Abstract
This study examines the association between insulin resistance, schizophrenia polygenic risk, and treatment outcomes in first-episode, antipsychotic-naive patients with schizophrenia.
Patients with schizophrenia show an increased risk of impaired glucose metabolism,1 yet the mechanism behind this association remains unknown. Multiple studies have attempted to identify the determinants of insulin resistance (IR) in schizophrenia, with evidence suggesting that it cannot be fully explained by disease duration, symptom severity, medication effects, obesity, or hormonal stress axis activation, and other interacting factors are likely involved.1 While family and genome-wide association studies have suggested a shared genetic vulnerability between schizophrenia and abnormal glucose metabolism,2 to our knowledge, a direct link between schizophrenia genetic risk and insulin resistance has not been investigated. Here, we examine the association between IR, schizophrenia polygenic risk, and treatment outcomes in first-episode, antipsychotic-naive patients with schizophrenia and matched healthy individuals while controlling for demographic, lifestyle, and clinical factors.
Methods
First-episode, antipsychotic-naive patients with schizophrenia and matched unaffected control individuals (58 patients with schizophrenia and 58 control individuals; Table) were recruited at the University Hospital Marqués de Valdecilla (Santander, Spain) as described previously.3 The study procedures were approved by the medical faculty ethical committee, and written informed consent was obtained from all study participants. Schizophrenia polygenic risk scores (PRS) were calculated based on 108 genome-wide significant schizophrenia loci4 from the Illumina Infinium PsychArray Bead-Chip genotyping data imputed using IMPUTE2/SHAPEIT. The updated Homeostasis Model Assessment (HOMA2)5 was used to infer IR, β cell function, and insulin sensitivity from clinical measurements of fasting serum glucose and insulin levels. Switching antipsychotic medication at least once during the initial 12 months of treatment was used as a heuristic long-term treatment outcome measure. All statistical tests were 2-sided and are described in figure legends. A P value of less than .05 was considered significant. Analyses were conducted in R, version 3.5.0 (R Foundation).
Table. Demographic and Clinical Data of Study Participants.
| Characteristic | No. (%) | P Valuea | Missing, No. (%) | ||
|---|---|---|---|---|---|
| Control (n = 58) | Schizophrenia (n = 58) | Control | Schizophrenia | ||
| Sex | |||||
| Male | 35 (60) | 36 (62) | >.99 | NA | NA |
| Female | 23 (40) | 22 (38) | |||
| Age, mean (SD), y | 31.8 (7.6) | 29.5 (8.5) | .15 | NA | NA |
| BMI, mean (SD) | 24.4 (3.7) | 23.0 (5.0) | .07 | NA | NA |
| Race/ethnicity | |||||
| White | 58 (100) | 52 (90) | .03b | NA | NA |
| Hispanic | 0 (0) | 5 (9) | |||
| Romani | 0 (0) | 1 (2) | |||
| Smokingc | |||||
| No | 28 (48) | 25 (43) | .73 | NA | NA |
| Yes | 30 (52) | 33 (57) | |||
| Alcoholc | |||||
| No | 42 (72) | 28 (48) | .02b | NA | NA |
| Yes | 16 (28) | 30 (52) | |||
| Cannabisc | |||||
| No | 44 (76) | 32 (55) | .04b | NA | NA |
| Yes | 14 (24) | 26 (45) | |||
| Family history of diabetes | |||||
| No | 3 (5) | 26 (45) | .04b | 49 (84) | 22 (38) |
| Yes | 6 (10) | 10 (17) | |||
| Family history of psychiatric diseased | |||||
| No | 58 (100) | 45 (78) | <.001c | NA | NA |
| Yes | 0 | 13 (22) | |||
| Previous psychiatric medication | |||||
| No | 58 (100) | 45 (78) | <.001b | NA | NA |
| Yes | 0 (0) | 13 (22)e | |||
| Age at onset of psychosis, mean (SD), y | NA | 28.4 (8.4) | NA | NA | 1 (2) |
| First antipsychoticf | |||||
| Aripiprazole | NA | 28 (48) | NA | NA | NA |
| Olanzapine | NA | 1 (2) | |||
| Risperidone | NA | 29 (50) | |||
| PRS, mean (SD) | 0.46 (0.18) | 0.49 (0.13) | .38 | 1 (2) | 7 (12) |
| HOMA2, mean (SD) | |||||
| IR | 1.04 (0.59) | 1.72 (1.77) | .004b | 4 (7) | 2 (3) |
| %B | 112.3 (44.3) | 144.5 (85.5) | .02b | 4 (7) | 2 (3) |
| %S | 131.3 (78.8) | 111.7 (83.8) | .20 | 4 (7) | 2 (3) |
| Glucose, mean (SD), mg/dL | 84.2 (10.3) | 84.8 (10.6) | .76 | 3 (5) | 2 (3) |
| Insulin, mean (SD), μIU/mL | 8.1 (4.6) | 13.6 (14.8) | .004b | 3 (5) | NA |
| Insulin resistantg | |||||
| No | 55 (95) | 49 (84) | .005b | 3 (5) | NA |
| Yes | 0 | 9 (16) | |||
| BPRS, mean (SD) | NA | 68.6 (14.9) | NA | NA | 1 (2) |
| SAPS, mean (SD) | NA | 15.2 (4.1) | NA | NA | NA |
| SANS, mean (SD) | NA | 6.4 (6.4) | NA | NA | 1 (2) |
Abbreviations: %B, beta cell function (%); BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BPRS, Brief Psychiatric Rating Scale; HOMA2, the updated Homeostasis Model Assessment; IR, insulin resistance; NA, not applicable; PRS, schizophrenia polygenic risk score; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; %S, insulin sensitivity (%).
