Key Points
Question
What is the effect of a use-tracking incentive spirometer (IS) reminder on patient adherence and clinical outcomes following coronary artery bypass grafting surgery?
Findings
In this randomized clinical trial that included 160 patients undergoing coronary artery bypass grafting, the patient reminder improved IS use adherence, atelectasis severity, early postoperative fevers, noninvasive positive pressure ventilation use, intensive care unit length of stay by a day, and 6-month mortality rates.
Meaning
With the reminder, IS appears to be clinically effective when used appropriately.
Abstract
Importance
Incentive spirometers (ISs) were developed to reduce atelectasis and are in widespread clinical use. However, without IS use adherence data, the effectiveness of IS cannot be determined.
Objective
To evaluate the effect of a use-tracking IS reminder on patient adherence and clinical outcomes following coronary artery bypass grafting (CABG) surgery.
Design, Setting, and Participants
This randomized clinical trial was conducted from June 5, 2017, to December 29, 2017, at a tertiary referral teaching hospital and included 212 patients who underwent CABG, of whom 160 participants were randomized (intent to treat), with 145 completing the study per protocol. Participants were stratified by surgical urgency (elective vs nonelective) and sex (men vs women).
Interventions
A use-tracking, IS add-on device (SpiroTimer) with an integrated use reminder bell recorded and timestamped participants’ inspiratory breaths. Patients were randomized by hourly reminder “bell on” (experimental group) or “bell off” (control group).
Main Outcomes and Measures
Incentive spirometer use was recorded for the entire postoperative stay and compared between groups. Radiographic atelectasis severity (score, 0-10) was the primary clinical outcome. Secondary respiratory and nonrespiratory outcomes were also evaluated.
Results
A total of 145 per-protocol participants (112 men [77%]; mean age, 69 years [95% CI, 67-70]; 90 [62%] undergoing a nonelective procedure) were evaluated, with 74 (51.0%) in the bell off group and 71 (49.0%) in the bell on group. The baseline medical and motivation-to-recover characteristics of the 2 groups were similar. The mean number of daily inspiratory breaths was greater in bell on (35; 95% CI, 29-43 vs 17; 95% CI, 13-23; P < .001). The percentage of recorded hours with an inspiratory breath event was greater in bell on (58%; 95% CI, 51-65 vs 28%; 95% CI, 23-32; P < .001). Despite no differences in the first postoperative chest radiograph mean atelectasis severity scores (2.3; 95% CI, 2.0-2.6 vs 2.4; 95% CI, 2.2-2.7; P = .48), the mean atelectasis severity scores for the final chest radiographs conducted before discharge were significantly lower for bell on than bell off group (1.5; 95% CI, 1.3-1.8 vs 1.8; 95% CI, 1.6-2.1; P = .04). Of those with early postoperative fevers, fever duration was shorter for bell on (3.2 hours; 95% CI, 2.3-4.6 vs 5.2 hours; 95% CI, 3.9-7.0; P = .04). Having the bell turned on reduced noninvasive positive pressure ventilation use rates (37.2%; 95% CI, 24.1%-52.5% vs 19.2%; 95% CI, 10.2%-33.0%; P = .03) for participants undergoing nonelective procedures. Bell on reduced the median postoperative length of stay (7 days; 95% CI, 6-9 vs 6 days; 95% CI, 6-7; P = .048) and the intensive care unit length of stay for patients undergoing nonelective procedures (4 days; 95% CI, 3-5 vs 3 days; 95% CI, 3-4; P = .02). At 6 months, the bell off mortality rate was higher than bell on (9% vs 0%, P = .048) for participants undergoing nonelective procedures.
Conclusions and Relevance
The incentive spirometer reminder improved patient adherence, atelectasis severity, early postoperative fever duration, noninvasive positive pressure ventilation use, ICU and length of stay, and 6-month mortality in certain patients. With the reminder, IS appears to be clinically effective when used appropriately.
Trial Registration
ClinicalTrials.gov identifier: NCT02952027
This randomized clinical trial evaluates the effect of an audible, use-tracking incentive spirometer reminder for US patients who have undergone coronary artery bypass grafting.
Introduction
Atelectasis is one of the most common postoperative pulmonary complications,1 affecting up to 90% of patients after surgery.2 The condition of deflated alveoli has been associated with early postoperative fevers,3 increased admission to the intensive care unit (ICU),4 early postoperative mortality,4 and an increased length of stay.4 First developed in the 1970s to reduce atelectasis, incentive spirometers (ISs) work to open alveoli through sustained maximal inspiration.5 Although IS is routinely prescribed and poses an annual cost of $1.04 billion6 in postoperative patients, to our knowledge, its effectiveness has yet to be demonstrated,7 largely because of the inability to reliably track use adherence.8,9 If a patient is not using his or her IS, then its clinical effectiveness cannot be ascertained. Therefore, to establish the effectiveness of IS, the effect of IS use adherence must be studied.
Incentive spirometer use adherence cannot be controlled or randomized a priori; thus, an intervention to increase adherence, which can be randomized, can be used. In this case, increases in adherence should then translate into better outcomes if IS is effective. Reminders have been demonstrated to increase patient adherence with various self-administered therapies.10 The objective of this study was to evaluate the effects of an IS reminder on adherence and, in turn, clinical outcomes in patients recovering from coronary artery bypass grafting (CABG) surgery. The reminder was hypothesized to improve IS use adherence and, as a result, postoperative atelectasis and secondary clinical outcomes. Patients undergoing nonelective surgery were hypothesized to demonstrate the greatest benefit, as they were at higher risk for postoperative respiratory complications than patients undergoing elective surgery.11
Methods
Experimental Design and Sample
This was a single-center, randomized clinical trial. The study was performed at a tertiary referral teaching hospital with 4 experienced, board-certified cardiothoracic surgeons. Consecutive patients undergoing CABG surgery between June 5, 2017, and December 29, 2017, were approached for preoperative consent and all participants who gave written informed consent were enrolled in the study. As standard of care after CABG, following extubation all participants were prescribed IS as part of a standardized pulmonary hygiene regimen that also included ambulation, an oscillating positive expiratory pressure device, and pain control. Standard nursing IS orders were to remind patients to use the device routinely every 4 hours.
Participants were randomized to either the experimental “bell on” group (the bell sound turned on) or the control “bell off” group (the bell sound turned off) for their entire postoperative hospital stay. Without a bell, the bell off group was representative of current IS treatment practices. Participants were permuted block randomized by prespecified factors that could affect adherence and/or clinical outcomes, such as sex12,13 (male or female) and operative urgency14,15 (elective vs nonelective). A list of computer-randomized group assignments was generated before the study. After their surgery, sequential participants were randomized by accessing this list while concealing their subsequent allocation. The exclusion criteria were individuals who did not or were not able to give informed consent themselves or individuals who were unable to hear the researcher during the consent process conversation, given the audible nature of the reminder. This study was approved by the Rhode Island Hospital institutional review board and the reporting of this trial was done in accordance with Consolidated Standards of Reporting Trials guidelines (Supplement 1). Because the motivation to recover may influence IS use adherence, factors related to patient motivation (self-efficacy,16 effort attribution,17 implicit theory of change,18 and locus of control19) were assessed before surgery using a questionnaire (eTable 1 in Supplement 2) to ensure that the randomization of groups included this prespecified potential confounder.
Use Tracking With the IS Reminder Device
A use-tracking IS add-on device (SpiroTimer) was developed to record and timestamp patient inspiratory breaths greater than a daily target threshold inspiratory volume using a light-based sensor. The SpiroTimer attached to the participants’ IS (4000 mL PORTEX Coach2; Smiths Medical). The SpiroTimer was set up and turned on every postoperative morning after participants were extubated and not receiving noninvasive positive pressure ventilation (continuous or bilevel positive airway pressure) while in the hospital. Each morning, the target threshold inspiratory volume was set at the greatest of 3 initial attempts by the study staff members at the bedside to successively approximate the participants’ postoperative inspiratory volumes. The SpiroTimer had an integrated 75-decibel bell that could sound for up to 2 minutes every hour, which is the clinically suggested IS use frequency.20,21 The bell could be silenced if the participant completed an inspiratory breath greater than his or her target threshold volume. The SpiroTimer recorded every inspiratory breath regardless of whether the bell was turned on or off, thereby tracking the use adherence for both groups. To avoid sleep-wake cycle disruption and to recreate how IS is currently implemented, the SpiroTimer was turned on each morning (between 6:30 am-8:30 am) and continued to record use until bedtime (8 pm-9 pm) each day.
Some days IS data could not be collected or were unreliable because of events such as delirium, air leaks from chest tubes, SpiroTimer power interruptions, and mouth obstruction from the presence of a supplemental oxygen device, such as a face mask, that precluded IS use. In addition, data were unreliable on the days that participants moved rooms or were discharged because of moving-related activities that affected patient IS performance and measurement (eg, the SpiroTimer was temporarily located in a different room from the patient) and were thus excluded for all participants.
Power Analysis
Because IS was developed to open alveoli and reduce atelectasis, it is presumed that increased IS adherence would lead to a reduction in atelectasis relative to treatment as usual. Thus, this study was powered for superiority, in which those receiving bell on would have less atelectasis relative to those with bell off (treatment as usual). Given the ubiquity2 of post-CABG22 atelectasis, validated23 atelectasis severity scores were used to evaluate more subtle differences than a binary outcome.
In a study of post-CABG atelectasis, Joyce et al24 reported a mean (SD) Wilcox25 severity score of 2.5 (1.8) for all patient chest radiograph (CXR) results. For this study, the mean (SD) atelectasis severity score was assumed to be 2.5 (1.8) for the treatment as usual condition (bell off) at discharge; for the bell on group, a 1-point difference from this was selected, as it is the smallest increment of change on the Wilcox scale. To achieve 80% power using an α of .05 with a 10% adjustment for attrition and crossover for each condition, the total sample size was calculated to be 160 participants.
Outcome Measures
Incentive spirometer adherence was hypothesized to drive clinical improvement; thus, there were 2 primary outcomes: a nonclinical and clinical outcome. The primary nonclinical outcome measure was IS use adherence, measured by the number of inspiratory breaths achieved per day and the proportion of recorded hours during which participants successfully completed at least 1 inspiratory breath.
The primary clinical outcome measure was atelectasis severity on the first postextubation postoperative CXR (anteroposterior view) (excluding any obtained during noninvasive positive pressure ventilation therapy) and the final CXR (anteroposterior or posterioranterior view) results obtained before discharge. Each CXR was scored by 2 experienced, thoracic radiology fellowship–trained, board-certified radiologists who were masked to the intervention using the straightforward and validated23 modified24 Wilcox25 score per lung (eTable 2 in Supplement 2).
Febrile episodes (period of time with temperatures ≥38°C) during the first 4 postoperative days were evaluated.26 The characteristics of the febrile episodes were compared between groups. The following additional secondary clinical outcome measures were examined: length of stay (ICU and total postoperative days), rate of return to baseline pulmonary function (forced expiratory volume in the first second [FEV1], forced vital capacity [FVC], FEV1/FVC ratio, and peak expiratory flow [PEF]), IS inspiratory volumes, supplemental oxygen use (use rates and duration), pulmonary complication rates (eg, reintubation, tracheostomy, pneumonia, pneumothorax, pulmonary edema severity, pleural effusion, bronchospasm, and pulmonary emboli), discharge location, and mortality (in-hospital and 6-month). All primary and secondary outcome measures, along with operative urgency subgroups (elective and nonelective), were planned analyses a priori.
To assess the effect of the IS bell on nursing workload, questionnaires were distributed in person at 6:45 pm daily to nurses who worked the 7 am to 7 pm shift caring for participants enrolled in the study. For each patient, nurses were asked to answer these questions: (1) how many times did you remind this patient to use their IS? (“episodes”) and (2) how much time did you spend educating or reminding this patient to use their IS? (“minutes”).
Statistical Methods
All analyses were conducted using SAS, version 9.4 (SAS Institute) and all modeling examined the effects between conditions (bell on vs bell off) by elective and nonelective status using the GLIMMIX procedure (SAS). The data were analyzed by the first 2 authors (A.E. M.E. and G.L.B.). The differences in baseline characteristics were assessed using the Fisher exact test and the t test for categorical and normal data, respectively. Use adherence was examined for the number of inspiratory breaths achieved per day and the proportion of recorded hours per day that participants successfully completed at least 1 breath using generalized mixed modeling (GMM) with sandwich estimation, in which observations were nested within participants, assuming negative binomial and binomial distributions, respectively. Wilcox atelectasis severity scores were examined at 2 separate points, first and final CXR, also using GMM, in which scores were nested within participants. Radiologist agreement was assessed using the Kendall coefficient of concordance with the %MAGREE SAS macro. The count and times of reminders from nursing, number of fevers and duration of fevers, duration of high-flow cannula and non-rebreather mask, and ventilation were all modeled using GMM assuming a negative binomial distribution; motivational questions, reintubation, tracheostomy, and mortality rates were modeled using GMM assuming a binomial distribution. Differences between conditions and surgical electively status for ICU and total postoperative length of stay were examined using Kaplan-Meier estimation with the LIFETEST procedure (SAS); because censoring existed due to in-hospital death, the log-rank test was used. Incentive spirometer volumes and pulmonary function tests (eg, FEV1, FVC, FEV/FVC, and PEF δ from baseline) were modeled using GMM assuming a normal distribution. Pulmonary complications, discharge location, and 6-month mortality were assessed using a Fisher exact test. The α was established, a priori, at the .05 level and interval estimates were calculated for 95% confidence; Tukey corrections were used for post hoc comparisons when appropriate. All analyses were conducted for the per-protocol (n = 145) and intent-to-treat (n = 160) populations.
