Abstract
This randomized clinical trial evaluates the use of atorvastatin combined with narrowband UV-B therapy vs narrowband UV-B therapy alone in adult patients with active vitiligo.
Studies have reported the anti-inflammatory, immunomodulatory, and antioxidant effects of statins. Targeting motif chemokine CXCL10 using antibodies has been shown to prevent the depigmentation in the vitiligo mouse model.1 In the same model, simvastatin was effective in the regression of vitiligo.2 Simvastatin has also been shown to protect melanocytes from oxidative stress by activating nuclear factor (erythroid-derived 2)-like 2,3 and atorvastatin can reduce plasma levels of CXCL10 in patients with Crohn disease.4 Therefore, there is a rationale for using statins to halt the progression of active vitiligo.
Methods
We conducted an interventional, bicentric, prospective, randomized single-blind trial to evaluate the efficacy of a combination of atorvastatin and narrowband (NB) UV-B phototherapy vs NB-UV-B phototherapy alone in active vitiligo. Patients with nonsegmental active vitiligo were recruited from Nice, France, and Singapore. The trial was conducted from May 2015 to February 2017. The trial was reviewed and approved by the Sud-Meditérannée and National Healthcare Group institutional review boards (NCT02432534). The participants provided written informed consent; there was no financial compensation. The trial protocol is available in the Supplement.
Active vitiligo was defined as new patches or extension of old lesions in the past 3 months and the presence of hypochromic borders and/or confetti-like depigmentation under a Wood lamp examination. Centralized block randomization, stratified by center, at methodologic center was assigned in a 1:1 ratio to either group A: atorvastatin (40 mg/d for 1 month followed by 80 mg/d for 5 months) and NB-UV-B phototherapy twice a week for 6 months or group B: NB-UV-B phototherapy twice a week for 6 months. Clinical examination, blood tests, and photography were done at baseline, 1 month, 3 months, and 6 months. The primary end point was the percentage of decrease in Vitiligo Area Scoring Index (VASI) score at 6 months, evaluated using standardized pictures by 3 physicians blinded to the treatment. The VASI measures the body surface affected by vitiligo, with scores ranging from 0 (lowest) to 100 (highest). The secondary end points included the Vitiligo European Task Force (VETF) Vitiligo Extent Score (extent, 0%-100%; stage, 0-20; and disease progression, −5 to 5; all lowest to highest) and Dermatology Life Quality Index (0 [lowest]-30 [highest]). Sample size calculation determined the recruitment of 28 patients.
Results
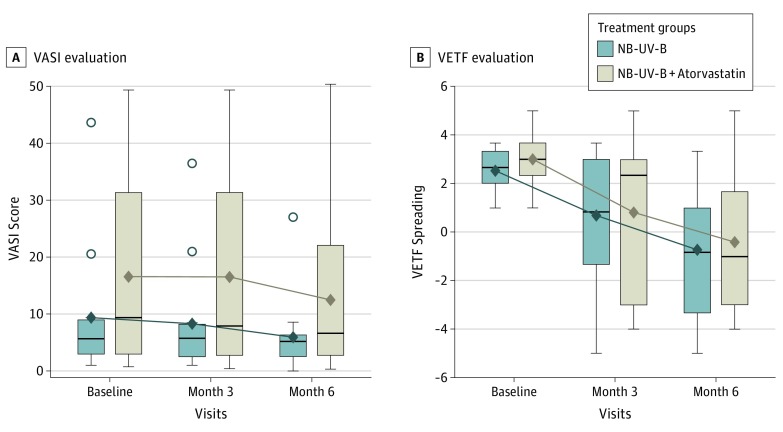
A total of 30 patients were recruited. The median age was 38 (range, 30-51) years for the UV-B group and 45 (range, 27-63) years for the NB-UV-B plus atorvastatin group. The male/female ratio was 7/8 and 6/9 for group A and group B, respectively. Sixteen patients have European ancestries and 14 were Asian. Of the 30 patients randomized, 29 patients completed the study and were included in the modified intent-to-treat analysis (1 patient refused randomization and did not receive any treatment) (Figure 1). Baseline characteristics were similar across treatment groups. The mean (SD) VASI score evolution between baseline and month 6 for group A was −2.95 (1.39) (range, −5.81 to −0.09; P = .04) and −4.38 (1.44) (range, −7.35 to −1.42; P = .005) for group B. The difference in the mean VASI changes between the 2 groups was not statistically significant (1.43; range, −2.76 to 5.62; P = .49, analysis of covariance adjusted by center and VASI at baseline). The VETF spreading score evolution at month 6 was −3.31 (0.69) (range, −4.73 to −1.89; P < .001) in group A and −3.41 (0.72) (range, −4.89 to −1.94; P < .001) in group B. The difference in the mean VETF spreading changes between the 2 groups was not statistically significant (0.11; range, −1.98 to 2.19; P = .92, analysis of covariance adjusted by center and VASI at baseline) (Figure 2). Ten patients in group A had adverse events that included diarrhea, abdominal pain, myalgia, and headache. One patient developed transient elevation of muscular enzyme levels that normalized after the atorvastatin dose was reduced to 40 mg/d. No adverse effects were reported in group B.
