This cross-sectional study compares the value of determining serum levels of soluble interleukin 2 receptor with that of angiotensin-converting enzyme for diagnosing sarcoidosis in patients with uveitis.
Key Points
Question
What is the diagnostic value of soluble interleukin 2 receptor compared with that of angiotensin-converting enzyme in sarcoidosis-associated uveitis?
Findings
In this cross-sectional study of 249 patients with uveitis, the sensitivity and specificity of using serum-soluble interleukin 2 receptor levels alone for diagnosing sarcoidosis were 81% and 64%, respectively. Combined with chest radiography, the sensitivity and specificity for soluble interleukin 2 receptor were 92% and 58%, respectively; for angiotensin-converting enzyme, 70% and 79%, respectively.
Meaning
These findings support the usefulness of soluble interleukin 2 receptor level for diagnosing sarcoidosis in patients with uveitis and suggest that this biomarker may be slightly better than angiotensin-converting enzyme.
Abstract
Importance
New and improved diagnostic tests for sarcoidosis-associated uveitis are needed because the currently available laboratory diagnostic biomarkers (eg, lysozyme and angiotensin-converting enzyme [ACE]) are lacking in high sensitivity and specificity.
Objective
To compare the value of soluble interleukin 2 receptor (sIL-2R) with ACE as diagnostic biomarkers of sarcoidosis in patients with uveitis.
Design, Setting, and Participants
A cross-sectional retrospective study was conducted using data collected from 249 consecutive patients with uveitis at the Erasmus University Medical Center uveitis outpatient clinic, Rotterdam, the Netherlands, from April 3, 2013, through November 25, 2015. Measurements of sIL-2R and ACE in serum samples and data extraction from patient files were conducted from December 2016 through February 2017, and analysis from April to May 2017.
Main Outcomes and Measures
Serum levels of sIL-2R and ACE and chest radiographic findings were assessed. Receiver operating characteristics analysis was used to determine the probability that individual tests correctly identified patients with sarcoidosis. The Youden Index was used to determine the optimal cutoff points for serum sIL-2R and ACE levels to define sarcoidosis in patients with uveitis.
Results
Data were analyzed from 249 patients with uveitis who had their serum sIL-2R and ACE levels determined and underwent chest radiography. Mean (SD) age at the time of sampling was 51 (16) years, 161 patients (64.7%) were women, and 191 (76.7%) were white. Although patients with sarcoidosis-associated uveitis had the highest mean (SD) serum sIL-2R (6047 [2533] pg/mL) and ACE (61 [38] U/L) levels, elevated serum sIL-2R levels were also found in patients with HLA-B27–associated (4460 [2465] pg/mL) and varicella-zoster virus–associated (5386 [1778] pg/mL) uveitis. Serum sIL-2R and ACE levels were significantly correlated (Pearson correlation coefficient, 0.205; P = .001, 2-sided), but no association was found between uveitis activity and sIL-2R (Spearman rank correlation coefficient [ρ], 0.070, P = .27) nor uveitis activity and ACE (ρ, −0.071; P = .27). The highest Youden index for sIL-2R alone was 0.45, corresponding to an optimal cutoff of 4000 pg/mL and providing 81% (95% CI, 74%-89%) sensitivity and 64% (95% CI, 56%-72%) specificity alone but combined with chest radiography yielded 92% sensitivity and 58% specificity. Chest radiography combined with sIL-2R at a cutoff of 6000 pg/mL resulted in 77% sensitivity and 73% specificity. Combined chest radiography and serum ACE levels at the standard cutoff of 68 U/L resulted in 70% sensitivity and 79% specificity.
Conclusions and Relevance
This cross-sectional study demonstrates that sIL-2R is a useful marker for diagnosing sarcoidosis in patients with uveitis and has slightly better diagnostic value than ACE.
Introduction
Sarcoidosis is a major cause of uveitis worldwide.1 An accurate diagnosis of sarcoidosis in patients with uveitis has consequences for the management of the patients’ care and their vison outcomes as well as the choice of medication. Determining whether a patient with uveitis also has sarcoidosis is usually assessed using chest imaging in combination with biochemical measures and is preferably confirmed by biopsy results.2
The lack of a highly sensitive and specific screening test for sarcoidosis in patients with uveitis poses a substantial problem for diagnosing sarcoidosis because undetected sarcoidosis can lead to substantial systemic and ocular morbidity.3 Although serum angiotensin-converting enzyme (ACE) is the most commonly used diagnostic and activity marker for sarcoidosis, this biomarker has low sensitivity.4,5,6
The soluble interleukin 2 receptor (sIL-2R; also termed CD25) is a truncated protein that is released from activated T cells; hence, it is a surrogate marker for T-cell activation.7 Activation of T cells is a main component of the inflammatory process in sarcoidosis, and sIL-2R serum levels indeed correlate with disease activity in sarcoidosis.8,9,10,11,12 However, sIL-2R has been rarely investigated in sarcoidosis-associated uveitis, and its diagnostic value in patients with uveitis is unclear.5
We assessed the value of sIL-2R as a diagnostic biomarker for sarcoidosis-associated uveitis, determining its sensitivity and specificity and comparing these results with those of ACE.