Results
Participants
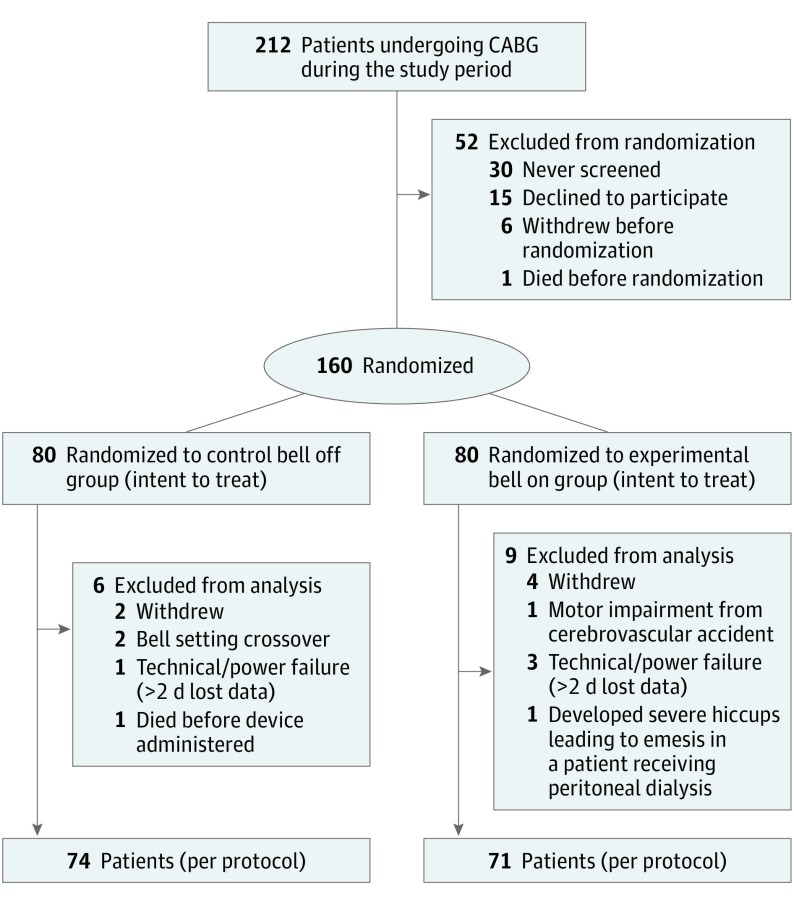
From June 5, 2017, to December 29, 2017, 212 patients underwent CABG. A total of 160 participants consented and were randomized, with 80 in each group (intent to treat), thereby satisfying the sample size requirement. After nonadherence and/or crossover, 145 (68.4%) completed the study per-protocol (112 men [77%]; mean age, 68.7 years; 95% CI, 67.2-70.2), with 74 participants randomized to the control bell off group and 71 to the experimental bell on group (Figure).
Figure. Study Design Flow Diagram.
CABG indicates coronary artery bypass grafting.
The baseline medical and surgical characteristics of the groups were not observed to be different (Table 1; eTable 3 in Supplement 2). There was no difference observed between groups in the assessed baseline motivational factors of adherence (eTable 4 in Supplement 2), thereby confirming a successful overall randomization and that of the motivational factors.
Table 1. Study Participant Characteristics (Intent to Treat).
| Variable | Control Bell Off (n = 80) | Experimental Bell On (n = 80) |
|---|---|---|
| Preoperative factors | ||
| Patients, No. (%) | ||
| Nonelective | 45 (56.3) | 51 (63.8) |
| Elective | 35 (43.8) | 29 (36.3) |
| Age, mean (95% CI), y | ||
| Overall | 69.9 (67.7-72.1) | 67.5 (65.4-69.6) |
| Nonelective | 69.0 (66.2-71.9) | 66.8 (64.3-69.3) |
| Elective | 70.9 (67.5-74.4) | 68.7 (64.6-72.8) |
| Male, No. (%) | 62 (77.5) | 61 (76.3) |
| Nonelective | 37 (46.3) | 39 (48.8) |
| Elective | 25 (31.3) | 22 (27.5) |
| BMI, mean (95% CI) | ||
| Overall | 31.0 (29.9-32.2) | 30.7 (29.6-31.8) |
| Nonelective | 30.6 (29.0-32.1) | 30.7 (29.4-31.9) |
| Elective | 31.6 (29.7-33.6) | 30.9 (28.8-33.0) |
| ASA class, mean (95% CI) | ||
| Overall | 4.0 (3.9-4.0) | 4.0 (3.9-4.0) |
| Nonelective | 4.0 (3.9-4.0) | 3.9 (3.8-4.0) |
| Elective | 4.0 (3.9-4.0) | 4.0 (4.0-4.0) |
| FEV1, mean (95% CI), L | ||
| Overall | 2.0 (1.8-2.2) | 2.0 (1.9-2.2) |
| Nonelective | 2.0 (1.7-2.2) | 2.0 (1.7-2.2) |
| Elective | 2.1 (1.8-2.3) | 2.2 (1.9-2.5) |
| FEV1 predicted, mean (95% CI), % | ||
| Overall | 70.9 (66.1-75.6) | 68.7 (63.3-74.1) |
| Nonelective | 67.4 (61.1-73.6) | 68.2 (61.3-75.2) |
| Elective | 76.6 (70.4-82.9) | 73.2 (65.9-80.5) |
| FVC, mean (95% CI), L | ||
| Overall | 2.5 (2.3-2.7) | 2.6 (2.4-2.8) |
| Nonelective | 2.4 (2.1-2.6) | 2.5 (2.2-2.8) |
| Elective | 2.6 (2.3-2.8) | 2.8 (2.5-3.1) |
| FVC predicted, mean (95% CI), % | ||
| Overall | 65.1 (61.3-68.9) | 66.1 (61.9-70.4) |
| Nonelective | 60.6 (55.5-65.6) | 64.0 (57.9-70.1) |
| Elective | 70.9 (65.7-76.1) | 69.9 (65.2-74.5) |
| OSA, No. (%) | 16 (20.0) | 18 (22.5 |
| Nonelective | 9 (11.3) | 12 (15.0) |
| Elective | 7 (8.8) | 6 (7.5) |
| Asthma, No. (%) | 5 (6.3) | 6 (7.5) |
| Nonelective | 1 (12.5) | 3 (3.8) |
| Elective | 4 (5.0) | 3 (3.8) |
| Smoker, No. (%) | ||
| Current smoker | 8 (10.0) | 6 (7.5) |
| Nonelective | 6 (7.5) | 5 (6.3) |
| Elective | 2 (2.5) | 1 (1.3) |
| Former smoker | 43 (53.8) | 42 (52.5) |
| Nonelective | 22 (27.5) | 28 (35.0) |
| Elective | 21 (26.3) | 14 (17.5) |
| Never smoked | 29 (36.3) | 32 (40.0) |
| Nonelective | 17 (21.3) | 18 (22.5) |
| Elective | 12 (15.0) | 14 (17.5) |
| Pack-years, mean (95% CI) | ||
| Overall | 12.3 (6.8-17.9) | 7.3 (4.1-10.4) |
| Nonelective | 6.0 (1.9-10.1) | 6.7 (3.0-10.5) |
| Elective | 20.5 (9.3-31.8) | 8.2 (2.3-14.0) |
| COPD, No. (%) | 11 (13.8) | 12 (15.0) |
| Nonelective | 7 (8.8) | 8 (10.0) |
| Elective | 4 (5.0) | 4 (5.0) |
| Diabetes, No. (%) | 41 (51.3) | 35 (43.8) |
| Nonelective | 27 (33.8) | 22 (27.5) |
| Elective | 14 (17.5) | 13 (16.3) |
| GERD, No. % | 20 (25.0) | 20 (25.0) |
| Nonelective | 9 (11.3) | 8 (10.0) |
| Elective | 11 (13.8) | 12 (15.0) |
| SpO2, mean (95% CI) | ||
| Overall | 97.2% (96.8-97.6) | 97.3% (96.8-97.7) |
| Nonelective | 97.0 (96.4-97.6) | 97.1 (96.6-97.7) |
| Elective | 97.4% (96.9-98.0) | 97.5 (96.8-98.2) |
| Perioperative factors | ||
| Duration of anesthesia, mean (95% CI), min | ||
| Overall | 294 (282-312) | 300 (282-318) |
| Nonelective | 294 (276-312) | 294 (276-312) |
| Elective | 300 (276-318) | 300 (264-342) |
| Duration of cardiopulmonary bypass, mean (95% CI), min | ||
| Overall | 114 (102-126) | 114 (102-120) |
| Nonelective | 114 (96-126) | 114 (102-120) |
| Elective | 114 (96-132) | 108 (90-132) |
