Key Points
Question
To what extent is the familial risk of heart failure caused by shared genetic factors?
Findings
In this nationwide study, adoptees showed an increased risk of heart failure (odds ratio, 1.45 [95% CI, 1.04-2.03]) if they had a biological parent with the condition; an affected adoptive parent conferred no such risk. Heritability (h2) of heart failure per Falconer regression was 26%; if cardiomyopathies were excluded, heritability was 34%.
Meaning
A history of heart failure in a biological parent is associated with heart failure in adopted children, which suggests that a genetic susceptibility should be considered in clinical practice and should motivate further genetic studies.
This nationwide study of Swedish adoptees and their adoptive and biological parents aimed to determine the heritability of heart failure.
Abstract
Importance
Heart failure (HF) aggregates in families, but the heritability of HF has not been determined. Discerning the genetic and environmental contributions to HF risk is important to further helping to identify individuals at risk. Adoption studies may establish the genetic contribution to HF.
Objective
This nationwide adoption study aimed to determine the heritability of HF.
Design, Setting, and Participants
This case-control study and cohort study design used logistic regression for calculating risks of HF in adoptees. Adoptees who were born in Sweden between 1942 and 1990 were linked to their adoptive parents and biological parents. The Swedish Multi-Generation Register was linked to the Swedish Patient Register for information on hospital inpatient and outpatient admissions and to the Swedish Cause of Death Register for the period 1964 through 2015. Heritability (h2 with a standard error) for HF was determined both with Falconer regression and with tetrachoric correlation. Data analysis was completed from July 2017 to April 2018.
Exposures
Heart failure in biological parents and/or adoptive parents.
Main Outcomes and Measures
Heritability; risk of HF, expressed as odds ratios.
Results
A total of 21 643 adoptees were included (of whom 10 626 [49.1%] were female), as well as 35 016 adoptive parents (14 872 [42.5%] female) and 43 286 biological parents (21 643 [50.0%] female). There were 194 cases of HF in adoptees, 3972 cases of HF in adoptive parents, and 3657 cases of HF in biological parents. The cohort study odds ratio (OR) for heart failure was 1.45 in adoptees (95% CI, 1.04-2.03) for biological parents with HF, compared with those without an affected biological parent. If cardiomyopathies were excluded, this OR was 1.58 (95% CI, 1.03-2.42). The corresponding OR associated with an affected adoptive parent were nonsignificant, both with cardiomyopathies included (OR, 0.83 [95% CI, 0.57-1.20]) and with cardiomyopathies excluded (OR, 0.79 [95% CI, 0.49-1.29]). The heritability of HF per Falconer regression (h2) was 26% (SE, 14%). With exclusion of cardiomyopathies the heritability using Falconer regression was 34% (SE, 18%).
Conclusions and Relevance
Heart failure in a biological parent is an HF risk factor that is worth clinical consideration. The increased heritability of HF suggests that genetic factors are important in HF pathogenesis.
Introduction
Heart failure (HF) is a major global health burden with an associated severe prognosis.1,2,3,4 Heart failure is acknowledged to be the result of risk factors such as hypertension, coronary heart disease (CHD), diabetes, obesity, and valvular heart disease.1,2,3 The condition also aggregates in families.5,6 The Framingham Offspring Study cohort reported an offspring HF hazard ratio of 1.70 in individuals who had 1 parent affected and 1.92 in individuals with both parents affected with HF.5 Recently, a Swedish nationwide sibling study has confirmed the importance of familial aggregation of HF.6 In the Swedish study, the familial relative risk of HF was 1.62 for individuals with 1 affected sibling and 15.46 for individuals with 2 affected siblings.6 The familial relative risk associated with a sibling history of HF was age dependent.6 In these prior studies, the inheritance of HF is consistent with nonmendelian inheritance5,6 (ie, a complex trait), in contrast to the often rarer mendelian familial cardiomyopathies.7,8
This familial aggregation of HF may be because of both genetic and shared familial environmental factors. Genome-wide studies of HF have so far been largely limited in sample size and underpowered for detection of common susceptibility variants,9,10 though a few candidate genes10,11 and epigenetic factors10 have been implicated in the pathogenesis of HF. Twin studies are a classic method to disentangle genetic factors from shared environmental factors and to determine heritability for a disease.12 An alternative approach is adoption studies.12 To the best of our knowledge, heritability has not been determined for HF. The aim of the present nationwide Swedish adoption study was to determine the familial risk and heritability of HF for Swedish-born adopted offspring.
