Abstract
This study combines data from 3 existing functional magnetic resonance imaging data sets of people with schizophrenia spectrum disorders and healthy controls to assess the association of schizophrenia spectrum disorders with connectome stability.
Accumulating neuroimaging evidence has documented group-level abnormalities in brain structure and function in individuals with severe mental disorders.1 However, the diversity of findings on localization, magnitude, and clinical sensitivity; the lack of robust associations with polygenic risk scores; and poor diagnostic classification using machine learning2 suggest that the discriminatory power of neuroimaging data alone is limited and that the individual brain patterns are highly heterogeneous. Although disappointing from a clinical point of view, this lack of direct translation of group-level findings to the individual is in line with the substantial individual patient heterogeneity in clinical and cognitive profiles.
The brain functional connectome is highly individualized3,4,5 and has been metaphorically called a fingerprint.3 This suggests that important individual-level information may be overlooked in common imaging approaches, which has limited the specificity and clinical utility of functional magnetic resonance imaging (fMRI).6 The stability of the connectome captures system-level, within-participant properties of the connectome and has recently shown promise as a marker of mental health in adolescents.4 Thus, we hypothesized that stability is also lower in adults with schizophrenia spectrum disorders and in individuals with high cumulative polygenic risk for schizophrenia.
Methods
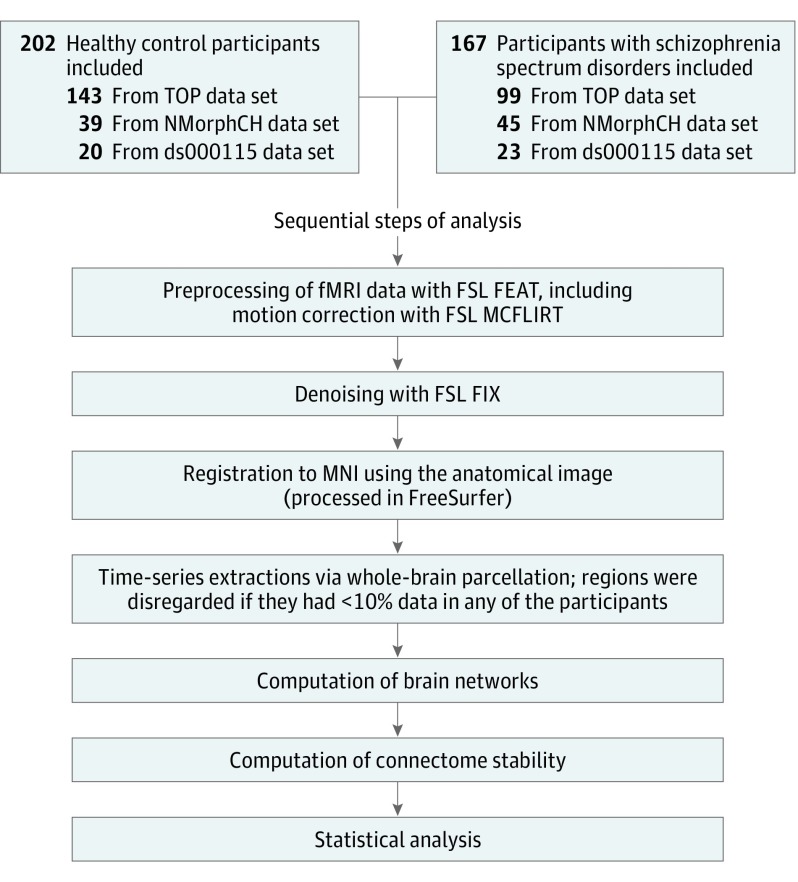
We reanalyzed task fMRI data sets (OpenfMRI ds000115, Neuromorphometry by Computer Algorithm Chicago, and Thematically Organized Psychosis) from 3 previous studies on working memory in individuals with schizophrenia spectrum disorders. Data sets included 167 individuals with these disorders (aged 16-52 years) and 202 healthy controls (aged 13-59 years) who had been scanned during a 0-back and a 2-back version of similar blocked n-back tasks. Figure 1 describes the analysis. As in procedures described elsewhere,4 we computed individual brain networks of the full brain and 9 subnetworks and calculated connectome stability as the within-participant Spearman correlation coefficient of 0-back and 2-back networks. We assessed effect sizes of diagnosis (Cohen d) and tested for associations with task performance (n = 365) and polygenic risk for schizophrenia (n = 165). Effect sizes were regarded significant at Bonferroni level (P = .005, adjusted for the number of networks).