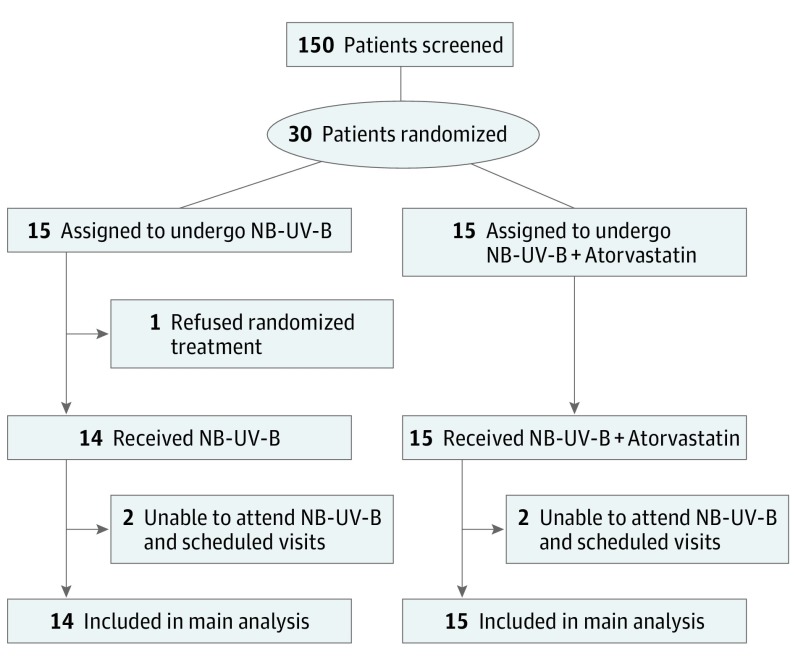
Figure 1. Flowchart of the Studya.
Of the 30 patients randomized, 29 patients completed the study and were included in the modified intent-to-treat analysis (1 patient refused randomization and did not receive any treatment). NB-UV-B indicates narrowband UV-B phototherapy.
aThe exact number of patients screened is unavailable and 150 is presented as an estimate. The inclusion criteria included patients aged 18 to 75 years, active nonsegmental vitiligo, and patient registered to French social security. The exclusion criteria included segmental or mixed vitiligo, allergy to statin medications, and use of statin or fibrate medications owing to cardiac risks.
Figure 2. Evolution of Vitiligo Area Scoring Index (VASI) and Vitiligo European Task Force (VETF) Spreading Scores From Baseline to Each Study Visit.
These results show no statistically significant difference in the mean VASI changes between the 2 groups and a significant decrease in the spreading of vitiligo 6 months after the 2 treatments, but no statistically significant difference between the 2 groups. Horizontal line in each box indicates the median. The diamond within each box indicates the mean. Top and bottom borders of the boxes indicate 75th and 25th percentiles. The whiskers that extend from each box indicate the range of values that are outside of the interquartile range, but are close enough not to be considered outliers (a distance less than or equal to ≤1.5 • IQR). Points beyond whiskers are outliers. NB-UV-B indicates narrowband UV-B phototherapy.
Discussion
These data confirm that NB-UV-B phototherapy can effectively decrease the spreading of active vitiligo. Despite a rationale suggesting that statins would be beneficial in active vitiligo, atorvastatin did not add any benefit to NB-UV-B treatment for repigmentation or halting disease progression. The main limitation of this study is the possible confounding role of NB-UV-B and the fact that only patients with active vitiligo were included. However, a previous trial showed no effect of simvastatin in monotherapy.5 In comparison with mouse studies, the doses used in these 2 clinical trials are at least 3 times lower. Higher doses of statins may thus be required, but their use is limited due to potential adverse effects. More specific treatment targeting the pathways involved in vitiligo may provide better results.
Trial Protocol
References
- 1.Rashighi M, Agarwal P, Richmond JM, et al. . CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6(223):223ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal P, Rashighi M, Essien KI, et al. . Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo. J Invest Dermatol. 2015;135(4):1080-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang Y, Li S, Guo W, et al. . Simvastatin protects human melanocytes from H2O2-induced oxidative stress by activating Nrf2. J Invest Dermatol. 2017;137(6):1286-1296. [DOI] [PubMed] [Google Scholar]
- 4.Grip O, Janciauskiene S. Atorvastatin reduces plasma levels of chemokine (CXCL10) in patients with Crohn’s disease. PLoS One. 2009;4(5):e5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanderweil SG, Amano S, Ko WC, et al. . A double-blind, placebo-controlled, phase-II clinical trial to evaluate oral simvastatin as a treatment for vitiligo. J Am Acad Dermatol. 2017;76(1):150-151.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol