This cohort study examines if activity status changes differ among races/ethnicities and levels of sensitization and how these differences are associated with transplant probability after implementing the Kidney Allocation System.
Key Points
Question
How do changes in a patient’s kidney transplant wait-list status impact their chances of getting a transplant, and has the new Kidney Allocation System improved access for all underserved populations?
Findings
In this cohort study of 42 558 patients, racial disparities were reduced for most patients after implementation of the Kidney Allocation System. However, there continue to be significant racial/ethnic differences in transplant probability for patients who are highly sensitized.
Meaning
Further analysis into the root causes of ongoing racial disparities in patients who are highly sensitized is required with potential modifications to the current allocation system to address this finding.
Abstract
Importance
Inactive patients on the kidney transplant wait-list have a higher mortality. The implications of this status change on transplant outcomes between racial/ethnic groups are unknown.
Objectives
To determine if activity status changes differ among races/ethnicities and levels of sensitization, and if these differences are associated with transplant probability after implementation of the Kidney Allocation System.
Design, Setting, and Participants
A multistate model was constructed from the Organ Procurement and Transplantation Network kidney transplant database (December 4, 2014, to September 8, 2016). The time interval followed Kidney Allocation System implementation and provided at least 1-year follow-up for all patients. The model calculated probabilities between active and inactive status and the following competing risk outcomes: living donor transplant, deceased donor transplant, and death/other. This retrospective cohort study included 42 558 patients on the Organ Procurement and Transplantation Network kidney transplant wait-list following Kidney Allocation System implementation. To rule out time-varying confounding from relisting, analysis was limited to first-time registrants. Owing to variations in listing practices, primary center listing data were used for dually listed patients. Individuals listed for another organ or pancreatic islets were excluded. Analysis began July 2017.
Main Outcome and Measures
Probabilities were determined for transitions between active and inactive status and the following outcome states: active to living donor transplant, active to deceased donor transplant, active to death/other, inactive to living donor transplant, inactive to deceased donor transplant, and inactive to death/other.
Results
The median (interquartile range) age at listing was 55.0 (18.0-89.0) years, and 26 535 of 42 558 (62.4%) were men. White individuals were 43.3% (n = 18 417) of wait-listed patients, while black and Hispanic individuals made up 27.8% (n = 11 837) and 19.5% (n = 8296), respectively. Patients in the calculated plasma reactive antibody categories of 0% or 1% to 79% showed no statistically significant difference in transplant probability among races/ethnicities. White individuals had an advantage in transplant probability over black individuals in calculated plasma reactive antibody categories of 80% to 89% (hazard ratio [HR], 1.8 [95% CI, 1.4-2.2]) and 90% or higher (HR, 2.4 [95% CI, 2.1-2.6]), while Hispanic individuals had an advantage over black individuals in the calculated plasma reactive antibody group of 90% or higher (HR, 2.5 [95% CI, 2.1-2.8]). Once on the inactive list, white individuals were more likely than Hispanic individuals (HR, 1.2 [95% CI, 1.17-1.3]) or black individuals (HR, 1.4 [95% CI, 1.3-1.4]) to resolve issues for inactivity resulting in activation.
Conclusions and Relevance
For patients who are highly sensitized, there continues to be less access to kidney transplant in the black population after the implementation of the Kidney Allocation System. Health disparities continue after listing where individuals from minority groups have greater difficulty in resolving issues of inactivity.
Introduction
Patients with end-stage renal disease, who are made inactive on the kidney transplant waiting list, have a higher mortality.1 There are several reasons an individual may be made inactive after listing for transplant including medical comorbidities, incomplete testing, psychosocial issues, or financial constraints.2,3,4 Patients work with their health care professionals, social workers, and transplant team to resolve these issues to attain active status, which confers the ability to obtain deceased donor organ offers. It is understandable that the ability to resolve the causes for inactivity may be more difficult for individuals with less access to health care, social support, or financial resources. Yet, to date, the implications and impact of a wait-list inactive status change on a patients’ future chances of obtaining a kidney transplant remain unknown.