Figure 1. Overview of Analysis.
FEAT indicates functional magnetic resonance imaging (fMRI) expert analysis tool; FIX, fMRI of the brain (FMRIB) independent component analysis–based Xnoiseifier; FSL, FMRIB software library; MCFLIRT, Motion Correction of FMRIB’s Linear Image Registration Tool; MNI, Montreal Neurological Institute; NMorphCH, Neuromorphometry by Computer Algorithm Chicago; TOP, Thematically Organized Psychosis study. ds000115 is a data set accession number.
Results
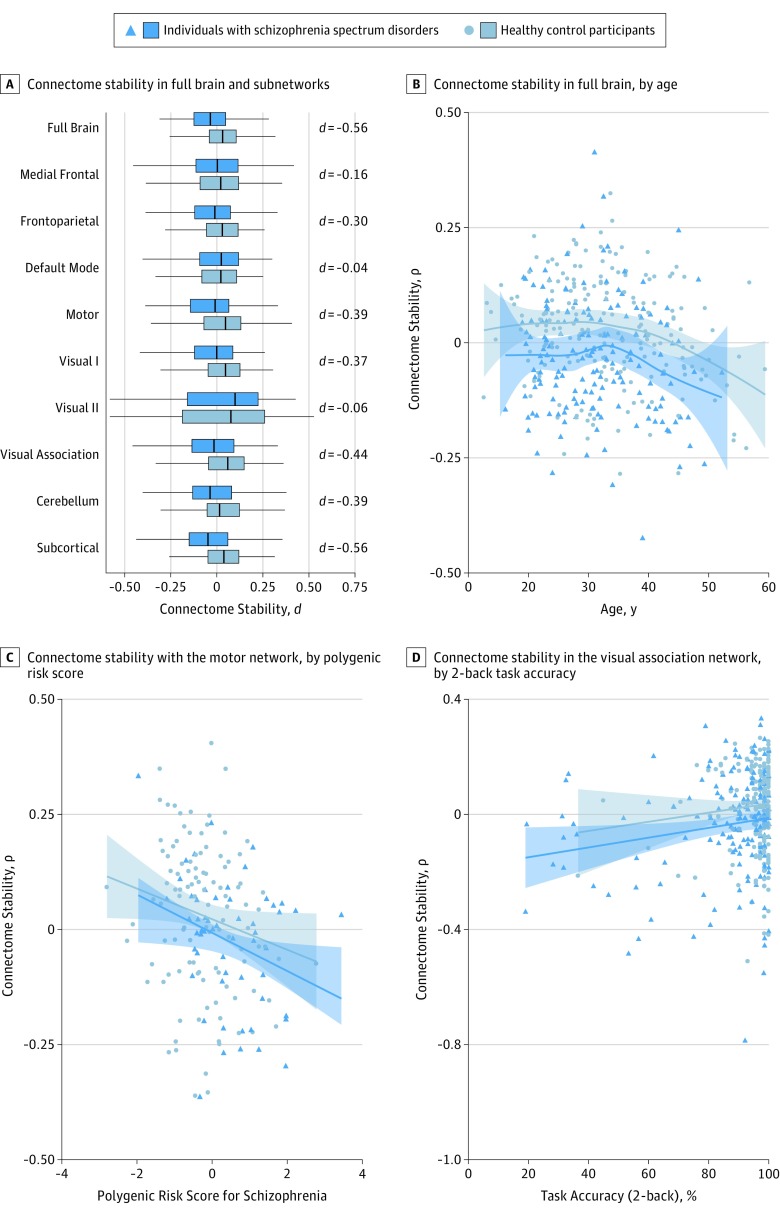
Compared with controls, individuals with schizophrenia spectrum disorders showed significantly lower stability of the connectome fingerprint for the full brain (Cohen d, −0.56; P = 2 × 10−7) and frontoparietal (d, −0.30; P = 5 × 10−3), motor (d, −0.39; P = 2 × 10−4), visual 1 (d, −0.37; P = 6 × 10−4), visual association (d, −0.44; P = 4 × 10−5), cerebellum (d, −0.39; P = 3 × 10−4), and subcortical (d, −0.56; P = 3 × 10−7) networks after accounting for age, age squared, sex, motion, temporal signal-to-noise ratio, and scanning site (Figure 2A). Figure 2B illustrates estimated age trajectories for full-brain connectome stability, matching previous reports of increasing stability as the brain develops and suggesting decreasing stability with advancing adult age in both groups. This was supported by a significant effect of age (d,−0.39; P = 3 × 10−4) and a nominally significant effect of age-orthogonalized age squared (d, −0.27; P = .01).
