,32–36). Biliary strictures in these patients can be isolated or involve non-contiguous areas of bile duct. Nine patients in our series had evidence of isolated (n = 6) or multi-focal biliary (n = 3) stricturing proximal to the intrapancreatic portion of the bile duct that responded to steroid therapy. Although histology specimens obtained were not helpful in establishing diagnosis (inadequate in five and inconclusive in one), other features (dramatic response to steroids, concomitant presence of steroid responsive pancreatic mass, elevated IgG4 in three, ANA in three) strongly suggest the diagnosis of IAC in these patients. The higher mean age (63 years) of patients with extrapancreatic biliary strictures in our study compared with the typical age for primary sclerosing cholangitis was also similar to previous observations (2,32–36). None of these patients were previously diagnosed with primary sclerosing cholangitis or inflammatory bowel disease. Our findings are also consistent with recent reports of a high relapse rate after initial steroid treatment among patients with proximal biliary strictures (2). We believe that in patients with proximal strictures, it may be reasonable to start steroid-sparing immunosuppressant with the initial steroid therapy.
In summary, we report the second largest series on AIP patients from the United States. We confirm several findings on clinical presentation and management of these patients. The differences in our series from previous reports highlight the evolution of our knowledge of this disease. We report that vascular findings and complications involving the peri-pancreatic vasculature occur in a subset of AIP patients.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Autoimmune pancreatitis (AIP) is a treatable form of pancreatitis that is increasingly recognized.
AIP is part of a systemic fibro-inflammatory syndrome complex known as immunoglobulin-4 (IgG4)-related systemic disease.
Serum IgG4 levels are increased in approximately two-thirds of AIP patients.
Approximately 40 % of AIP patients have extrapancreatic involvement.
WHAT IS NEW HERE
The currently used diagnostic criteria do not identify all AIP patients.
The sensitivity of IgG4 in AIP may be lower than previously reported.
Clinically significant vascular complications occur in a subset of AIP patients.
Because of higher recurrence rates, early use of steroidsparing agents should be considered in AIP patients who have proximal biliary strictures.
ACKNOWLEDGMENTS
David C. Whitcomb MD is supported by NIDDK grants DK054709–09, DK061451–06A1, and U01 DK075803–01A1 NIDDK
Financial support: None
Footnotes
Potential competing interests: None
REFERENCES
- 1.Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol 2006; 41: 613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghazale A, Chari ST, Zhang L et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology 2008; 134: 706–15. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Nakajima H, Egawa N et al. IgG4-related sclerosing disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis and retroperitoneal fibrosis with lymphadenopathy. Pancreatology 2006; 6: 132–7. [DOI] [PubMed] [Google Scholar]
- 4.Kamisawa T IgG4-positive plasma cells specifically infiltrate various organs in autoimmune pancreatitis. Pancreas 2004; 29: 167–8. [DOI] [PubMed] [Google Scholar]
- 5.Kamisawa T, Funata N, Hayashi Y et al. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut 2003; 52: 683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawa S, Hamano H, Umemura T et al. Sclerosing cholangitis associated with autoimmune pancreatitis. Hepatol Res 2007;37 (Suppl 3): S487–95. [DOI] [PubMed] [Google Scholar]
- 7.Church NI, Pereira SP, Deheragoda MG et al. Autoimmune pancreatitis: clinical and radiological features and objective response to steroid therapy in a UK series. Am J Gastroenterol 2007; 102: 2417–25. [DOI] [PubMed] [Google Scholar]
- 8.Hamano H, Arakura N, Muraki T et al. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol 2006; 41: 1197–05. [DOI] [PubMed] [Google Scholar]
- 9.Deheragoda MG, Church NI, Rodriguez-Justo M et al. The use of immunoglobulin g4 immunostaining in diagnosing pancreatic and extrapancreatic involvement in autoimmune pancreatitis. Clin Gastroenterol Hepatol 2007; 5: 1229–34. [DOI] [PubMed] [Google Scholar]
- 10.Ghazale A, Chari ST, Smyrk TC et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol 2007; 102: 1646–53. [DOI] [PubMed] [Google Scholar]
- 11.Okazaki K Autoimmune pancreatitis: etiology, pathogenesis, clinical findings and treatment. Th e Japanese experience. JOP 2005; 6 (1 Suppl): 89–96. [PubMed] [Google Scholar]