Wait-listed patients may experience several competing risk outcomes, including living donor transplant, deceased donor transplant, and death or removal from the list.5,6 Because patients on the inactive list cannot receive organ offers and therefore cannot “compete” for a deceased donor transplant, conventional approaches that use traditional Cox regression models are unable to reconcile implications of changing wait-list status.7 Multistate modeling, which uses a nested-competing risk approach, is a novel method that is capable of determining the chances of a patient going from active to inactive status, or vice versa, while simultaneously determining the probability of other competing risk transplant outcomes.6,8,9
Priorities of the new Kidney Allocation System (KAS) (Organ Procurement and Transplantation Network (OPTN) policy 8.3; effective December 4, 2014) were to increase transplant rates in individuals who are highly sensitized and to improve access to underserved populations.10,11 As minorities had longer dialysis exposure prior to listing, having wait time start at the date of dialysis initiation, instead of the date of listing, was intended to adjust for this access issue and improve transplant rates among minority groups. Since the implementation of KAS, there has been an observed rise in the number of black and Hispanic individuals receiving deceased donor transplants.12,13 These studies used transplant rates to show the improvement in the proportion of transplants in underserved populations compared with rates prior to KAS. However, they are limited as they are unable to account for inactive wait-listed patients who do not have the ability to obtain deceased donor offers and cannot provide a true assessment of the future chances of obtaining a transplant given specific variables, most notably race/ethnicity. Despite this, the United Network for Organ Sharing now claims that parity in organ allocation has been obtained across racial/ethnic groups.14 It is important to recognize that equity cannot be proven by simply showing that the proportion of transplants in a group is close to the group’s representation on the wait-list. Achieving equity requires equal opportunity for life-saving treatment,15 or more specifically equal probability of transplant, regardless of race or ethnicity, which must also account for wait-list activity status. Although transplant rates, calculated by including patients with active and inactive status, provide proportion of individuals receiving transplants in a given time frame, they cannot provide wait-listed patients the future chances of getting a transplant.
We created a unique analysis of OPTN kidney transplant wait-list data and analyzed competing risk transplant outcomes after the implementation of KAS. This model is able to account for changes in activity status; this is critical as health disparities and differences in social determinants of health do not stop following listing and likely persist while patients wait for a deceased donor transplant. Using this novel approach, we attempt to redefine how health disparities are measured for kidney transplant access, which to date has largely been based on access to the wait-list.16,17 We are now able to determine how wait-list status changes and the ability to convert from inactive to active status differs between racial/ethnic groups and how this factors into the probability of obtaining a transplant. Furthermore, the status change conversion metrics that are presented offer a new measurement for dialysis units and transplant centers to improve quality through improved care coordination of shared patients on the inactive list.
Methods
The Yale University institutional review board approved this study and did not require patient consent because deidentified data were used. We constructed a semiparametric, multistate model using OPTN wait-list data that started at KAS implementation and provided at least 1-year follow-up for all patients (December 4, 2014, to September 8, 2016). Analysis began in July 2017. Observations were censored if patients were lost to follow-up or still on the waiting list at the end of study. Patients entered the model through either an active or inactive state and could transition between these states before entering a final outcome state.