Figure 2. Connectome Stability in Schizophrenia Spectrum Disorders.
A, C, and D, Connectome stability data are residualized for age, age squared, sex, motion, temporal signal-to-noise ratio, and scanning site; B, Connectome stability data are residualized for sex, motion, temporal signal-to-noise ratio, and scanning site. A, Statistically significant differences were found in the full brain and several subnetworks. B, Age trajectories in data on 369 participants suggest a decrease in connectome stability with adult age. C, Association of connectome stability in the motor network of 165 participants with polygenic risk for schizophrenia. D, Association of connectome stability in the visual association network of 365 participants with 2-back task performance.
After accounting for age, age squared, sex, motion, temporal signal-to-noise ratio, scanning site, and diagnosis, we found that stability of the motor network was significantly associated with polygenic risk for schizophrenia (d,−0.46; P = .004; Figure 2C), and stability of the visual association network was significantly associated with task performance (d, 0.36; P = 8 × 10−4; Figure 2D). Associations with polygenic risk or task performance in other subnetworks did not remain significant after correction for multiple comparisons.
Discussion
Assessing brain network stability provides a window into within-participant variance, offering inference on the individual connectome. In conjunction with recent evidence from a neurodevelopmental study,4 our findings suggest that stability of the connectome increases during development, peaks in early adulthood, and decreases during adulthood. The reported delay in reaching adult-level stability in individuals with preclinical signs of mental illness4 match the trajectories observed for young individuals with schizophrenia spectrum disorders in this study. Further, our findings indicate decreased stability in adults with schizophrenia spectrum disorders and the trajectories jointly provide support for the hypothesis of developmental delay and accentuated aging as significant factors of decreased mental health. Taken together, our results encourage the use of system-level, within-participant properties of the connectome in mental health research.
References
- 1.Sprooten E, Rasgon A, Goodman M, et al. Addressing reverse inference in psychiatric neuroimaging: meta-analyses of task-related brain activation in common mental disorders. Hum Brain Mapp. 2017;38(4):1846-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfers T, Buitelaar JK, Beckmann CF, Franke B, Marquand AF. From estimating activation locality to predicting disorder: a review of pattern recognition for neuroimaging-based psychiatric diagnostics. Neurosci Biobehav Rev. 2015;57:328-349. [DOI] [PubMed] [Google Scholar]
- 3.Finn ES, Shen X, Scheinost D, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18(11):1664-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufmann T, Alnæs D, Doan NT, Brandt CL, Andreassen OA, Westlye LT. Delayed stabilization and individualization in connectome development are related to psychiatric disorders. Nat Neurosci. 2017;20(4):513-515. [DOI] [PubMed] [Google Scholar]
- 5.Mueller S, Wang D, Fox MD, et al. Individual variability in functional connectivity architecture of the human brain. Neuron. 2013;77(3):586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon EM, Laumann TO, Gilmore AW, et al. Precision functional mapping of individual human brains. Neuron. 2017;95(4):791-807.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]