SI conversion factors: To convert glucose to millimoles per liter, multiply by 0.0555; insulin to picomoles per liter, multiply by 6.945.
Data were analyzed using t test for continuous variables and Fisher exact test or χ2 test for categorical variables. P values were obtained by permutation testing (N = 1000 permutations).
Significant (<.05) P value.
Self-reported.
Assessed using the Comprehensive Assessment of Symptoms and History.
Antidepressant; mean (SD) duration, 7.8 (8.3) days.
Antipsychotic treatment was initiated after baseline data collection and used for determining switching medication status in Figure, C.
Based on the fasting insulin criteria (≥25 μIU/mL).
Results
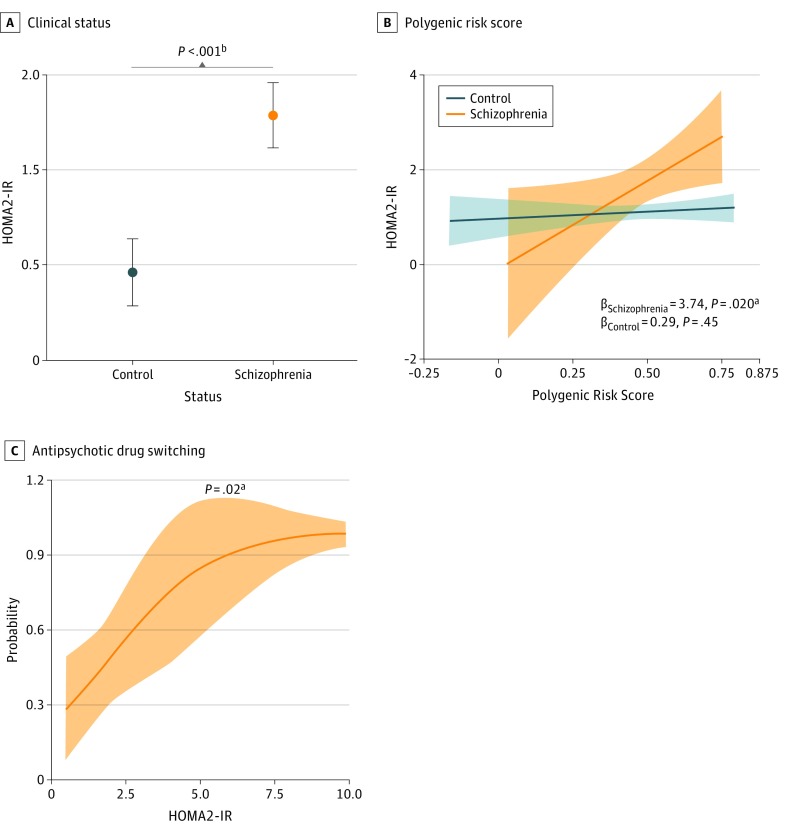
Consistent with previous reports,1 patients with schizophrenia showed increased baseline HOMA2-IR (mean difference [MD] [SE], 0.68 [0.25]; P = .004), HOMA2 β-cell function (MD [SE], 32.2 [13.1]; P = .02), and fasting insulin levels (MD [SE], 5.5 [2.1] μIU/mL [to convert to picomoles per liter, multiply by 6.945]; P = .004), whereas HOMA2 insulin sensitivity (MD [SE], −19.6 [15.5]; P = .20) and fasting glucose levels (MD [SE], 0.6 [2.0] mg/dL [to convert to millimoles per liter, multiply by 0.0555]; P = .76) did not differ significantly from control values (Table). After adjusting for covariates, HOMA2-IR remained significantly increased in patients with schizophrenia (MD [SE], 0.82 [0.25]; P < .001, adjusted for body mass index; Figure, A). The HOMA2-IR was positively associated with schizophrenia PRS in patients with schizophrenia (β [SE], 3.74 [1.68]; P = .02, adjusted for age; Figure, B) but not in the control group (adjusted β [SE], 0.29 [0.40]; P = .45), where body mass index was the most significant risk factor (adjusted β [SE], 0.071 [0.018]; P < .001). Baseline HOMA2-IR was significantly associated with switching antipsychotic medication during the initial 12 months of treatment, with an adjusted odds ratio (OR) of 1.77 (95% CI, 1.10-3.52; P = .02, adjusted for ethnicity; Figure, C). Of the 41 patients for whom complete follow-up information was available, all 8 patients who at baseline satisfied the fasting insulin criteria for IR (≥25 μIU/mL)6 required changing medication within the first year of treatment. Schizophrenia PRS was not significantly associated with medication switching status (adjusted OR, 183; 95% CI, 0.48-504 931; P = .12).