| Duration of aortic cross clamp, mean (95% CI), min | ||
| Overall | 92 (83-101) | 90 (81-99) |
| Nonelective | 88 (77-99) | 88 (78-98) |
| Elective | 97 (84-111) | 93 (75-112) |
| Vessels bypassed, No. (95% CI) | ||
| Overall | 3 (3-3) | 3 (3-3) |
| Nonelective | 3 (3-3) | 3 (3-3) |
| Elective | 3 (2-3) | 3 (2-3) |
| Graft type | ||
| Left internal mammary artery, No. (%) | 70 (87.5) | 78 (97.5) |
| Nonelective | 41 (91.1) | 51 (100) |
| Elective | 29 (82.9) | 27 (93.1) |
| Right internal mammary artery, No. (%) | 6 (7.5) | 12 (15.0 |
| Nonelective | 4 (8.9) | 9 (17.6) |
| Elective | 2 (5.7) | 3 (10.3) |
| Bilateral internal mammary artery, No. (%) | 5 (6.3) | 12 (15.0) |
| Nonelective | 3 (6.7) | 9 (17.6) |
| Elective | 2 (5.7) | 3 (10.3) |
| Saphenous vein, No. (%) | 73 (91.3) | 75 (93.8) |
| Nonelective | 42 (93.3) | 50 (98.0) |
| Elective | 31 (88.6) | 25 (86.2) |
| Radial artery, No. (%) | 9 (11.3) | 6 (7.5) |
| Nonelective | 7 (15.6) | 4 (7.8) |
| Elective | 2 (5.7) | 2 (6.9) |
| CABG performed with additional surgical procedure, No. (%) | 21 (26.3) | 23 (28.8) |
| Nonelective | 7 (8.8) | 11 (13.8) |
| Elective | 14 (17.5) | 12 (15.0) |
| Additional surgical procedures | ||
| Aortic valve replacement, No. (%) | 10 (12.5) | 15 (18.8) |
| Nonelective | 4 (8.9) | 6 (11.8) |
| Elective | 6 (17.1) | 9 (31.0) |
| Mitral valve replacement, No. (%) | 3 (3.8) | 2 (2.5) |
| Nonelective | 0 (0.0) | 2 (3.9) |
| Elective | 3 (8.6) | 0 (0.0) |
| Mitral valve repair, No. (%) | 4 (5.0) | 1 (1.3) |
| Nonelective | 1 (2.2) | 0 (0.0) |
| Elective | 3 (8.6) | 1 (3.4) |
| Maze, No. (%) | 5 (6.3) | 2 (2.5) |
| Nonelective | 1 (2.2) | 1 (2.0)] |
| Elective | 4 (11.4) | 1 (3.4) |
| Left atrial appendage closure, No. (%) | 5 (6.3) | 2 (2.5) |
| Nonelective | 2 (4.4) | 1 (2.0) |
| Elective | 3 (8.6) | 1 (3.4) |
| Coronary endarterectomy, No. (%) | 0 (0) | 4 (5.0) |
| Nonelective | 0 (0) | 2 (3.9) |
| Elective | 0 (0) | 2 (7.0) |
| Insertion of intra-aortic balloon pump, No. (%) | 2 (2.5) | 1 (1.3) |
| Nonelective | 2 (4.4) | 1 (2.0) |
| Elective | 0 (0) | 0 (0) |
| Carotid endarterectomy, No. (%) | 1 (1.3) | 1 (1.3) |
| Nonelective | 1 (2.2) | 1 (2.0) |
| Elective | 0 (0) | 0 (0) |
| Septal myectomy, No. (%) | 1 (1.3) | 0 (0) |
| Nonelective | 0 (0) | 0 (0) |
| Elective | 1 (2.9) | 0 (0.0) |
| Ligation of coronary artery to pulmonary artery fistula, No. (%) | 1 (1.3) | 0 (0) |
| Nonelective | 0 (0) | 0 (0) |
| Elective | 1 (2.9) | 0 (0) |
| Lung lobectomy, No. (%) | 1 (1.3) | 0 (0) |
| Overall | 1 (1.3) | 0 (0) |
| Nonelective | 0 (0) | 0 (0) |
| Elective | 1 (2.9) | 0 (0) |
| Redo sternotomy, No. (%) | 2 (2.5) | 1 (1.3) |
| Nonelective | 2 (4.4) | 1 (2.0) |
| Elective | 0 (0) | 0 (0) |
| Chest tube size, No. (%), F | ||
| Mediastinal | ||
| 32 | 77 (96.3) | 71 (88.8) |
| Nonelective | 42 (93.3) | 46 (90.2) |
| Elective | 35 (100.0) | 25 (86.2) |
| 28 | 40 (50.0) | 37 (46.3) |
| Nonelective | 27 (60.0) | 20 (39.2) |
| Elective | 13 (37.1) | 17 (58.6) |
| 24 | 1 (1.3) | 2 (2.5) |
| Nonelective | 1 (2.2) | 1 (2.0) |
| Elective | 0 (0) | 1 (3.4) |
| 19 | 1 (1.3) | 3 (3.8) |
| Nonelective | 0 (0.0) | 2 (3.9) |
| Elective | 1 (2.9) | 1 (3.4) |
| Pleural | ||
| 28 | 35 (43.8) | 35 (43.8) |
| Nonelective | 18 (40.0) | 25 (49.0) |
| Elective | 17 (48.6) | 10 (34.5) |
| 24 | 39 (48.8) | 42 (52.5) |
| Nonelective | 26 (57.8) | 26 (51.0) |
| Elective | 13 (37.1) | 16 (55.2) |
| 19 | 1 (1.3) | 1 (1.3) |
| Nonelective | 0 (0.0) | 1 (2.0) |
| Elective | 1 (2.9) | 0 (0.0) |
| Postoperative factors | ||
| Duration of mechanical ventilation, mean (95% CI), h | ||
| Overall | 23.5 (8.1-39.0) | 18.5 (14.6-22.40 |
| Nonelective | 31.0 (3.5-58.4) | 16.7 (13.7-19.7) |
| Elective | 14.0 (9.9-18.1) | 21.6 (11.9-31.3) |
| Presence of NGT or OGT, No. (%) | 39 (48.8) | 38 (47.5) |
| Nonelective | 25 (31.3) | 27 (33.8) |
| Elective | 14 (17.5)] | 11 (13.8) |
| Duration with ≥1 chest tube, mean (95% CI), d | 4.3 (3.7-4.9) | 3.9 (3.4-4.3) |
| Nonelective | 4.8 (3.9-5.7) | 3.8 (3.2-4.4) |
| Elective | 3.7 (2.9-4.5) | 3.9 (3.1-4.7) |
Abbreviations: ASA, American Society of Anesthesiologists, class range, 1 to 6; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; GERD, gastroesophageal reflux disease; NGT, nasogastric tube; OGT, orogastric tube; OSA, obstructive sleep apnea; SpO2, peripheral capillary oxygen saturation, last reading before intubation for surgery.