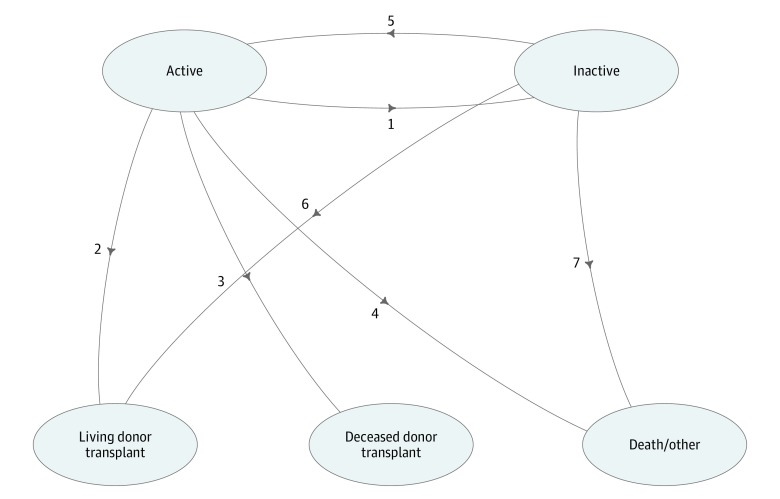
There were 7 transitions in this model: active to inactive status, active to living donor transplant, active to deceased donor transplant, active to death/other, inactive to active, inactive to living donor transplant, and inactive to death/other (Figure 1). “Other” included listing removal for refusal of transplant, improved condition, and other reasons. Direct transition from inactive to deceased donor transplant was not modeled because patients on the inactive list were not eligible to receive deceased donor transplant offers. Because the multistate process is actually a series of nested competing risk experiments, the estimation techniques used in hazard-based survival analysis also work in the complex multistate model. Specifically, the transition probability is derived by computing the product integral with respect to the estimated cumulative hazard for each transition, which could be estimated using the nonparametric method or the Cox regression model.18
Figure 1. Multistate Model Schematic of Transitions With Competing Risk Outcomes.
Activity status changes focus on transitions between 1 and 5. There is no direct transition from inactive status to deceased donor transplant; however, there is a path from inactive status through active status (transition 5) to deceased donor transplant (transition 3). “Other” includes listing removal for refusal of transplant, improved condition, and other reasons.
In our model, the association of race/ethnicity and initial calculated panel reactive antibody (cPRA) with each transition was evaluated independently by using a transition-specific Cox regression model adjusted for sex, diabetes status, dialysis status, blood type, and donor service area. Calculated panel reactive antibodies were categorized into the following groups: 0%, 1% to 79%, 80% to 89%, and 90% or more. The rationale for using these categories was to focus on differences in organ allocation in the higher cPRA groups of 80% to 89% and 90% or more, as KAS included changes in allocation for patients with a cPRA of more than 80% with a sliding scale distribution.10
The proportional hazards assumption was examined by checking the scaled Schoenfeld residuals against time.19 First, the null model was built with the regression coefficients for all risk factors constrained to be identical for all 7 transitions. Each covariate was then sequentially allowed to differ across transitions by creating transition-specific covariates, while the other covariates were kept identical across transitions. The likelihood ratio test was used to test the null hypothesis that the regression coefficients were identical for all transitions.19 The interactions between cPRA and race/ethnicity were tested for each transition. The details for the model selection process and the parameter estimates for final model are included in the supplemental material (eTables 1 and 2 in the Supplement). Cumulative hazards were then calculated from the final transition-specific Cox model, and probabilities were estimated using the Aalen-Johansen estimator.20 Direct transition hazard from inactive status to deceased donor transplant cannot be modeled; however, the transition probability during a given period could be calculated because a patient on the inactive list could subsequently move to active status and then receive a deceased donor transplant. For demographic characteristics, means and SDs were reported for continuous variables and frequencies, and percentages were reported for categorical variables. Data management was conducted using SAS, version 9.4 (SAS Institute Inc) and multistate modeling was performed in R statistical software, version 3.1.0 (R Foundation for Statistical Computing) using the mstate package.21
Results
Patient Demographics
The post-KAS wait-list population used in this study included 42 558 individuals from December 4, 2014, to September 8, 2016, for which demographics are presented in Table 1. Briefly, the median (interquartile) age at listing was 55 (18.0-89.0) years, and 26 535 (62.4%) were men. End-stage renal disease diagnoses at listing were diabetes mellitus (15 568 [36.6%]), other/unknown (12 257 [28.8%]), hypertension (9043 [21.3%]), glomerulonephritis (4836 [11.4%]), and graft failure (854 [2.0%]). White individuals were 43.3% (n = 18 417) of wait-listed patients, while black and Hispanic individuals made up 27.8% (n = 11 837) and 19.5% (n = 8296), respectively. There were 67.9% (n = 28 905) who were receiving dialysis treatments at listing, while 32.1% (n = 13 653) were not.
Table 1. Post–Kidney Allocation System Cohort Demographics (N = 42 558).
| Demographic | Total No. (%) |
|---|---|
| Age at baseline, y | |
| Mean (SD) | 52.74 (13.01) |
| Median (range) | 55.0 (18.0-89.0) |
| Sex | |
| Male | 26 535 (62.4) |
| Female | 16 023 (37.7) |
| Dialysis status | |
| No | 13 653 (32.1) |
| Yes | 28 905 (67.9) |
| BMI (n = 42 467) | |
| Mean (SD) | 29.10 (5.6) |
| Median (range) | 28.8 (3.6-54.0) |
| Race/ethnicity | |
| White | 18 417 (43.3) |
| Black | 11 837 (27.8) |
| Hispanic | 8296 (19.5) |
| Asian/othera | 4008 (9.4) |
| ABO blood group | |
| A | 14 178 (33.3) |
| AB | 1651 (3.9) |
| B | 6192 (14.6) |
| O | 20 537 (48.3) |
| cPRA, % | |
| 0 | 38 589 (90.7) |
| 1-79 | 3086 (7.3) |
| 80-89 | 286 (0.7) |
| 90-100 | 597 (1.4) |
| Primary diagnosis | |
| Diabetes | 15 568 (36.6) |
| Glomerulonephritis | 4836 (11.4) |
| Graft failure | 854 (2.0) |
| Hypertension | 9043 (21.3) |
| Other/unknown | 12 257 (28.8) |
| Donor service region | |
| 1 | 1990 (4.7) |
| 2 | 5213 (12.3) |
| 3 | 6083 (14.3) |
| 4 | 3852 (9.0) |
| 5 | 8138 (19.1) |
| 6 | 1456 (3.4) |
| 7 | 3333 (7.8) |
| 8 | 2397 (5.6) |
| 9 | 2899 (6.8) |
| 10 | 3383 (8.0) |
| 11 | 3814 (9.0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); cPRA, calculated panel reactive antibody.
Other includes American Indian/Alaska Native, Native Hawaiian/other Pacific Islander, and multiracial.
Wait-list Status and Activity Transitions
There were 31 643 patients (74%) with active status and 10 915 patients (26%) with inactive status on the day of listing. During the first year of listing, at least 1 inactivity status change was observed in 53.1% (n = 9779) of white individuals, 42.3% (n = 3506) of Hispanic individuals, and 49.3% (n = 5836) of black individuals. Given their representation on the wait-list (Hispanic individuals, 19.5% [n = 8296]; black individuals, 27.8% [n = 11 837]; Table 1), Hispanic and black individuals had a disproportionate number of patients experiencing an inactive status change. The association of race with transition 1 (active to inactive) and transition 5 (inactive to active) was evaluated separately (Figure 1). Although there was no statistically significant interaction between cPRA and race/ethnicity, once on the inactive list, white individuals were more successful than Hispanic or black individuals at resolving issues for inactivity resulting in wait-list activation (Table 2). There was no statistically significant difference between Hispanic and black individuals in activity status transitions (Table 2).
Table 2. Wait-list Status Changes Comparing Different Races.
| Racial Group Comparison | Status Change, HR (95% CI) | |
|---|---|---|
| Active to Inactive | Inactive to Active | |
| White vs black | 1.36 (1.30-1.42)a | 1.35 (1.29-1.41)a |
| White vs Hispanic | 1.32 (1.25-1.39)a | 1.24 (1.17-1.31)a |
| Hispanic vs black | 1.03 (0.98-1.09) | 1.03 (0.98-1.09) |
Abbreviation: HR, hazard ratio.
Statistically significant.