Figure. Analysis of the Homeostasis Model Assessment of Insulin Resistance (HOMA2-IR) in First-Episode, Antipsychotic-Naive Patients With Schizophrenia and Healthy Control Individuals.
A, Association of HOMA2-IR with clinical status. B, Association of HOMA2-IR with the 108 loci schizophrenia polygenic risk score. C, Association of antipsychotic drug switching during the initial 12 months of treatment with baseline HOMA2-IR. Plots show adjusted mean with standard error (A) and marginal effects with 95% confidence intervals (B and C). Statistical tests included analysis of covariance (A) and multivariable linear (B) and logistic (C) regression. Covariates were selected using bidirectional elimination and Bayesian information criterion from age, sex, body mass index, race/ethnicity, smoking, alcohol consumption, cannabis use, and previous psychiatric medication (A-C); baseline Brief Psychiatric Rating Scale, Scale for the Assessment of Positive Symptoms, and Scale for the Assessment of Negative Symptoms scores (B; schizophrenia group); and the initial treatment drug (C). Only cases with complete data were analyzed. P values were obtained by permutation testing (1000 permutations). P less than .05 was considered significant. Numbers: A, 54 control individuals and 56 patients with schizophrenia; B, 53 control individuals and 49 patients with schizophrenia; C, 20 patients with schizophrenia with no drug switch and 21 patients with schizophrenia with drug switch (13 owing to low efficacy, 5 owing to adverse effects, and 3 owing to noncompliance).
aP < .001.
bP < .05.
Discussion
We report that schizophrenia polygenic risk is significantly associated with insulin resistance in first-episode, antipsychotic-naive patients with schizophrenia independent from selected demographic, lifestyle, and clinical factors. This result suggests that IR is a hallmark of schizophrenia rather than a secondary effect of emerging symptoms and supports the hypothesis that multiple susceptibility genes might exert pleiotropic effects cooccurring between the 2 conditions. Furthermore, the results indicate a potential association of IR with diminished response to antipsychotic treatment. Hence, patients with schizophrenia presenting with IR might constitute a distinct patient subgroup and require personalized treatment tailored to this endophenotype.
Limitations
Limitations of this study include incomplete metadata for subsets of clinical variables and the fact that although nonresponse was the primary factor influencing medication switching, other clinical variables, such as adverse effects and treatment nonadherence, cannot be excluded in a minority of cases. Well-powered pharmacogenomic studies and more specific assays, such as the oral glucose tolerance and cortisol tests,1 are required to further examine the association between IR, schizophrenia, and antipsychotic treatment response, in addition to determining the effects of other lifestyle factors such as diet and exercise.
References
- 1.Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269. doi: 10.1001/jamapsychiatry.2016.3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chouinard VA, Henderson DC, Dalla Man C, et al. . Impaired insulin signaling in unaffected siblings and patients with first-episode psychosis. Mol Psychiatry. 2018. doi: 10.1038/s41380-018-0045-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lago SG, Tomasik J, van Rees GF, et al. . Exploring the neuropsychiatric spectrum using high-content functional analysis of single-cell signaling networks. Mol Psychiatry. 2018. doi: 10.1038/s41380-018-0123-4 [DOI] [PubMed] [Google Scholar]
- 4.Ripke S, Neale BM, Corvin A, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium . Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi: 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191-2192. doi: 10.2337/diacare.21.12.2191 [DOI] [PubMed] [Google Scholar]
- 6.Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed Philadelphia, PA: Elsevier Saunders; 2011. [Google Scholar]