Use Adherence
Overall, the mean number of daily inspiratory breaths (overall, 35.4 vs 17.1; P < .001) and percentage of recorded hours with at least 1 or more inspiratory breaths (overall, 58.2% vs 27.5%; P < .001) more than doubled for bell on vs bell off. This effect held for participants undergoing nonelective and elective surgery (Table 2).
Table 2. Outcomes.
| Characteristic | Per Protocol | Intent to Treat | ||||
|---|---|---|---|---|---|---|
| Control Bell Off, Mean (95% CI) | Experimental Bell On, Mean (95% CI) | P Value | Control Bell Off, Mean (95% CI) | Experimental Bell On, Mean (95% CI) | P Value | |
| Use adherence | ||||||
| Daily inspiratory breaths, No. | ||||||
| Overall | 17 (13-23) | 35 (29-43) | <.001 | 17 (12-22) | 34 (28-41) | <.001 |
| Nonelective | 16 (11-23) | 33 (26-42) | <.001 | 16 (11-22) | 32 (25-41) | <.001 |
| Elective | 19 (12-29) | 40 (29-56) | <.001 | 18 (12-28) | 37 (27-51) | <.001 |
| Hours with ≥1 inspiratory breath, % | ||||||
| Overall | 28 (23-32) | 58 (51-65) | <.001 | 28 (23-32) | 58 (51-65) | <.001 |
| Nonelective | 27 (21-33) | 59 (52-65) | <.001 | 26 (21-33) | 59 (52-66) | <.001 |
| Elective | 29 (23-36) | 57 (42-70) | <.001 | 29 (23-36) | 57 (44-69) | <.001 |
| Postoperative radiographic atelectasis severity scores | ||||||
| First CXR | 2.4 (2.2-2.7) | 2.3 (2.0-2.6) | .48 | 2.4 (2.2-2.7) | 2.4 (2.2-2.7) | .87 |
| Final CXR | 1.8 (1.6-2.1) | 1.5 (1.3-1.8) | .04 | 1.9 (1.6-2.1) | 1.5 (1.3-1.8) | .04 |
| Early postoperative fevers | ||||||
| Febrile episodes, rate (%) | ||||||
| Overall | 5 (2-11) | 3 (1-6) | .13 | 4 (2-10) | 3 (1-6) | .24 |
| Nonelective | 6 (2-14) | 3 (1-8) | .11 | 5 (2-13) | 3 (1-8) | .26 |
| Elective | 4 (1-11) | 2 (0-6) | .11 | 3 (1-9) | 2 (1-7) | .26 |
| Febrile duration, h | ||||||
| Overall | 5 (4-7) | 3 (2-5) | .04 | 5 (4-7) | 3 (3-5) | .047 |
| Nonelective | 5 (4-7) | 4 (2-6) | .01 | 5 (4-7) | 4 (2-6) | .02 |
| Elective | 6 (4-10) | 3 (2-4) | .01 | 6 (4-10) | 3 (2-4) | .02 |
| Febrile peak temperatures, °C | ||||||
| Overall | 38.3 (38.2-38.4) | 38.2 (38.1-38.2) | .01 | 38.3 (38.2-38.4) | 38.2 (38.1-38.2) | .01 |
| Nonelective | 38.3 (38.2-38.4) | 38.2 (38.1-38.2) | .048 | 38.3 (38.2-38.4) | 38.2 (38.1-38.2) | .05 |
| Elective | 38.4 (38.2-38.6) | 38.1 (38.1-38.2) | .10 | 38.4 (38.2-38.6) | 38.2 (38.1-38.3) | .11 |
| Noninvasive positive pressure ventilation | ||||||
| Use rate, No. (%) | ||||||
| Overall | 34 (24-45) | 24 (15-35) | .10 | 33 (23-44) | 25 (17-36) | .15 |
| Nonelective | 37 (24-53) | 19 (10-33) | .03 | 38 (25-53) | 22 (12-35) | .04 |
| Elective | 29 (16-47) | 33 (18-54) | .37 | 26 (14-43) | 31 (17-50) | .32 |
| Length of stay | ||||||
| Total postoperative length of stay, d | ||||||
| Overall | 7 (6-8) | 7 (6-7) | .31 | 7 (6-7) | 7 (6-7) | .49 |
| Nonelective | 7 (6-9) | 6 (6-7) | .048 | 7 (6-9) | 6 (6-7) | .05 |
| Elective | 6 (6-7) | 7 (6-7) | .48 | 6 (6-7) | 7 (6-7) | .16 |
| Intensive care unit length of stay, d | ||||||
| Overall | 4 (3-5) | 3 (3-4) | .06 | 4 (3-5) | 3.5 (3-4) | .08 |
| Nonelective | 4 (3-6) | 3 (3-4) | .02 | 4 (3-6) | 3 (3-4) | .02 |
| Elective | 4 (3-5) | 4 (2-5) | .65 | 4 (3-5) | 4 (2-5) | .49 |
| 6-mo Mortality | ||||||
| Deaths, No. (%) | ||||||
| Overall | 9.5 (3.9-18.5) | 2.8 (0.3-9.8) | .17 | 10.0 (4.4-18.8) | 2.5 (0.3-8.7) | .10 |
| Nonelective | 9.3 (2.6-22.1) | 0.0 (0.0-0.0) | .048 | 8.9 (2.5-21.2) | 0.0 (0.0-0.0) | .04 |
| Elective | 9.7 (2.0-25.8) | 8.3 (1.0-27.0) | .99 | 11.4 (3.2-26.7) | 6.9 (0.8-22.8) | .68 |
Abbreviation: CXR, chest radiographs.