Methods
Study Population and Sample Collection
We conducted a cross-sectional study in consecutive patients with uveitis who visited the Ophthalmology Department at the Erasmus University Medical Center, Rotterdam, the Netherlands. All participating patients visited the department from April 3, 2013, through November 25, 2015, for evaluation, treatment, or both and agreed to have samples included in a biobank for use in research studies. The study was designed in November 2016. Measurements of sIL-2R and ACE levels in serum samples were conducted from December 2016 through February 2017. Data from patient files were abstracted from December 2016 through April 2017. Data analysis was performed from April to May 2017.
The medical ethical committee of Erasmus University Medical Center approved the biobanking protocol and the associated procedures. Written informed consent was obtained for use of the biobank material, which adheres to the tenets of the Declaration of Helsinki.13
Assessment of Clinical Characteristics
Demographic data as well as the final diagnosis of uveitis and its onset, laterality, and location were recorded. Uveitis onset was defined as the date on which an ophthalmologist first documented uveitis. At the time of sampling, 152 of 249 patients (61.0%) had active uveitis. Use of immunosuppressive medications and ACE inhibitors were also recorded.
Assessment of Serum sIL-2R and ACE Levels
For missing routine diagnostic ACE or sIL-2R results, serum levels were determined with biobank samples (stored at −80°C) using the same standard diagnostic facilities and laboratory methods. For all patients, ACE and sIL-2R levels were measured in all serum samples on the same day.
Serum sIL-2R and ACE level assessments were performed by a laboratory with an ISO 15189:2012 accreditation. Both assays were within the scope of this certification and as such were subjected to periodic external quality assessment.
Serum sIL-2R levels were determined using an enzyme-linked immunosorbent assay (human sCD25/sIL-2R ELISA kit; Diaclone SAS) according to the manufacturer’s instructions. The interassay variation coefficient for the sIL-2R measurement was 12%. Freeze-thaw cycles of samples did not affect sIL-2R values until the third cycle. In addition, material could be stored at room temperature for 3 days without affecting the sIL-2R values. A value greater than 2500 pg/mL indicated a level that was elevated compared with that in a healthy population.
The interassay coefficient of variation for ACE, used as an internal quality control, was 2.2% at 46 U/L and 2.1% at 84 U/L (to convert ACE levels to nanokatals per liter, multiply by 16.667). This was below the manufacturer’s claim of 8.1% for interassay precision. The influence of freeze-thaw cycles on ACE was not investigated. The ACE levels were determined using a commercial ACE kinetic assay kit (Bühlmann Laboratories AG), which has CE (Conformité Européenne) marking, analyzed spectrophotometrically on an automated analyzer (Cobas 8000; Roche Diagnostics). The assay is based on the enzymatic cleavage by ACE of the synthetic substrate FAPGG (N-[3-(2-16 furyl)acryloyl]-l-phenylalanyl-l-glycyl-l-glycine) into an amino acid derivative and dipeptide. The kinetics of this reaction was measured by detecting the decrease in absorbance at a wavelength of 340 nm. The standard cutoff for serum ACE levels of greater than 68 U/L was used.
Chest Imaging Assessment
Chest radiography had been conducted in 190 of 249 participants (76.3%). When multiple images were available, the radiograph dated closest to that of the serum sampling was selected. However, chest radiography had to have been performed within 12 months (before or after) of blood sampling to be included in our analyses. Radiographic signs consistent with the diagnosis of sarcoidosis were symmetrical bilateral hilar lymphadenopathy or interstitial lung patterns suggestive of sarcoidosis or both. All other changes were classified according to current radiologic criteria.
Outcome Assessment
Only patients with definitive or presumed ocular sarcoidosis based on the International Workshop on Ocular Sarcoidosis criteria were included (biopsy or radiologic finding), and patients with probable or possible ocular sarcoidosis were classified as having unknown origin.2 A definite diagnosis of tuberculosis-associated uveitis was made in patients with a positive microbiology test result in samples obtained anywhere in the body without another explanation of uveitis.14 Other diagnoses were made based on current international criteria.15,16,17,18,19,20 Active uveitis was defined as the presence of anterior chamber cells or vitreous cells, opalescent anterior chamber, or vasculitis or retinitis as documented by ophthalmoscopy or fluorescence angiography.
Statistical Analysis
Patient characteristics were summarized using descriptive statistics, including means (SDs) and percentages. Unpaired t tests were used to compare characteristics between the groups. A 2-sided P < .05 was considered statistically significant. The test characteristics for sIL-2R as well as for ACE in the diagnosis of sarcoidosis (ie, sensitivity, specificity, positive predictive value [PPV]), and negative predictive value [NPV]) were calculated. Receiver operating characteristic (ROC) curves were plotted, and the C statistic (ie, the area under the ROC curve) for sIL-2R and for ACE were calculated. The ROC curve is a plot that shows the sensitivity and specificity of a test at all possible cutoff values that could be used to distinguish patients anticipated to have a disease from those who do not. The sensitivity and specificity are calculated at every observed value in the data set and are plotted to form the ROC curve. The area under this curve, termed the C statistic, describes the probability that the test will correctly identify patients with the disease and can vary between 1 (perfect sensitivity and specificity) and 0.5 (no better than chance and thus a useless test).
The sensitivity and specificity for the use of chest radiography in the diagnosis of sarcoidosis was also determined. In addition, the Youden indices (J = sensitivity + [specificity − 1]) for sIL-2R, ACE, and chest radiographic results were calculated. This index, which ranges from −1 to 1, indicates that the diagnostic test is useless when it equals zero because this would mean the same proportion of positive results were obtained for groups with and without the disease. A higher Youden index is more favorable because a value of 1 indicates no false-positives or false-negatives.21 The Youden index in the ROC curve analysis was used to determine the optimal cutoff levels for sIL-2R and ACE. Combined sensitivity and specificity were also calculated using the method for simultaneous testing according to Kanchanaraksa et al.22 The statistical analyses were conducted using Excel; IBM SPSS Statistics for Windows, version 21.0.0 (IBM Corp); and R, using the software package pROC.
Results
Patient inclusion is illustrated in the study flowchart (Figure 1). From April 3, 2013, through November 25, 2015, 266 patients with uveitis agreed to participate in our biobank study, of which 249 had their serum sIL-2R and ACE levels simultaneously measured.
Figure 1. Flow Diagram of Patients and Samples Included in the Study.
ACE indicates angiotensin-converting enzyme; sIL-2R, soluble interleukin 2 receptor.
Population Characteristics
Final diagnoses and demographic characteristics of the study cohort are given in Table 1. The mean (SD) age at uveitis onset was 46 (17) years and at sampling for the present study was 51 (16) years. Women had significantly higher mean (SD) serum ACE levels than men (49 [28] U/L vs 39 [24] U/L; P = .01), whereas serum sIL-2R levels were similar between the sexes (women, 4070 [2224] pg/mL vs men, 4509 [2490] pg/mL; P = .16). Age and serum sIL-2R levels showed a significant linear association (P = .045): serum sIL-2R increased approximately 18 pg/mL every year.
Table 1. Demographic Characteristics and Final Diagnoses in Patients With Uveitis.
| Characteristic | No. (%) | Level, Mean (SD) | |
|---|---|---|---|
| Serum ACE, U/L | Serum sIL-2R, pg/mL | ||
| Total included patients | 249 (100) | 46 (27) | 4225 (2326) |
| Unilateral involvement | 87 (35) | 39 (22) | 4212 (2432) |
| Bilateral involvement | 162 (65) | 49 (28) | 4233 (2275) |
| Men | 88 (35) | 39 (24) | 4509 (2490) |
| Women | 161 (65) | 49 (28) | 4070 (2224) |
| Race | |||
| White | 191 (77) | 47 (28) | 4198 (2218) |
| Non-White | 58 (23) | 41 (22) | 4316 (2672) |
| Anatomical localization of uveitis | |||
| Anterior | 37 (15) | 44 (23) | 4373 (2585) |
| Intermediate | 21 (8) | 43 (20) | 4236 (2086) |
| Posterior | 77 (31) | 47 (25) | 3848 (2230) |
| Panuveitis | 103 (41) | 47 (32) | 4554 (2416) |
| Scleritis | 11 (4) | 36 (17) | 3257 (918) |
| Use of medication | |||
| Immunosuppressive medication | 89 (36) | 47 (27) | 3872 (2456) |