Methods
Study Design
To determine HF heritability among adopted individuals in Sweden, Swedish nationwide registers and health care data were linked.13,14,15,16,17,18 The study was approved by the Ethics Committee of Lund University, Sweden, and was performed in compliance with the Helsinki Declaration. The ethics committee waived informed consent as a requirement because this was a retrospective analysis of anonymized data from Statistics Sweden and the National Board of Health and Welfare.
Data were included from the Swedish Multi-Generation Register, which contains information on family relationships including adoptions and provides information on index persons registered in Sweden from January 1, 1961, who were born on January 1, 1932, or later. This was combined with the LISA Register from Statistics Sweden,19 which contains annual data on education status. It also holds information on the Swedish Standard Classification of Occupations 1996, which is a national version of the International Standard Classification of Occupations.
In addition, data were linked to the Swedish National Patient Register, which includes data on inpatients and hospital-based outpatients. The Inpatient Register (which is also called the Hospital Discharge Register) contains all hospital diagnoses for residents in Sweden from 1964 through 2015, with a nationwide coverage since 1987. The Outpatient Register contains information on diagnoses on all hospital-based outpatients in Sweden from 2001 through 2015.
These data were combined with data from the Swedish Cause of Death Register, which holds data on dates and causes of death from 1964 through 2015, as well as the Migration Register, which contains data on immigration and emigration registered from 1892 onwards. Census registers that included address information for all individuals in Sweden were also linked. (The census is updated and made available every 5 years for the period 1960 through 1990.)
Finally, Small Area Market Statistics (SAMS) data were included. From 1991, SAMS data have been available to define municipal subareas to assist researchers in characterizing neighborhoods. A neighborhood deprivation index was created per Winkleby et al20 and was based on educational status, income, unemployment, and social welfare recipiency. High socioeconomic neighborhood status was defined as a z score less than 1 SD below the mean (low deprivation).20
Study Sample
The resulting data set contained data on all Swedish-born adoptive children born between 1942 and 1990 and their biological or adoptive parents (eFigure in the Supplement). Adoptees and their biological and adoptive parents were excluded from the study if adoptees met any of the following criteria: they (1) died before the age of 16 years or before 1964, (2) emigrated from Sweden before the age of 16 years, (3) could not be linked to both biological parents and to at least 1 adoptive parent, or (4) registered with HF before the age of 10 years(n = 1). Outliers, defined as individuals who had been registered as living longer than 115 years (n = 18 biological parents and 30 adoptive parents), were also excluded.
To maintain validity, adoptees were excluded if the adoptee or any linked biological or adoptive parent had a registered diagnosis of HF in the Swedish Cause of Death Register that was not found in the Inpatient Register or in the Outpatient Register. Additionally, adoptees who had lived with their adoptive or biological grandparent, aunt or uncle, sibling, stepparents, or biological parent were excluded as well, according to data retrieved from the census (1960-1990) or SAMS (from 1991) data sets. For adoptees born between 1942 and 1959, the status in the 1960 census was used.
After exclusions, a total of 21 643 adoptees constituted the study population in the cohort study. These adoptees could be linked to 35 016 adoptive parents and 43 286 biological parents. From these numbers, 1730 adoptees (8.0%), 17 740 adoptive parents (50.7%), and 20 885 biological parents (48.2%) had died or emigrated without having a diagnosis of HF at the end of the follow-up period. Another 292 adoptees (1.32%) had not been registered with any form of education and were therefore regarded as having low educational status.
Definition of Heart Failure
Inpatients and outpatients diagnosed with HF were identified in the Swedish National Patient Register by the following International Statistical Classification of Diseases and Related Health Problems (ICD) codes: from the Seventh Revision (ICD-7), codes 434.10 and 434.20; from the Eighth Revision (ICD-8), codes 427.00 and 428; from the Ninth Revision (ICD-9), code 428; and from the Tenth Revision (ICD-10) code I50. Primary care records were not included. Only registrations with a main diagnosis of HF were included as a method to increase diagnostic validity, which has been shown to have a positive predictive value of approximately 95% when the main diagnosis in the Inpatient Register is used,21 which was the approach used in this study. Patients with HF who had a main or secondary diagnosis in the National Patient Register of cardiomyopathy were registered with ICD-8 code 425, ICD-9 code 425, and ICD-10 codes I42 and I43.