Atelectasis
No differences were observed for mean atelectasis severity scores between bell on and bell off (2.3 vs 2.4; P = .48) on the first postoperative CXR. For the final CXR before discharge, the mean atelectasis severity scores were significantly lower for bell on than bell off (1.5 vs 1.8; P = .04) (Table 2). Such a finding was particularly notable given that the first scores of both groups were already low relative to what was hypothesized, and thus the final score had little room to decrease (ie, the floor effect). There was good concordance between the radiologists for the first CXR (k = 0.72) and final CXR (k = 0.75).
Early Postoperative Fevers
Although the prevalence of early postoperative fevers was low (3%-5%), of those with fevers, the reminder bell significantly shortened the duration (5 vs 3 hours; P = .04) of the febrile episodes. The reminder also significantly lowered the peak febrile temperatures (38.3°C vs 38.2°C; P = .01) (Table 2).
IS Inspiratory Volumes and Pulmonary Function Tests
No differences were observed regarding mean IS inspiratory volumes (overall bell off vs bell on, 977 mL vs 1023 mL; P = .55) (eTable 5 in Supplement 2) or the rate of IS volume change (δ = 8.57; 95% CI, −35.4 to 52.53; per-protocol P = .70; δ = 6.60; 95% CI, −36.0 to 49.2; intent-to-treat P = .76). Between the bell off and bell on groups, no differences were observed between the rates of change to preoperative baseline for FEV1, FVC, FEV1/FVC, and PEF (eTable 6 in Supplement 2).
Oxygen Supplementation and Noninvasive Ventilation
Having the reminder bell turned on reduced noninvasive positive pressure ventilation use rates (37% vs 19%; P = .03) for participants undergoing nonelective surgery (Table 2). Having the bell turned on reduced the use duration of high-flow nasal cannula (23 vs 11 hours; P = .049) for participants undergoing nonelective surgery (eTable 7 in Supplement 2). There were no differences observed between the bell off vs bell on groups regarding noninvasive positive pressure ventilation use duration (9 vs 6 hours; P = .24) (eTable 8 in Supplement 2), reintubation rates (12% vs 9%; P = .28), and intubation duration (8 vs 6 hours; P = .40) or tracheostomy rates (0% vs 0%; P = .99) (eTable 9 in Supplement 2).
Postoperative Pulmonary Complications
There were no differences observed between the bell off vs bell on groups regarding pneumonia (5% vs 1%; P = .18), pneumothorax (20% vs 23%; P = .45), pleural effusion (8% vs 10%; P = .47), bronchospasm (5% vs 13%; P = .11), pulmonary emboli (1% vs 0%; P = .51), or pulmonary edema severity (first CXR, 0.7 vs 0.7; P = .97; final CXR, 0.3 vs 0.3; P = .99) (eTable 10 in Supplement 2).
Discharge Location
Participants were either discharged home or to a nursing facility (skilled nursing or rehabilitation center). There were no differences observed between the bell off and bell on groups regarding the discharge location (home, 61% vs 59%; P = .49; nursing facility, 35% vs 39%; P = .36) (eTable 11 in Supplement 2).
Length of Stay
Having the bell on reduced the total postoperative (7 vs 6 days; P = .048) for participants underdoing nonelective surgery. Having the bell on also reduced the ICU length of stay (4 vs 3 days; P = .02) for participants undergoing nonelective surgery (Table 2).
Mortality
There were no differences observed between the bell off vs bell on regarding in-hospital mortality rates (4% vs 1%; P = .62) (eTable 12 in Supplement 2). At 6 months, the bell on group had fewer deaths (0% vs 9%; P = .048) for participants undergoing nonelective surgery (Table 2).
Nursing Workload
There was no evidence of an effect of the bell on the daily nursing workload (overall bell off vs bell on, 3 vs 3 episodes; P = .77; 6 vs 7 minutes; P = .32). This suggests that the increased use adherence occurred independently of increased clinician efforts (eTable 13 in Supplement 2).
Discussion
A single-center randomized superiority clinical trial was completed to evaluate the effect of an audible, use-tracking IS reminder after CABG. As was hypothesized, the SpiroTimer reminder improved IS adherence and, in turn, atelectasis and multiple subsequent clinical outcomes. The reminder reduced the duration and peak temperatures of early postoperative fevers. For participants undergoing nonelective surgery, the reminder reduced noninvasive positive pressure ventilation use rates and the duration of high-flow nasal cannula use. Most notably, the reminder reduced postoperative and ICU lengths of stay by 1 day and 6-month mortality rates for participants undergoing nonelective surgery.
The bell off group demonstrated low IS use adherence. As this is, to our knowledge, the first study to reliably track IS use,8 the results from the bell off condition are the best estimates available to represent current IS use adherence, although a Hawthorne effect (improved adherence due to study participation) cannot be excluded. The most commonly cited reason for poor adherence is patient forgetfulness.9 Patients may not even know how to use their IS properly. A meta-analysis of patient reminder interventions has shown that adherence significantly increases in the reminded group.10 For this study, the silencing of the reminder alarm when adequate IS inspiration was achieved functions under an operant conditioning paradigm of negative reinforcement in which a desirable behavior (ie, sustained maximal inspiration) is strengthened by removing an aversive stimulus (ie, the bell sound).27
Despite the already low atelectasis severity scores at first CXR, the reminder bell still reduced atelectasis relative to those without the bell. Atelectasis is one of the most common postoperative pulmonary complications,28 affecting up to 90% of patients after surgery.29 Atelectasis could be considered a sentinel diagnosis that decreases lung compliance30 and reduces functional residual capacity,31 necessitating greater work to open the lungs with each inspiration. Atelectatic hypoxia leads to increased pulmonary vascular resistance, which may result in right ventricular dysfunction if not appropriately treated.32 Atelectatic loss of lung volume with maintained total tidal volumes can lead to acute lung injury33 and an activation of inflammatory mediators because of atelectrauma from repeated inflation and deflation of alveoli during the respiratory cycle.34 Atelectasis has been linked to early postoperative mortality.4 Interestingly, in a clinical trial of IS in participants who underwent laparotomy, Tyson et al35 reported a similar trend toward decreased mortality in the intervention arm as observed in this investigation.
Atelectasis is frequently associated with early postoperative fevers.3 However, the data supporting this association as causal are equivocal.26 In this study, neither the first postoperative atelectasis severity scores nor the incidence of early postoperative fevers were different between groups. In addition to reducing atelectasis severity, the reminder bell on reduced fever duration and peak temperatures. To more clearly assess the relationship between atelectasis and fever, the onset timing of the fevers with its relationship to atelectasis severity needs to be investigated along with interventions to reduce atelectasis.
Length of stay in the ICU is an important measure of resource use. The ICU represents approximately one-third of inpatient health care costs despite accounting for only 10% of hospital beds.36 Daily ICU costs of care are increasing,37 amounting to an estimated $9000 per day in 2017.38 Thus, the SpiroTimer’s reduction in the ICU length of stay for participants undergoing nonelective surgery and 6-month mortality rates highlights the overall strength of the intervention despite its simplicity (ie, the addition of a bell). Without such a reminder in place, the more than $1 billion annual costs of IS may be without benefit. For individual patients, a reduced length of stay reduces the risk of various hospital-associated conditions, which may in turn affect postdischarge mortality. For hospitals, an intervention that could more broadly reduce the length of stay for many patients would offer important opportunities for quality improvement and cost savings. The absence of length of stay reduction for participants undergoing elective surgery may highlight the presence of fundamental differences between participants undergoing elective vs nonelective surgery. Participants undergoing nonelective surgery start at a more medically compromised baseline and may have greater room for improvement from IS use. This is supported by the observed reductions on the duration of high-flow nasal cannula, 6-month mortality rates, and use of noninvasive positive pressure ventilation specifically in participants undergoing nonelective surgery. Further studies of the SpiroTimer powered for length of stay are warranted, with a particular focus on patients with greater illness severity.
Limitations
This investigation has several potential limitations. This study did not directly evaluate effectiveness of IS on outcomes. To do so, a future study would need to experimentally compare a treatment group (high-adherence reminder IS) with a negative control condition (no IS). Such a study may not be feasible given the widespread use and promotion of IS as standard of care and may be not be permissible in light of the data from this study due to the improved outcomes in the bell on group. Masking the reminder bell sound (the intervention) is not possible for participants, clinicians, and researchers with intact hearing. The objective of the study was to evaluate the effect of the unmaskable reminder on clinical outcomes in a real clinical setting. Atelectasis severity, as the primary clinical outcome measure, was scored by 2 masked radiologists. The primary nonclinical outcome measure, IS use, was measured by the SpiroTimer machine. Clinician-directed end points (eg, continuous positive airway pressure/bilevel positive airway pressure use and length of stay) were determined by standardized objective criteria and clinical protocols that were the same between groups. The research team was independent from the clinicians and not involved in patient treatment decisions. If unmasked clinicians responded to the reminder bell (consciously or unconsciously) to help patients use their IS more, use continuous positive airway pressure/bilevel positive airway pressure less, or be more aggressive in discharge timing, then the reminder bell still caused the observed clinical improvements. Additionally, although the target threshold inspiratory volume may underestimate the number of breath events (eg, participants may have taken subthreshold breaths), the method was used in both groups and represents how clinicians instruct patients.20 Several secondary outcomes were not observed to be different between the conditions. The lack of observed effects on certain secondary outcomes was not unexpected, given that the study was only powered to detect a difference in atelectasis between groups. It would be interesting to assess the effect of various perioperative factors (eg, surgical techniques, graft type, anesthesia, postoperative pain scores, transverse sternal fracture, rib fracture, type of sternal closure, circulatory arrest times, and skeletonized vs pedicle fashion graft) on immediate postoperative atelectasis, IS use, and SpiroTimer effectiveness. For example, future IS studies of patients undergoing CABG could randomize on variables such as bilateral internal mammary artery use and/or skeletonization of internal mammary artery. Studies aimed at different patient populations that are powered to detect additional outcomes are needed to help further clarify and define IS applications and indications. Furthermore, IS protocol optimization studies are warranted to test what parameters have the greatest effect on outcomes.
Conclusions
The results from this investigation help inform the debate regarding the clinical effectiveness of IS in which some believe that IS is not beneficial while others believe that it is. The findings suggest that both arguments may be correct. Participants with the bell were more adherent with IS use and experienced an improved length of stay and 6-month mortality rates. These results indicate the benefit of IS when a reminder is present compared with the current, no-bell standard of care. Incentive spirometers can be clinically effective, but perhaps only when adherence is high. To our knowledge, the benefit of IS without a reminder is still unknown. Further studies are needed to evaluate if IS without a reminder is cost-effective or of any clinical use.
Trial protocol
eTable 1. Subject motivational factors
eTable 2. Modified Wilcox radiographic atelectasis severity scoring per lung
eTable 3. Subject characteristics
eTable 4. Subject motivational factors
eTable 5. Mean daily IS inspiratory volumes
eTable 6. Rate of change of pulmonary function tests
eTable 7. High-flow nasal cannula and non-rebreather mask use duration
eTable 8. Noninvasive positive pressure ventilation use duration
eTable 9. Reintubation and tracheostomy
eTable 10. Postoperative pulmonary complication rates
eTable 11. Discharge location
eTable 12. In-hospital causes of death
eTable 13. Daily nursing workload
Data sharing statement
References
- 1.Xue FS, Li BW, Zhang GS, et al. . The influence of surgical sites on early postoperative hypoxemia in adults undergoing elective surgery. Anesth Analg. 1999;88(1):213-219. [DOI] [PubMed] [Google Scholar]
- 2.Carter AR, Sostman HD, Curtis AM, Swett HA. Thoracic alterations after cardiac surgery. AJR Am J Roentgenol. 1983;140(3):475-481. doi: 10.2214/ajr.140.3.475 [DOI] [PubMed] [Google Scholar]
- 3.Doherty G. Postoperative complications In: Doherty G, ed. Current Diagnosis & Treatment: Surgery. 13th ed New York, NY: McGraw-Hill; 2010:35-45. [Google Scholar]
- 4.Fernandez-Bustamante A, Frendl G, Sprung J, et al. . Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the Perioperative Research Network Investigators. JAMA Surg. 2017;152(2):157-166. doi: 10.1001/jamasurg.2016.4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett RH, Krop P, Hanson EL, Moore FD. Physiology of yawning and its application to postoperative care. Surg Forum. 1970;21:222-224. [PubMed] [Google Scholar]
