Abstract
This cohort study uses data from the ongoing Adolescent Brain Cognitive Development (ABCD) study to assess the association of maternal use of cannabis before and after knowledge of pregnancy with psychosis proneness in children.
Mirroring increases in the general population, the prevalence of past-month marijuana use among pregnant mothers in the United States increased by 75% between 2002 (2.85%) and 2016 (4.98%).1 Although cannabis use has been linked to psychosis, little is known about prenatal exposure.2,3 Unprecedented increases in marijuana use during pregnancy, alongside evidence that cannabis use is correlated with psychosis and that endocannabinoids play an important role in neurodevelopment, highlight the importance of evaluating potential long-term consequences of prenatal exposure.4
Methods
We used data from the ongoing Adolescent Brain Cognitive Development (ABCD) study (data release 1.0; https://abcdstudy.org/) to test whether maternal report of cannabis use during pregnancy is associated with psychosis proneness (Prodromal Questionnaire–Brief Child Version total score) among 4361 children aged 8.9 to 11.0 years who were born between 2005 and 2008 to 3774 mothers through 3926 pregnancies (Table). All parents provided written informed consent, and all children provided assent to a research protocol approved by the institutional review board at each data collection site (https://abcdstudy.org/study-sites/). The Prodromal Questionnaire-Brief Child version is a 21-item self-report questionnaire designed for children that assesses psychoticlike experiences; total scores range from 0 to 21, with higher scores indicating more psychoticlike experiences.5 Because the sample contains twin and nontwin siblings as well as 21 research sites, linear mixed-effects models were used to nest data on these parameters using the lme4 package in R, version 3.5.0 (The R Foundation). Analyses examining the association between offspring psychosis proneness and prenatal marijuana exposure before (0/1) and after (0/1) maternal knowledge of pregnancy (entered into the regression simultaneously) were conducted using 3 statistical models: (1) no fixed-effect covariates (FECs); (2) potentially confounding FECs (ie, child-, mother-, and pregnancy-related variables; eg, maternal alcohol and tobacco use during pregnancy, maternal education, household income, familial history of psychosis, unplanned pregnancy, child substance exposure; see footnote to the Table); and (3) only FECs significantly associated with psychosis proneness (Table).
Table. Regression Results and Descriptive Statistics for Prenatal Cannabis Exposure and Child Psychosis Proneness Among 4054 Children.
| Risk Factora | Sample Descriptive Statistic | b (95% CI) | P Value |
|---|---|---|---|
| Maternal education, mean (SD), y | 16.99 (2.49) | −0.15 (−0.19 to −0.10) | <.001 |
| Unplanned pregnancy, No. (%) | 1368 (33.7) | 0.50 (0.26 to 0.74) | <.001 |
| Family history of psychosis, No. (%) | 98 (2.4) | 1.21 (0.50 to 1.92) | <.001 |
| Female child, No. (%) | 1908 (47.1) | −0.23 (−0.44 to −0.02) | .03 |
| Child tobacco puff, No. (%) | 25 (0.6) | 1.32 (−0.01 to 2.65) | .05 |
| Child alcohol sip, No. (%) | 1069 (26.4) | 0.63 (0.39 to 0.88) | <.001 |
| Cannabis use during pregnancy, No. (%) | |||
| Before knowledge of pregnancy | 189 (4.7) | −0.06 (−0.66 to 0.53) | .84 |
| After knowledge of pregnancy | 56 (1.4) | 1.41 (0.34 to 2.48) | .01 |
Results represent the model including only covariates that were significantly associated with child psychosis proneness. All covariates considered were (1) household income; (2) maternal educational level; (3) length of time pregnant before maternal knowledge of pregnancy; (4) whether the pregnancy was unplanned (0 = planned, 1 = unplanned); (5) maternal age at birth; (6) prenatal vitamin use, (7-11) first-degree familial history of mental illness: depression, psychosis, anxiety, mania, antisocial behavior; (12-13) prenatal exposure to alcohol or tobacco before maternal knowledge of pregnancy; (14-15) prenatal exposure to alcohol or tobacco after maternal knowledge of pregnancy; (16-22) child race/ethnicity: white, African American, Asian, Native American, Pacific Islander, Hispanic, other; (23) birth weight; (24) child age; (25) child sex (0 = male, 1 = female); (26-27) child substance use: tobacco puff, alcohol sip. A model including all covariates produced similar results (n = 3308): marijuana use after (b = 1.88 P = .008), but not before (b = −0.41, P = .26), maternal knowledge of pregnancy was associated with increased psychosis proneness. Owing to the limited endorsement of ever having a marijuana puff among children (n = 1), we did not include this variable as a covariate. However, excluding this individual does not meaningfully alter association, nor does using log-transformed values for psychosis proneness.