| ACE inhibitor | 16 (6) | 46 (27) | 4628 (1861) |
| Activity of uveitis | |||
| Active uveitis | 152 (61) | 46 (28) | 4430 (2474) |
| Remission of uveitis | 97 (39) | 45 (25) | 3905 (2045) |
| Associated with systemic disease | 77 (31) | 48 (32) | 4823 (2502) |
| Sarcoidosis, total | 37/77 (48) | 61 (38) | 6047 (2533) |
| Definitive sarcoidosis | 23/77 (30) | 55 (27) | 5521 (2232) |
| Presumed sarcoidosis | 14/77 (18) | 70 (51) | 6911 (2835) |
| Multiple sclerosis | 13/77 (17) | 37 (18) | 3487 (1431) |
| HLA-B27–associated uveitis | 10/77 (13) | 37 (23) | 4460 (2465) |
| VKH syndrome | 4/77 (5) | 28 (11) | 2834 (1694) |
| Miscellaneousa | 13/77 (17) | 35 (22) | 3569 (1847) |
| Infectious uveitis | 50 (20) | 37 (19) | 4268 (2011) |
| Rubella virus | 16/50 (32) | 30 (14) | 3915 (1885) |
| Toxoplasmosis | 12/50 (24) | 39 (21) | 3378 (1654) |
| Cytomegalovirus | 7/50 (14) | 37 (23) | 4537 (1611) |
| Varicella-zoster virus | 7/50 (14) | 50 (26) | 5386 (1778) |
| Miscellaneousb | 8/50 (16) | 36 (15) | 5092 (2780) |
| Established clinical entity | 47 (19) | 44 (23) | 3738 (2442) |
| BSCR | 25/47 (53) | 49 (26) | 2980 (1174) |
| Masquerade syndromec | 9/47 (19) | 43 (22) | 5741 (3701) |
| APMPE | 3/47 (6) | 38 (13) | 5184 (4180) |
| Miscellaneousd | 10/47 (21) | 38 (16) | 3395 (2076) |
| Unknowne | 75 (30) | 50 (27) | 3889 (2163) |
| IGRA-positive | 20/75 (27) | 50 (30) | 4147 (2292) |
| IGRA-negative or not performed | 55/75 (73) | 50 (25) | 3717 (2081) |
Abbreviations: ACE, angiotensin-converting enzyme; APMPE, acute posterior multifocal placoid pigment epitheliopathy; BSCR, birdshot chorioretinopathy; CMV, cytomegalovirus; FHUS, Fuchs heterochromic uveitis syndrome; IBD, inflammatory bowel disease; IGRA, interferon-γ release assay; sIL-2R, soluble interleukin 2 receptor; VKH, Vogt-Koyanagi-Harada.
SI conversion factor: To convert ACE to nanokatals per liter, multiply by 16.667.
Includes granulomatosis with polyangiitis (n = 3), juvenile idiopathic arthritis (n = 2), Sjögren disease (n = 1), IBD (n = 1), polychondritis (n = 1), morphea (n = 1), Kikuchi disease (n = 1), Behçet disease (n = 1), ankylosing spondylitis (n = 1), and giant cell arteritis (n = 1).
Includes herpes simplex virus (n = 3), HIV (n = 1), tuberculosis (n = 1), Staphylococcus aureus (n = 1), Streptococcus pneumoniae (n = 1), and Aspergillus niger (n = 1).
Includes lymphoma (n = 7), retinitis pigmentosa (n = 1), and uveitis suspected to be caused by intravesical bacille Calmette-Guérin immunotherapy for bladder cancer (n = 1).
Includes Fuchs heterochromic uveitis syndrome (n = 3), ampiginous choroiditis (n = 2), sympathetic ophthalmia (n = 2), acute zonal occult outer retinopathy (n = 1), presumed ocular histoplasmosis syndrome (n = 1), and posttraumatic uveitis (n = 1).
In 10 patients with uveitis of unknown cause, no IGRA test was performed.
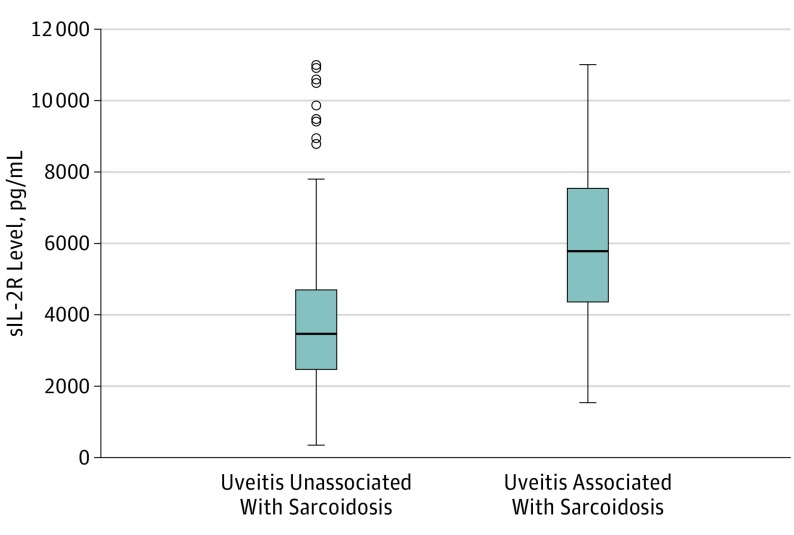
The results of serum sIL-2R levels are illustrated in Figure 2 and given in Table 1. The mean (SD) sIL-2R level of patients with uveitis associated with systemic noninfectious disease was 4823 (2502) pg/mL and in patients with infectious uveitis was 4268 (2011) pg/mL. Within the systemic disease group, the highest mean (SD) serum sIL-2R (6047 [2533] pg/mL) as well as serum ACE (61 [38] U/L) levels were noted in patients with sarcoidosis. Patients with HLA-B27–associated uveitis also exhibited high mean (SD) sIL-2R values (4460 [2465] pg/mL; P = .08 compared with patients with sarcoidosis). Within the samples obtained from patients with infectious uveitis, varicella-zoster virus–associated uveitis had the highest mean (SD) sIL-2R (5386 [1778] pg/mL) and ACE (50 [26] U/L) serum levels. Low mean (SD) sIL-2R levels were found in patients with birdshot chorioretinopathy (2980 [1174] pg/mL; P < .05, compared with patients with sarcoidosis). Mean sIL-2R serum levels did not differ between those patients using any form of systemic immunosuppressant therapy and those who did not (for the whole population: 3872 [2456] pg/mL vs 4422 [2235] pg/mL, P = .91; for patients with sarcoidosis: 5450 [2955] pg/mL vs 6333 [2314] pg/mL, P = .33).
Figure 2. Plot Comparing Soluble Interleukin 2 Receptor (sIL-2R) Serum Levels in Patients With Sarcoidosis-Associated Uveitis and With Uveitis Unassociated With Sarcoidosis.
Boxes indicate the interquartile range; bold horizontal lines, medians; whiskers, the minimum and maximum, excluding the outliers; and open circles, outliers.
Determining the Optimal Cutoff for Serum sIL-2R and ACE Levels to Define Sarcoidosis-Associated Uveitis
Use of a sIL-2R cutoff value of 2500 pg/mL resulted in a relatively low Youden index of 0.17 (Table 2). Therefore, to calculate the optimal cutoff for the diagnosis of sarcoidosis-associated uveitis, we maximized the Youden index in our ROC curve. The highest Youden index for sIL-2R was 0.45, which yielded an optimal cutoff of 4000 pg/mL. Corresponding sensitivity for the diagnosis of sarcoidosis was 81% (95% CI, 65%-92%), and the corresponding specificity was 64% (95% CI, 56%-72%). To ensure a fair comparison of sIL-2R and ACE levels, we also calculated an optimal cutoff point for serum ACE levels in the population with uveitis. The optimal cutoff point for ACE was 51 U/L, which was marginally lower than the currently used standard cutoff of 68 U/L.
Table 2. Assessment of the Value of the Various Diagnostic Tests and Their Combinations for Uveitis Associated With Sarcoidosis.
| Diagnostic Strategy | Sensitivity, % (95% CI) | Specificity % (95% CI) | Youden Index | C Statistic (95% CI) | PPV | NPV |
|---|---|---|---|---|---|---|
| sIL-2R ≥2500 pg/mL | 92 (78-98) | 26 (20-32) | 0.17 | 0.76 (0.68-0.84) | 0.18 | 0.95 |
| sIL-2R ≥4000 pg/mL | 81 (74-89) | 64 (56-72) | 0.45 | 0.76 (0.68-0.84) | 0.28 | 0.95 |
| sIL-2R ≥6000 pg/mL | 47 (31-63) | 79 (73-86) | 0.27 | 0.76 (0.68-0.84) | 0.28 | 0.90 |
| ACE ≥68 U/L | 30 (16-47) | 85 (80-90) | 0.15 | 0.65 (0.55-0.74) | 0.26 | 0.87 |
| ACE ≥51 U/L | 54 (37-71) | 70 (63-76) | 0.23 | 0.65 (0.55-0.74) | 0.24 | 0.90 |
| Chest radiographa | 56 (38-73) | 98 (94-100) | 0.48 | NA | 0.83 | 0.93 |
| ACE ≥68 U/L + chest radiographa,b | 70 (NA) | 79 (NA) | 0.49 | NA | 0.42 | 0.92 |
| ACE ≥51 U/L + chest radiographa,b | 82 (NA) | 64 (NA) | 0.45 | NA | 0.33 | 0.94 |
| ACE ≥68 U/L + sIL-2R ≥4000 pg/mLb | 87 (NA) | 55 (NA) | 0.42 | NA | 0.30 | 0.95 |
| ACE ≥51 U/L + sIL-2R ≥4000 pg/mLb | 92 (NA) | 44 (NA) | 0.36 | NA | 0.26 | 0.96 |
| sIL-2R ≥4000 pg/mL + chest radiographa,b | 92 (NA) | 58 (NA) | 0.50 | NA | 0.32 | 0.97 |
| sIL-2R ≥6000 pg/mL + chest radiographa,b | 77 (NA) | 73 (NA) | 0.50 | NA | 0.38 | 0.94 |
| sIL-2R ≥3195 pg/mL in the present study | 92 (78-98) | 43 (36-50) | 0.35 | NA | 0.20 | 0.96 |
| sIL-2R ≥3195 pg/mL in study by Gundlach et alc | 98 (87-100) | 94 (90-97) | 0.92 | NA | NA | NA |
Abbreviations: ACE, angiotensin-converting enzyme; NA, not applicable or not available, cannot be calculated for this combination of tests; NPV, negative predictive value; PPV, positive predictive value; sIL-2R, soluble interleukin 2 receptor.
SI conversion factor: To convert ACE to nanokatals per liter, multiply by 16.667.