- 6.Eltorai AEM, Baird GL, Pangborn J, et al. . Financial impact of incentive spirometry. Inquiry. 2018;55:46958018794993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freitas ER, Soares BG, Cardoso JR, Atallah AN. Incentive spirometry for preventing pulmonary complications after coronary artery bypass graft. Cochrane Database Syst Rev. 2012;(9):CD004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayanan AL, Hamid SR, Supriyanto E. Evidence regarding patient compliance with incentive spirometry interventions after cardiac, thoracic and abdominal surgeries: a systematic literature review. Can J Respir Ther. 2016;52(1):17-26. [PMC free article] [PubMed] [Google Scholar]
- 9.Eltorai AEM, Baird GL, Eltorai AS, et al. . Incentive spirometry adherence: a national survey of provider perspectives. Respir Care. 2018;63(5):532-537. doi: 10.4187/respcare.05882 [DOI] [PubMed] [Google Scholar]
- 10.Fenerty SD, West C, Davis SA, Kaplan SG, Feldman SR. The effect of reminder systems on patients’ adherence to treatment. Patient Prefer Adherence. 2012;6:127-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung DA, Sharples LD, Nashef SA. A case-control analysis of readmissions to the cardiac surgical intensive care unit. Eur J Cardiothorac Surg. 2002;22(2):282-286. doi: 10.1016/S1010-7940(02)00303-2 [DOI] [PubMed] [Google Scholar]
- 12.Sung JC, Nichol MB, Venturini F, Bailey KL, McCombs JS, Cody M. Factors affecting patient compliance with antihyperlipidemic medications in an HMO population. Am J Manag Care. 1998;4(10):1421-1430. [PubMed] [Google Scholar]
- 13.Vaccarino V, Lin ZQ, Kasl SV, et al. . Sex differences in health status after coronary artery bypass surgery. Circulation. 2003;108(21):2642-2647. doi: 10.1161/01.CIR.0000097117.28614.D8 [DOI] [PubMed] [Google Scholar]
- 14.Stamou SC, Hill PC, Haile E, Prince S, Mack MJ, Corso PJ. Clinical outcomes of nonelective coronary revascularization with and without cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2006;131(1):28-33. doi: 10.1016/j.jtcvs.2005.08.059 [DOI] [PubMed] [Google Scholar]
- 15.Sobolev BG, Fradet G, Hayden R, Kuramoto L, Levy AR, FitzGerald MJ. Delay in admission for elective coronary-artery bypass grafting is associated with increased in-hospital mortality. BMC Health Serv Res. 2008;8:185. doi: 10.1186/1472-6963-8-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandura A. Self-efficacy mechanism in human agency. Am Psychol. 1982;37(2):122-147. doi: 10.1037/0003-066X.37.2.122 [DOI] [Google Scholar]
- 17.Weiner B. Attribution theory, achievement motivation, and the educational process. Rev Educ Res. 1972;42(2):203-215. doi: 10.3102/00346543042002203 [DOI] [Google Scholar]
- 18.Hong Y, Chiu C, Dweck CS, Lin DMS, Wan W. Implicit theories, attributions, and coping: a meaning system approach. J Pers Soc Psychol. 1999;77:588-599. doi: 10.1037/0022-3514.77.3.588 [DOI] [Google Scholar]
- 19.Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr. 1966;80(1):1-28. doi: 10.1037/h0092976 [DOI] [PubMed] [Google Scholar]
- 20.Eltorai AEM, Baird GL, Eltorai AS, et al. . Perspectives on incentive spirometry utility and patient protocols. Respir Care. 2018;63(5):519-531. doi: 10.4187/respcare.05872 [DOI] [PubMed] [Google Scholar]
- 21.Wilkins R. Lung expansion therapy In: Wikins RLSJ, Kacmarek RM, eds. Egan’s Fundamentals of Respiratory Care. 9th ed St Louis: Mosby Elsevier; 2009:903-920. [Google Scholar]
- 22.Jensen L, Yang L. Risk factors for postoperative pulmonary complications in coronary artery bypass graft surgery patients. Eur J Cardiovasc Nurs. 2007;6(3):241-246. doi: 10.1016/J.EJCNURSE.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 23.Staehr AK, Meyhoff CS, Henneberg SW, Christensen PL, Rasmussen LS. Influence of perioperative oxygen fraction on pulmonary function after abdominal surgery: a randomized controlled trial. BMC Res Notes. 2012;5:383. doi: 10.1186/1756-0500-5-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joyce CJ, Baker AB, Chartres S. Influence of inspired nitrogen concentration during anaesthesia for coronary artery bypass grafting on postoperative atelectasis. Br J Anaesth. 1995;75(4):422-427. doi: 10.1093/bja/75.4.422 [DOI] [PubMed] [Google Scholar]
- 25.Wilcox P, Baile EM, Hards J, et al. . Phrenic nerve function and its relationship to atelectasis after coronary artery bypass surgery. Chest. 1988;93(4):693-698. doi: 10.1378/chest.93.4.693 [DOI] [PubMed] [Google Scholar]
- 26.Mavros MN, Velmahos GC, Falagas ME. Atelectasis as a cause of postoperative fever: where is the clinical evidence? Chest. 2011;140(2):418-424. doi: 10.1378/chest.11-0127 [DOI] [PubMed] [Google Scholar]
- 27.Skinner BF. About Behaviorism. New York, NY: Vintage; 1976. [Google Scholar]
- 28.Browne DR, Rochford J, O’Connell U, Jones JG. The incidence of postoperative atelectasis in the dependent lung following thoracotomy: the value of added nitrogen. Br J Anaesth. 1970;42(4):340-346. doi: 10.1093/bja/42.4.340 [DOI] [PubMed] [Google Scholar]
- 29.Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, Hedenstierna G. Prevention of atelectasis during general anaesthesia. Lancet. 1995;345(8962):1387-1391. doi: 10.1016/S0140-6736(95)92595-3 [DOI] [PubMed] [Google Scholar]
- 30.Bendixen HH, Hedley-Whyte J, Laver MB. Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation: a concept of atelectasis. N Engl J Med. 1963;269:991-996. doi: 10.1056/NEJM196311072691901 [DOI] [PubMed] [Google Scholar]
- 31.Froese AB, Bryan AC. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology. 1974;41(3):242-255. doi: 10.1097/00000542-197409000-00006 [DOI] [PubMed] [Google Scholar]
- 32.Marshall BE. Importance of hypoxic pulmonary vasoconstriction with atelectasis. Adv Shock Res. 1982;8:1-12. [PubMed] [Google Scholar]
- 33.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149(5):1327-1334. doi: 10.1164/ajrccm.149.5.8173774 [DOI] [PubMed] [Google Scholar]
- 34.Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):109-116. doi: 10.1164/ajrccm.160.1.9803046 [DOI] [PubMed] [Google Scholar]
- 35.Tyson AF, Kendig CE, Mabedi C, Cairns BA, Charles AG. The effect of incentive spirometry on postoperative pulmonary function following laparotomy: a randomized clinical trial. JAMA Surg. 2015;150(3):229-236. doi: 10.1001/jamasurg.2014.1846 [DOI] [PubMed] [Google Scholar]
- 36.Shorr AF. An update on cost-effectiveness analysis in critical care. Curr Opin Crit Care. 2002;8(4):337-343. doi: 10.1097/00075198-200208000-00011 [DOI] [PubMed] [Google Scholar]
- 37.Halpern NA, Pastores SM. Critical care medicine in the United States 2000-2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38(1):65-71. doi: 10.1097/CCM.0b013e3181b090d0 [DOI] [PubMed] [Google Scholar]
- 38.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266-1271. doi: 10.1097/01.CCM.0000164543.14619.00 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Subject motivational factors
eTable 2. Modified Wilcox radiographic atelectasis severity scoring per lung
eTable 3. Subject characteristics
eTable 4. Subject motivational factors
eTable 5. Mean daily IS inspiratory volumes
eTable 6. Rate of change of pulmonary function tests
eTable 7. High-flow nasal cannula and non-rebreather mask use duration
eTable 8. Noninvasive positive pressure ventilation use duration
eTable 9. Reintubation and tracheostomy
eTable 10. Postoperative pulmonary complication rates
eTable 11. Discharge location
eTable 12. In-hospital causes of death
eTable 13. Daily nursing workload
Data sharing statement