Probability of Transplant by cPRA and Race/Ethnicity
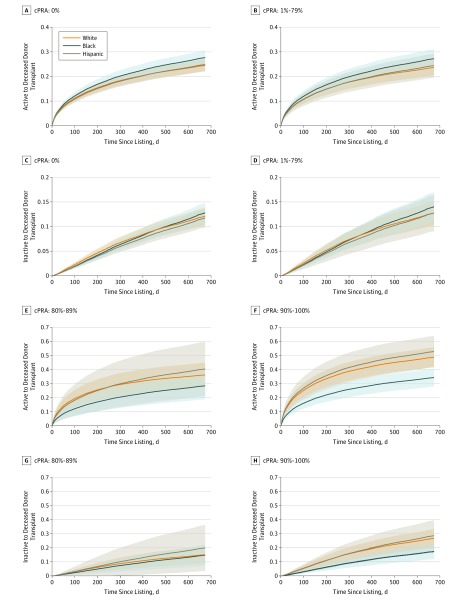
Most patients, who are represented in the cPRA groups of 0% or 1% to 79%, showed no statistically significant differences in transplant probability by race/ethnicity (Figure 2A-D). However, for patients initially listed as active, white individuals had a significant advantage in transplant probability over black individuals in cPRA categories of 80% to 89% (hazard ratio [HR], 1.8 [95% CI, 1.4-2.2]) and 90% or more (HR, 2.36 [95% CI, 2.1-2.6]), while Hispanic individuals had a statistically significant advantage over black individuals in the cPRA group of 90% or more (HR, 2.5 [95% CI, 2.1-2.8]) but not at a cPRA of 80% to 89% (HR, 1.6 [95% CI, 0.9-2.2]) (Figure 2E and F). Similar differences in transplant probability were noted in individuals initially listed as inactive, although the effect size between races/ethnicities was less pronounced (Figure 2C, D, G, and H), which was reasonable because inactively listed patients had to transition to active status before receiving a deceased donor transplant. When considering how changes in activity status affected these observed differences in transplant probability, white patients who were highly sensitized were more likely to move to the active group than black patients in the cPRA group of 90% or more (HR, 1.4 [95% CI, 1.2-1.6]); however, the changes in status were not significant in the cPRA group of 80% to 89% (HR, 0.9 [95% CI, 0.6-1.3]), which could account for the limited differences in transplant probability between black and white individuals who were listed inactive (Figure 2G). DR 0-mismatch provides 2 additional allocation points and these points were preferentially awarded to white individuals in these categories: cPRA group of 80% to 89%: white individuals, 64%; Hispanic individuals, 7%; black individuals, 28%; cPRA group of 90% or more: white individuals, 60%; Hispanic individuals, 13%; black individuals, 23%.
Figure 2. Predicted Transplant Probabilities by Calculated Panel Reactive Antibody (cPRA) Group.
Probabilities of deceased donor transplant for cPRA 0% (A), cPRA 1% to 79% (B), cPRA 80% to 89% (E), and cPRA 90% to 100% (F) stratified by race/ethnicities for patients listed as active. Transplant probabilities for cPRA 0% (C), cPRA 1% to 79% (D), cPRA 80% to 89% (G), and cPRA 90% to 100% (H) for inactively listed patients, stratified by race/ethnicity. Black individuals have a lower predicted probability of deceased donor transplant if they have a cPRA greater than 80% (E and F).
Changes in Activity Status and the Dynamic Prediction of Transplant Outcomes
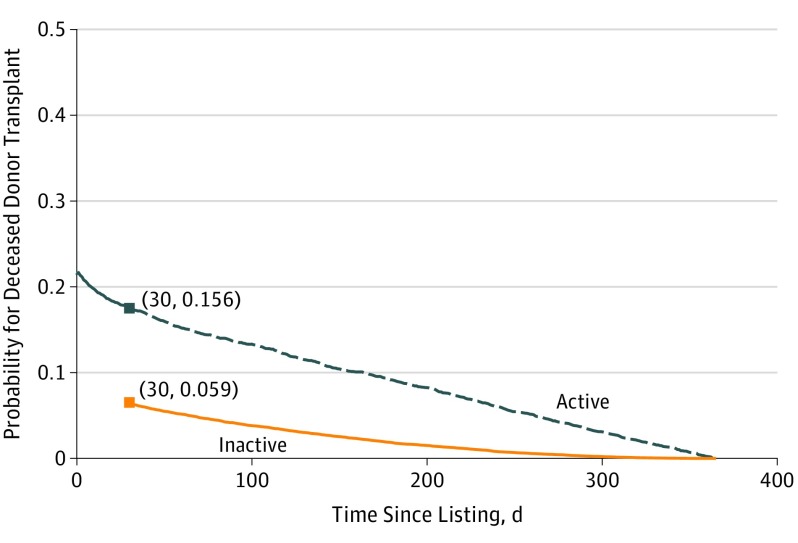
This modeling approach provides the ability to predict transplant probability on any day after patients were listed and shows how an activity status change affects their chances of obtaining a transplant. For example, a white man in his early 40s with diabetes receiving dialysis from region 6 with 0 cPRA was initially on the active list at day 0 and transitioned to inactive status at day 30. We were interested in the degree to which the activity status change would affect the patient’s probability of getting a deceased donor transplant at 1 year. As shown in Figure 3, on the day the patient was listed, the predicted probability of transplant at year 1 was 19.7%. If the activity status had not changed at day 30, this probability would decrease to 15.6%, likely caused by individuals who did not receive living donor transplants. However, given the patient’s transition to inactive status at day 30, the probability of getting the deceased donor transplant at year 1 instead decreased to 5.9%. As this example shows predicted probability over the first year after listing, the probability for patients on the active and inactive lists decrease over time owing to a lack of sufficient wait time accumulation (Figure 3).