- 12.Chari ST, Smyrk TC, Levy MJ et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol 2006; 4: 1010–6; quiz 934. [DOI] [PubMed] [Google Scholar]
- 13.Hamano H, Kawa S, Horiuchi A et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001; 344: 732–8. [DOI] [PubMed] [Google Scholar]
- 14.Song Y, Liu QD, Zhou NX et al. Diagnosis and management of autoimmune pancreatitis: experience from China. World J Gastroenterol 2008; 14: 601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang DH, Kim KW, Kim TK et al. Autoimmune pancreatitis: radiologic findings in 20 patients. Abdom Imaging 2006; 31: 94–102. [DOI] [PubMed] [Google Scholar]
- 16.Krasinskas AM, Raina A, Khalid A et al. Autoimmune pancreatitis. Gastroenterol Clin North Am 2007; 36: 239–57, vii. [DOI] [PubMed] [Google Scholar]
- 17.Okazaki K, Uchida K, Matsushita M et al. Autoimmune pancreatitis. Intern Med 2005; 44: 1215–23. [DOI] [PubMed] [Google Scholar]
- 18.Kim KP, Kim MH, Kim JC et al. Diagnostic criteria for autoimmune chronic pancreatitis revisited. World J Gastroenterol 2006; 12: 2487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okazaki K, Kawa S, Kamisawa T et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol 2006; 41: 626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon SH, Kim MH, Park DH et al. Is a two-week steroid trial after initial negative workup for malignancy useful in differentiating autoimmune pancreatitis from pancreatic cancer? A prospective outcome study. Gut 2008; 57: 1704–12. [DOI] [PubMed] [Google Scholar]
- 21.Kim T, Grobmyer SR, Dixon LR et al. Isolated lymphoplasmacytic sclerosing pancreatitis involving the pancreatic tail. Am Surg 2008; 74: 654–8. [PubMed] [Google Scholar]
- 22.Prasad P, Salem RR, Mangla R et al. Lymphoplasmacytic sclerosing pancreato-cholangitis: a case report and review of the literature. Yale J Biol Med 2004; 77: 143–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Witkiewicz AK, Kennedy EP, Kennyon L et al. Synchronous autoimmune pancreatitis and infiltrating pancreatic ductal adenocarcinoma: case report and review of the literature. Hum Pathol 2008; 39: 1548–51. [DOI] [PubMed] [Google Scholar]
- 24.Kim KP, Kim M, Lee YJ et al. [Clinical characteristics of 17 cases of autoimmune chronic pancreatitis]. Korean J Gastroenterol 2004; 43: 112–9. [PubMed] [Google Scholar]
- 25.Raina A, Krasinskas AM, Greer JB et al. Serum immunoglobulin G fraction 4 levels in pancreatic cancer: elevations not associated with autoimmune pancreatitis. Arch Pathol Lab Med 2008; 132: 48–53. [DOI] [PubMed] [Google Scholar]
- 26.Farrell JJ, Garber J, Sahani D et al. EUS findings in patients with autoimmune pancreatitis. Gastrointest Endosc 2004; 60: 927–36. [DOI] [PubMed] [Google Scholar]
- 27.Deshpande V, Mino-Kenudson M, Brugge WR et al. Endoscopic ultrasound guided fine needle aspiration biopsy of autoimmune pancreatitis: diagnostic criteria and pitfalls. Am J Surg Pathol 2005; 29: 1464–71. [DOI] [PubMed] [Google Scholar]
- 28.Levy MJ, Reddy RP, Wiersema MJ et al. EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointest Endosc 2005; 61: 467–72. [DOI] [PubMed] [Google Scholar]
- 29.Hirano K, Tada M, Isayama H et al. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut 2007; 56: 1719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park do H, Kim MH, Oh HB et al. Substitution of aspartic acid at position 57 of the DQbeta1 affects relapse of autoimmune pancreatitis. Gastroenterology 2008; 134: 440–6. [DOI] [PubMed] [Google Scholar]
- 31.Kamisawa T, Yoshiike M, Egawa N et al. Treating patients with autoimmune pancreatitis: results from a long-term follow-up study. Pancreatology 2005; 5: 234–8; discussion 238–240. [DOI] [PubMed] [Google Scholar]
- 32.Bjornsson E Immunoglobulin G4-associated cholangitis. Curr Opin Gastroenterol 2008; 24:389–94. [DOI] [PubMed] [Google Scholar]
- 33.Bjornsson E, Chari ST, Smyrk TC et al. Immunoglobulin G4 associated cholangitis: description of an emerging clinical entity based on review of the literature. Hepatology 2007; 45: 1547–54. [DOI] [PubMed] [Google Scholar]
- 34.Angulo P, Lindor KD. Primary sclerosing cholangitis. Hepatology 1999; 30: 325–32. [DOI] [PubMed] [Google Scholar]
- 35.Nakazawa T, Ohara H, Sano H et al. Cholangiography can discriminate sclerosing cholangitis with autoimmune pancreatitis from primary sclerosing cholangitis. Gastrointest Endosc 2004; 60: 937–44. [DOI] [PubMed] [Google Scholar]
- 36.Zen Y, Harada K, Sasaki M et al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol 2004; 28: 1193–203. [DOI] [PubMed] [Google Scholar]