In total, 190 of 249 (76.3%) underwent chest radiography. Of these, 37 patients (19.5%) did not have a chest radiograph for diagnostic purposes of uveitis screening, but 9 underwent chest radiography for follow-up of uveitis and 28 for any medical reason other than uveitis. Only lymphadenopathy or interstitial lung patterns suggestive of sarcoidosis were considered signs of sarcoidosis.
To determine the combined sensitivity and specificity of the various combinations of sIL-2R, ACE, and chest radiograph results, the sensitivity and specificity of sIL-2R, ACE, and chest radiograph calculated in a subcohort of 190 patients (of whom 34 [17.9%] had sarcoidosis) who underwent all 3 tests were used.
In the study by Gundlach et al,5 patients without histologic or radiologic evidence of sarcoidosis were also included; thus, some included patients had elevated serum sIL-2R and ACE levels. A conversion factor of 5 was assumed for the serum sIL-2R levels.
Evaluating the Value of the Various Diagnostic Tests for Sarcoidosis-Associated Uveitis
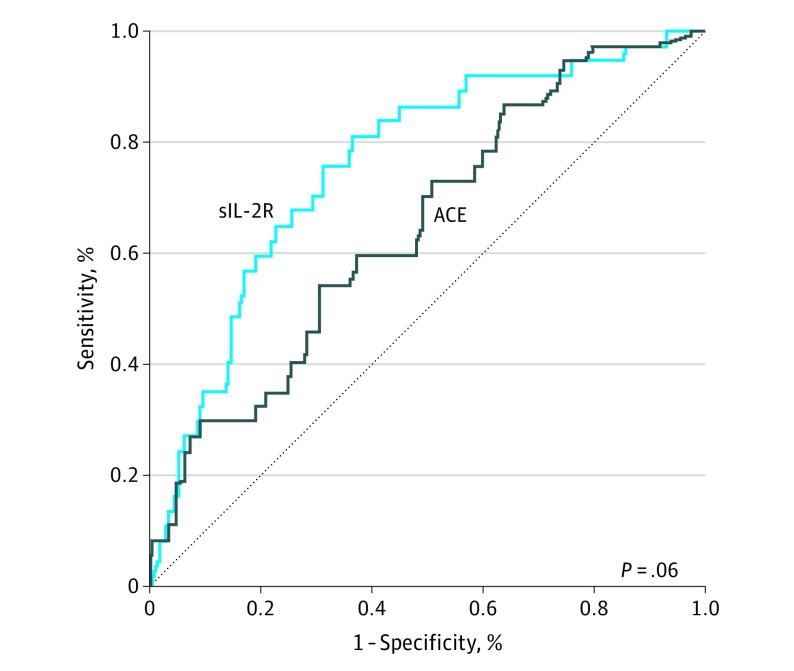
Table 2 provides the results of the various diagnostic tests using the reference values and optimized cutoffs. The Youden index (with optimized cutoffs) was higher for sIL-2R than for ACE levels (0.45 vs 0.23). In addition, the C statistic (area under the ROC curve) also favored sIL-2R over ACE (0.76 [95% CI, 0.68-0.84] vs 0.65 [95% CI, 0.55-0.74]; P = .06, 2-sided, DeLong test; P < .05 considered statistically significant) (Figure 3). We compared different diagnostic strategy combinations by using the Youden index (Table 2). The combination that yielded the highest Youden index was sIL-2R and chest radiography. Along with chest radiography, the cutoffs for sIL-2R levels of at least 4000 pg/mL and at least 6000 pg/mL both resulted in a Youden index of 0.50. The cutoff of 4000 pg/mL or greater corresponded to a sensitivity of 92% and specificity of 58%, whereas the cutoff of 6000 pg/mL or greater resulted in a more balanced sensitivity of 77% and specificity of 73%. The combination of ACE (standard cutoff) and chest radiography yielded a sensitivity of 70% and specificity of 79% and a Youden index of 0.49.
Figure 3. Receiver Operating Characteristic Curves Comparing Soluble Interleukin 2 Receptor (sIL-2R) and Angiotensin-Converting Enzyme (ACE) Levels.
Area under the curve for sIL-2R levels is 0.76 (95% CI, 0.68-0.84); for ACE levels, 0.65 (95% CI, 0.55-0.74) (P = .06, 2-sided, Delong test; P < .05 considered statistically significant).
We also calculated the PPVs and the NPVs for the various diagnostic tests and combinations. Both PPV and NPV favored sIL-2R levels with a cutoff equal to or greater than 4000 pg/mL over ACE with a cutoff equal to or greater than 51 U/L, with values for PPV and NPV of 0.28 and 0.95, respectively, for sIL-2R and of 0.24 and 0.90, respectively, for ACE.
In addition to these data, we also examined a cohort that excluded all patients who used any therapy that might have influenced the outcomes of the sIL-2R or ACE assays (ie, any systemic immunomodulatory therapy or ACE inhibitor therapy). In this cohort of 157 patients (including 24 patients [15.3%] with sarcoidosis), we calculated the sensitivity, specificity, and the C statistic for the serum sIL-2R and ACE levels. The results for the original cohort of 249 participants and this smaller cohort of 157 did not differ for the sIL-2R assay, but the sensitivity of the ACE assay increased from 54% to 71% in the smaller cohort. The C statistic for sIL-2R and for ACE did not differ (0.80 vs 0.73; P = .27, 2-sided, Delong test) and was similar to the results for the original cohort of 249 patients (0.76 vs 0.65; P = .06; 2-sided, Delong test).
Serum sIL-2R and ACE Levels and Uveitis Activity
A positive correlation was observed between serum sIL-2R and ACE levels (Pearson correlation coefficient, 0.205; P = .001, 2-sided). No correlation between uveitis activity and sIL-2R or ACE levels was observed for the whole study population (Spearman rank correlation coefficient [ρ], 0.070, P = .27, vs −0.071, P = .27, 2-sided) or for only those patients with sarcoidosis (ρ, 0.260, P = .12, vs 0.127, P = .45).
Discussion
This cross-sectional study revealed that the level of sIL-2R was slightly better than that of ACE in its diagnostic performance of sarcoidosis in a population of patients with uveitis. Serum sIL-2R levels also showed slightly better C statistic outcomes and had a slightly higher Youden index than for ACE. In addition, sIL-2R had higher sensitivity but lower specificity than ACE. Both PPV and NPV values favored sIL-2R (cutoff ≥4000 pg/mL) over ACE (cutoff ≥51 U/L).
The sensitivity of sIL-2R reported herein for the diagnosis of sarcoidosis in patients with uveitis was lower than that reported by Gundlach et al5 (81% vs 98%). This discrepancy might be explained by the lower cutoff level for sIL-2R used in that study. Gundlach et al also reported higher sIL-2R specificity (94% vs our finding of 64%), which might be explained by their inclusion of patients (20 of 42 [48%]) with probable and possible sarcoidosis, diagnoses that are based solely on laboratory and clinical signs.2,5 The inclusion of patients with only presumed and definitive ocular sarcoidosis in the present study, which was based on histologic and radiologic criteria, enabled an unbiased evaluation of sIL-2R and ACE levels, giving a lower proportion of true-negatives and thus lower specificity.2
Our results showed that high sIL-2R levels also occurred in patients with uveitis that was not associated with sarcoidosis, indicating a high proportion of T-cell–mediated disease in the population with uveitis. Our study results highlighted the need for using different cutoffs for diagnostic tests in diverse populations. An sIL-2R level above the reference value of 2500 pg/mL indicates increased T-cell activity compared with that in a healthy population. However, an optimized cutoff should be determined for diagnostic purposes in diseased populations.
We found low serum sIL-2R levels in patients with birdshot chorioretinopathy and with Vogt-Koyanagi-Harada syndrome, a finding that may help distinguish these ocular disorders from sarcoidosis. However, because the numbers of patients with birdshot chorioretinopathy and Vogt-Koyanagi-Harada syndrome were limited in our study, the low sIL-2R levels should be confirmed in larger studies.
The clinically most useful diagnostic test combination for sarcoidosis in patients with uveitis was the determination of serum sIL-2R levels combined with chest radiography (sensitivity and specificity of 92% and 58%, respectively). The high sensitivity of this combination reduces the chance of missing sarcoidosis compared with that afforded by the current clinical practice of determining serum ACE levels and obtaining a chest radiograph (sensitivity of 70%).23
Limitations
Our study has some shortcomings inherent in retrospective studies. Not all samples were obtained during active ocular disease, which might be associated with lower levels of sIL-2R and ACE.12 In our study, no association was found between ocular disease activity and elevated serum sIL-2R or ACE levels. These serum measurements reflected overall disease activity, but disease activity limited to the eyes may not be accurately reflected by these serum factors.
Not all of the patients in the present study underwent chest radiography shortly after the onset of uveitis, which might have influenced the percentage of positive and negative chest radiographic findings and certainly influenced the elevated PPV of chest radiography found in the present study. Levels of serum sIL-2R and ACE fluctuate over time with the activity of sarcoidosis and are not associated with changes in the same way as those observed over time on chest radiography.
We detected high variability in the serum sIL-2R levels that could not be explained by sex or age nor by the interassay variation coefficient. The high variability may reflect the systemic diseases represented in this cohort. Standard deviation can be influenced by the individual and mean values as well as by the sample size. The high variability in the individual values of patients included in the nonsarcoidosis-related groups likely increased the SD of the whole cohort.
Conclusion
This study indicates that the serum sIL-2R level is a useful biomarker for diagnosing sarcoidosis in patients with uveitis, showing an overall diagnostic performance slightly better than that of serum ACE levels.
References
- 1.Tsirouki T, Dastiridou A, Symeonidis C, et al. . A focus on the epidemiology of uveitis. Ocul Immunol Inflamm. 2016:1-15. [DOI] [PubMed] [Google Scholar]
- 2.Herbort CP, Rao NA, Mochizuki M; members of Scientific Committee of First International Workshop on Ocular Sarcoidosis . International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop on Ocular Sarcoidosis (IWOS). Ocul Immunol Inflamm. 2009;17(3):160-169. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP, Lower EE, Kaufman AH. Ocular sarcoidosis. Semin Respir Crit Care Med. 2010;31(4):452-462. [DOI] [PubMed] [Google Scholar]
- 4.Ungprasert P, Carmona EM, Crowson CS, Matteson EL. Diagnostic utility of angiotensin-converting enzyme in sarcoidosis: a population-based study. Lung. 2016;194(1):91-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gundlach E, Hoffmann MM, Prasse A, Heinzelmann S, Ness T. Interleukin-2 receptor and angiotensin-converting enzyme as markers for ocular sarcoidosis. PLoS One. 2016;11(1):e0147258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groen F, van Laar JAM, Rothova A. Chest radiographic screening for sarcoidosis in the diagnosis of patients with active uveitis. Ann Am Thorac Soc. 2017;14(6):912-918. [DOI] [PubMed] [Google Scholar]
- 7.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180-190. [DOI] [PubMed] [Google Scholar]
- 8.Ziegenhagen MW, Benner UK, Zissel G, Zabel P, Schlaak M, Müller-Quernheim J. Sarcoidosis: TNF-α release from alveolar macrophages and serum level of sIL-2R are prognostic markers. Am J Respir Crit Care Med. 1997;156(5):1586-1592. [DOI] [PubMed] [Google Scholar]
- 9.Ziegenhagen MW, Rothe ME, Schlaak M, Müller-Quernheim J. Bronchoalveolar and serological parameters reflecting the severity of sarcoidosis. Eur Respir J. 2003;21(3):407-413. [DOI] [PubMed] [Google Scholar]
- 10.Gungor S, Ozseker F, Yalcinsoy M, et al. . Conventional markers in determination of activity of sarcoidosis. Int Immunopharmacol. 2015;25(1):174-179. [DOI] [PubMed] [Google Scholar]
- 11.Grutters JC, Fellrath JM, Mulder L, Janssen R, van den Bosch JM, van Velzen-Blad H. Serum soluble interleukin-2 receptor measurement in patients with sarcoidosis: a clinical evaluation. Chest. 2003;124(1):186-195. [DOI] [PubMed] [Google Scholar]
- 12.Vorselaars AD, van Moorsel CH, Zanen P, et al. . ACE and sIL-2R correlate with lung function improvement in sarcoidosis during methotrexate therapy. Respir Med. 2015;109(2):279-285. [DOI] [PubMed] [Google Scholar]
- 13.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society Diagnostic standards and classification of tuberculosis. Am Rev Respir Dis. 1990;142(3):725-735. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum JT. New developments in uveitis associated with HLA B27. Curr Opin Rheumatol. 2017;29(4):298-303. [DOI] [PubMed] [Google Scholar]
- 16.Okada AA, Stanford M, Tabbara K. Ancillary testing, diagnostic/classification criteria and severity grading in Behçet disease. Ocul Immunol Inflamm. 2012;20(6):387-393. [DOI] [PubMed] [Google Scholar]
- 17.Levinson RD, Brezin A, Rothova A, Accorinti M, Holland GN. Research criteria for the diagnosis of birdshot chorioretinopathy: results of an international consensus conference. Am J Ophthalmol. 2006;141(1):185-187. [DOI] [PubMed] [Google Scholar]
- 18.de Boer JH, Verhagen C, Bruinenberg M, et al. . Serologic and polymerase chain reaction analysis of intraocular fluids in the diagnosis of infectious uveitis. Am J Ophthalmol. 1996;121(6):650-658. [DOI] [PubMed] [Google Scholar]
- 19.Snyder DA, Tessler HH. Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol. 1980;90(1):69-75. [PubMed] [Google Scholar]
- 20.Crawford CM, Igboeli O. A review of the inflammatory chorioretinopathies: the white dot syndromes. ISRN Inflamm. 2013;2013:783190. doi: 10.1155/2013/783190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32-35. [DOI] [PubMed] [Google Scholar]
- 22.Evaluation of diagnostic and screening tests: validity and reliability. The John Hopkins University and Sukon Kanchanaraksa. http://ocw.jhsph.edu/courses/fundepi/PDFs/lecture11.pdf. Published 2008. Accessed July 19, 2017.
- 23.Forooghian F, Gupta R, Wong DT, Derzko-Dzulynsky L. Anterior uveitis investigation by Canadian ophthalmologists: insights from the Canadian National Uveitis Survey. Can J Ophthalmol. 2006;41(5):576-583. [DOI] [PubMed] [Google Scholar]