Figure 3. Dynamic Transplant Prediction Model.
Predicted probabilities of deceased donor transplant at 1 year after listing given activity status changes. The probabilities were calculated for a white man in his early 40s with diabetes receiving dialysis from region 6 with 0 calculated panel reactive antibody. This patient was initially actively listed at day 0 and transitioned to inactive status at day 30, which markedly reduced the probability of obtaining a transplant long term. As this example shows predicted probability during the first year following listing, the probability for active and inactive patients decrease over time owing to a lack of sufficient wait time accumulation.
Discussion
One of the principal goals of KAS was to address known racial disparities to kidney transplant access.10,11 Although the United Network for Organ Sharing has declared success achieving this through early analyses, the methods used to determine this achievement do not consider how changes in activity status impact transplant probability.13,22,23 We observed that there continue to be differences in transplant probability between white and black patients who are highly sensitized, although Hispanic individuals now have a similar likelihood of transplant as white individuals across all levels of sensitization (Figure 2). Analyses prior to KAS implementation show that levels of sensitization are associated with wait-list mortality.24 If black individuals in the post-KAS era continue to have a lower probability of transplant at higher cPRA levels, as we observed, their mortality will likely be higher than other races/ethnicities when longer follow-up analyses are conducted. One way KAS aimed to improve transplant access to underserved populations was to start waiting time from the time of first dialysis treatment, rather than the date of listing. It is unclear why this did not mitigate disparities for black individuals who are highly sensitized, although it is possible that inactive status, particularly in the cPRA group of 90% or more, where white individuals had a statistically significant advantage over black individuals in the ability to move to the active list (HR, 1.4 [95% CI, 1.2-1.6]), could have contributed to the observed racial disparity. Further study on both the cPRA groups of 80% to 89% and 90% or more is necessary to determine the cause of this health disparity and how allocation rules, including the disproportionate number of white individuals receiving additional DR 0-mismatch points, could have contributed to it.
We noted that once white patients were on the inactive list, they were more likely to be moved to the active list than their Hispanic or black counterparts (Table 2). Because reasons for inactive status could include medical contraindications, incomplete testing, social problems, or financial clearance, it is not surprising that underserved populations who have less access to health care,4 less social support,25 and lower income levels,2 would be less likely to resolve these issues. Access to kidney transplant and the role of race/ethnicity has largely focused on how patients get on the wait-list.16,17 Our study shows that access issues in underserved populations continue after listing. The noted difference between races/ethnicities in transitioning from inactive to active states suggests that this metric could be used as a surrogate marker for measuring health disparities in wait-listed patients. New polices and oversight from the US Centers for Medicare and Medicaid Services have suggested that greater coordination between transplant centers and dialysis units could be achieved through aligned value-based compensation models.26 One option is to incorporate an inactive to active quality measure for dialysis units and transplant centers to incentivize care coordination of shared patients on the inactive list.
Multistate modeling provides a dynamic assessment of wait-list activity status changes over time and how these changes are associated with transplant outcomes. Most analyses performed on OPTN wait-list data use Cox regression models that provide hazards, which are unable to reconcile the important fact that patients with inactive status are unable to receive deceased donor transplants; this is critical in conducting competing risk assessments. Using active listing status or the number of wait-listed patients at the time of deceased donor outcome does not adjust for this factor, and because roughly a third of wait-listed patients remain inactive,27 transplant rates are likely inaccurate as they include individuals who are not able to or may never obtain deceased donor organ offers. Hazards provide the risk of an outcome at a specific point, so when determining the chances of the ability of a patients with inactive status to obtain a deceased donor transplant, the instantaneous hazard is understandably 0. However, patients with inactive status may become active, and the ability to provide the probability of this status change, which may lead to receiving a transplant, allows for counseling patients on the importance of converting their inactive status to active status.
Limitations
We note the following limitations in this study. First, as a retrospective cohort study, robust causal inference is difficult to establish. Second, allocation points for patients who are highly sensitized used a sliding scale starting at cPRA more than 80%.10,11 Our analysis could not use the assignment of specific allocation points in this group as a continuous variable. Third, allocation priorities and geographical organ offer distribution differed in patients with a cPRA of 98%, 99%, and 100%.10,11 Including each of these risk factors would have increased the complexity of the model considerably, and given the small number of patients in each cPRA category, the analysis would have lacked sufficient power to detect a meaningful difference in outcomes. Fourth, there are additional factors impacting access to transplant, including socioeconomic status and referral rates to transplant centers that our model, which was limited to OPTN data, cannot account for. Lastly, it is unclear if the addition of DR 0-mismatch allocation points resulted in the racial/ethnic disparities we observed, as white individuals have this advantage across all levels of sensitization. Future studies will focus on how sensitization sliding scale allocation and DR 0-mismatch allocation points specifically contribute to the observed disparity in transplant probability in patients who are highly sensitized.
Conclusions
In this study, we used a new analytic approach to OPTN data that included the association of wait-list status with transplant outcomes. For the first time to our knowledge, we are able to determine the association of postlisting status changes with the overall probability of obtaining a deceased donor kidney transplant. By including inactive patients in our analysis, we provide greater accuracy and provide new information to patients and health care professionals about the impact of being made inactive. We confirm that for most patients, racial/ethnic differences in obtaining a deceased donor transplant have decreased. However, barriers to transplant continue to exist in the higher cPRA groups, where higher transitions from inactive to active status and greater access to DR matching allocation points for white patients are likely contributory factors. We urge the monitoring of status changes as a quality measure for transplant centers and dialysis providers to encourage care coordination of shared patients, particularly in underserved populations.
eTable 1. Table for testing whether the effect of covariates varies across transitions
eTable 2. Final full model: parameter estimates and standard errors for all covariates and all transitions
References
- 1.Norman SP, Kommareddi M, Luan FL. Inactivity on the kidney transplant wait-list is associated with inferior pre- and post-transplant outcomes. Clin Transplant. 2013;27(4):E435-E441. doi: 10.1111/ctr.12173 [DOI] [PubMed] [Google Scholar]