Results
Among 4361 children born (mean [SD] age, 10.0 [0.6] years; 2062 [47.28%] girls), 201 (4.61%) were prenatally exposed to marijuana (138 were exposed to marijuana prenatally only before maternal knowledge, 61 were exposed to marijuana prenatally before and after maternal knowledge, and 2 were exposed to marijuana prenatally only after maternal knowledge of pregnancy). Marijuana use after knowledge of pregnancy was associated with increased offspring psychosis proneness in models with and without FECs (with FECs and missing data listwise deletion, there were 4054 children, of whom 56 were exposed to cannabis prenatally after maternal knowledge of pregnancy (unstandardized β coefficients [b] > 1.41; P < .01; Figure and Table). Post hoc specificity analyses with FECs revealed no link between prenatal exposure after pregnancy knowledge and offspring internalizing, externalizing, or attention symptoms reported on the Child Behavior Checklist (b < 1.01 and P > .46 for all comparisons).6 Marijuana use before maternal knowledge of pregnancy was associated with increased psychosis proneness without FECs (b = 0.73; P = .004), but not with their inclusion (|b|< 0.41; P > .25; Table).
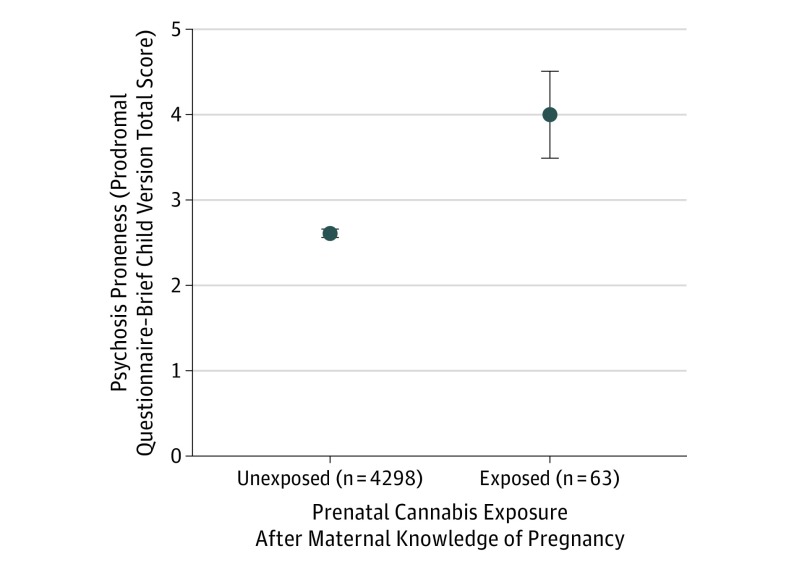
Figure. Association of Prenatal Cannabis Exposure After Maternal Knowledge of Pregnancy With Psychosis Proneness During Childhood.
Those exposed to marijuana prenatally after maternal knowledge of pregnancy had elevated levels of psychosis proneness in middle childhood (figure depicts raw data means and standard error of mean; corresponding mean [SD] were 4.06 [4.04] for exposed children and 2.61 [3.53] for unexposed children; statistics with nested, but not fixed, effect covariates: b = 1.56, P < .001). The exposed group includes individuals born to mothers who reported marijuana use after knowing they were pregnant who may also have used marijuana during their pregnancy before knowing they were pregnant. The unexposed group includes individuals born to mothers who reported not using marijuana at any point during their pregnancy or who reported using it prior to knowledge of pregnancy.
Discussion
These findings suggest that prenatal cannabis exposure after, but not before, maternal knowledge of pregnancy may be associated with a small increase in psychosis proneness during middle childhood. Study limitations include the small sample of prenatal cannabis–exposed offspring; potential maternal underreporting of use during pregnancy; imprecise data on timing and amount, frequency, and potency of cannabis exposure; absence of data on whether childhood psychosis proneness is associated with conversion to psychosis; and lack of data on some potential confounders (eg, maternal psychosis proneness, stress). Because associations between offspring psychosis proneness and marijuana use after pregnancy knowledge were robust to the inclusion of other potential confounding factors and preceded offspring marijuana use,3 these data increase the plausibility that prenatal cannabis exposure may increase offspring psychosis risk. One possible explanation for the finding that marijuana use following, but not before, knowledge of pregnancy (mean [SD], 7.70 [5.91] weeks’ gestation) was associated with offspring psychosis proneness is endocannabinoid system ontogeny. Among mice, the endocannabinoid type 1 receptor, through which cannabis primarily affects the brain, is expressed at the equivalent of 5 to 6 weeks in humans, with gradual expression increases.7 Thus, prenatal cannabis exposure may be associated with later psychosis proneness in offspring only when there is sufficient fetal endocannabinoid type 1 receptor expression, which may not occur until after many mothers learn they are pregnant. In the context of increasing cannabis accessibility and potency, perceptions of safety, and the potential use of cannabis to combat pregnancy-related nausea,4 these data suggest that cannabis use by pregnant women should be discouraged until more is known.
References
- 1.Agrawal A, Rogers CE, Lessov-Schlaggar CN, Carter EB, Lenze SN, Grucza RA. Alcohol, cigarette, and cannabis use between 2002 and 2016 in pregnant women from a nationally representative sample [published online November 5, 2018]. JAMA Pediatr. doi: 10.1001/jamapediatrics.2018.3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zammit S, Thomas K, Thompson A, et al. . Maternal tobacco, cannabis and alcohol use during pregnancy and risk of adolescent psychotic symptoms in offspring. Br J Psychiatry. 2009;195(4):294-300. doi: 10.1192/bjp.bp.108.062471 [DOI] [PubMed] [Google Scholar]
- 3.Day NL, Goldschmidt L, Day R, Larkby C, Richardson GA. Prenatal marijuana exposure, age of marijuana initiation, and the development of psychotic symptoms in young adults. Psychol Med. 2015;45(8):1779-1787. doi: 10.1017/S0033291714002906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkow ND, Compton WM, Wargo EM. The risks of marijuana use during pregnancy. JAMA. 2017;317(2):129-130. doi: 10.1001/jama.2016.18612 [DOI] [PubMed] [Google Scholar]
- 5.Karcher NR, Barch DM, Avenevoli S, et al. . Assessment of the Prodromal Questionnaire–Brief Child Version for measurement of self-reported psychoticlike experiences in childhood. JAMA Psychiatry. 2018;75(8):853-861. doi: 10.1001/jamapsychiatry.2018.1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Achenbach TM. The Achenbach System of Empirically Based Assessment (ASEBA): Development, Findings, Theory and Applications. Burlington, VT: University of Vermont Research Center for Children, Youth, and Families; 2009. [Google Scholar]
- 7.Wu CS, Jew CP, Lu HC. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol. 2011;6(4):459-480. doi: 10.2217/fnl.11.27 [DOI] [PMC free article] [PubMed] [Google Scholar]