- 2.Patzer RE, Perryman JP, Schrager JD, et al. The role of race and poverty on steps to kidney transplantation in the Southeastern United States. Am J Transplant. 2012;12(2):358-368. doi: 10.1111/j.1600-6143.2011.03927.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ladin K, Rodrigue JR, Hanto DW. Framing disparities along the continuum of care from chronic kidney disease to transplantation: barriers and interventions. Am J Transplant. 2009;9(4):669-674. doi: 10.1111/j.1600-6143.2009.02561.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol. 2010;5(12):2276-2288. doi: 10.2215/CJN.04940610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart A, Salkowski N, Snyder JJ, Israni AK, Kasiske BL. Beyond “median waiting time”: development and validation of a competing risk model to predict outcomes on the kidney transplant waiting list. Transplantation. 2016;100(7):1564-1570. doi: 10.1097/TP.0000000000001185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni S, Hall I, Formica R, et al. Transition probabilities between changing sensitization levels, waitlist activity status and competing-risk kidney transplant outcomes using multi-state modeling. PLoS One. 2017;12(12):e0190277. doi: 10.1371/journal.pone.0190277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grams ME, Massie AB, Schold JD, Chen BP, Segev DL. Trends in the inactive kidney transplant waitlist and implications for candidate survival. Am J Transplant. 2013;13(4):1012-1018. doi: 10.1111/ajt.12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen PK, Keiding N. Multi-state models for event history analysis. Stat Methods Med Res. 2002;11(2):91-115. doi: 10.1191/0962280202SM276ra [DOI] [PubMed] [Google Scholar]
- 9.Cortese G, Andersen PK. Competing risks and time-dependent covariates. Biom J. 2010;52(1):138-158. [DOI] [PubMed] [Google Scholar]
- 10.Israni AK, Salkowski N, Gustafson S, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol. 2014;25(8):1842-1848. doi: 10.1681/ASN.2013070784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedewald JJ, Samana CJ, Kasiske BL, et al. The kidney allocation system. Surg Clin North Am. 2013;93(6):1395-1406. doi: 10.1016/j.suc.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 12.Massie AB, Luo X, Lonze BE, et al. Early changes in kidney distribution under the new allocation system. J Am Soc Nephrol. 2016;27(8):2495-2501. doi: 10.1681/ASN.2015080934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melanson TA, Hockenberry JM, Plantinga L, et al. New kidney allocation system associated with increased rates of transplants among black and Hispanic patients. Health Aff (Millwood). 2017;36(6):1078-1085. doi: 10.1377/hlthaff.2016.1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UNOS KN . Odds for receiving a kidney transplant now equal for black, white and Hispanic candidates. 2017; https://unos.org/odds-equal-of-kidney-transplant-for-minorities/. Accessed April 24, 2018.
- 15.Daniels N. Justice, health, and healthcare. Am J Bioeth. 2001;1(2):2-16. doi: 10.1162/152651601300168834 [DOI] [PubMed] [Google Scholar]
- 16.Patzer RE, Plantinga LC, Paul S, et al. Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia. JAMA. 2015;314(6):582-594. doi: 10.1001/jama.2015.8897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM. Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol. 2009;20(6):1333-1340. doi: 10.1681/ASN.2008030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyersmann JA, Allignol A, Schumacher M. Competing Risks and Multistate Models in R. New York, NY: Springer; 2011. [Google Scholar]
- 19.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. [Google Scholar]
- 20.Andersen PK, Borgan Ø, Gill RD, Keiding N. Statistical Models Based on Counting Processes. New York, NY: Springer; 1993. [Google Scholar]
- 21.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389-2430. doi: 10.1002/sim.2712 [DOI] [PubMed] [Google Scholar]
- 22.Stewart DE, Klassen DK. Early experience with the new kidney allocation system: a perspective from UNOS. Clin J Am Soc Nephrol. 2017;12(12):2063-2065. doi: 10.2215/CJN.06380617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, Aeder MI. Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant. 2016;16(6):1834-1847. doi: 10.1111/ajt.13770 [DOI] [PubMed] [Google Scholar]
- 24.Sapir-Pichhadze R, Tinckam KJ, Laupacis A, Logan AG, Beyene J, Kim SJ. Immune sensitization and mortality in wait-listed kidney transplant candidates. J Am Soc Nephrol. 2016;27(2):570-578. doi: 10.1681/ASN.2014090894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladin K, Hanto DW. Understanding disparities in transplantation: do social networks provide the missing clue? Am J Transplant. 2010;10(3):472-476. doi: 10.1111/j.1600-6143.2009.02963.x [DOI] [PubMed] [Google Scholar]
- 26.Hippen BE, Maddux FW. Integrating kidney transplantation into value-based care for people with renal failure. Am J Transplant. 2018;18(1):43-52. doi: 10.1111/ajt.14454 [DOI] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services. National Data. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. Accessed May 10, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Table for testing whether the effect of covariates varies across transitions
eTable 2. Final full model: parameter estimates and standard errors for all covariates and all transitions