The Swedish Cause of Death Register is considered to be valid for epidemiological studies for many diagnoses, such as CHD and cerebrovascular diseases, but might be lower for other heart diseases, such as HF.22,23 For that reason, HF registered in the Swedish Cause of Death Register but with no entry in the National Patient register was considered a criterion for exclusion, as specified.
Comorbidities
The following main and secondary comorbidities from the National Patient register were used: coronary heart disease (CHD), hypertension, valvular heart disease, diabetes mellitus, and chronic obstructive pulmonary disease. Comorbidities were defined by ICD codes (eTable 1 in the Supplement). The validity in the Inpatient Register of the National Patient Register is generally regarded to be between 85% and 95% for all diagnoses.16
Statistical Analysis
Data were collected on adoptees and their biological and adoptive parents from 1964 to 2015. Two different study designs were used: a case-control study and a cohort study. For each study design, 2 main analyses were performed: the association between HF in adoptees and HF in their adoptive parents, and the association between HF in adoptees and HF in their biological parents.
For the case-control study, a case-control matching method (with a 1:5 ratio) was used by drawing a sample of affected adoptees as HF cases and a matched number of unaffected adoptees as a control group.24 The control group was matched to the group of affected individuals based on sex, birth year (±5 years), county of birth, and level of education. Affected individuals, as well as control individuals, were linked both to their biologic and adoptive parents.25 For the case-control study, odds ratios (ORs) were calculated using conditional logistic regression.
For the cohort study, no such matching process was applied, and we therefore used unconditional logistic regression to calculate ORs. In the multivariable model of the cohort study, the adoptee characteristics of birth year, sex, education of adoptees, and county of birth, as well as comorbidities, were incorporated in the model as covariates. In the cohort study, Cox regression was also performed.
In accordance with the definition of narrow-sense heritability (h2), we assumed the genetic proportion of the population phenotypic variance was exclusively attributable to additive allelic effects.26 Together with an assumption that there were no genetic effects on propagation and no common environmental exposure among relatives, this allowed for estimating the heritability as twice the calculated correlation of first-degree relatives.27 We used 2 methods for estimating this correlation, both of which were based on the case-control study design and on the liability threshold model of dichotomous diseases, which assumes a threshold of liability, above which all affected individuals are exclusively contained.28 We used the general method of tetrachoric correlation of disease in which the familial disease correlation is calculated by associating the observed frequencies of disease in relatives with the prevalence of disease in the general population.26,27 We also used Falconer regression method, which is described in great detail by Falconer.28
Statistical analysis performed with SAS version 9.3 (SAS Institute Inc) and for calculating heritability we used R software version 3.3.2 (R Foundation for Statistical Computing). A significance level of .05 was considered statistically significant. Data analysis was completed from July 2017 to April 2018.
Results
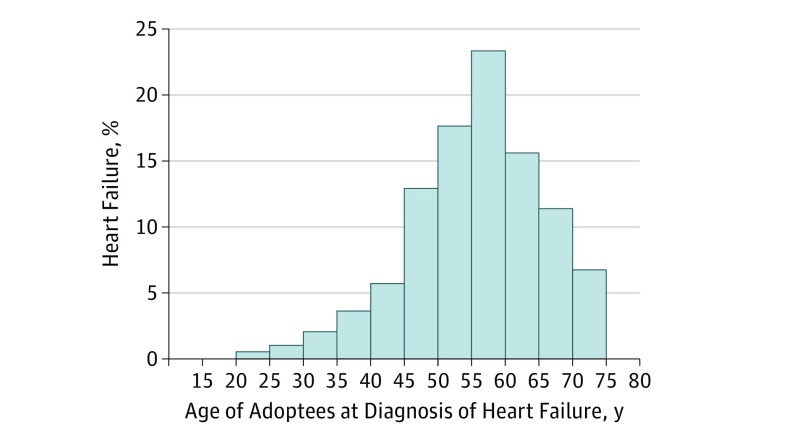
After exclusions, we identified 7823 individuals with HF (7.83%) in the study population during the study period, which ranged from January 1, 1961 (or thereafter, in case of a later birth date) to the date of death, emigration, or the end of study period (on December 31, 2015), whichever came first. Among adoptees, 194 cases of HF were found; 3972 cases of HF were found in adoptive parents, and 3657 cases of HF were found in biological parents. The Figure shows the age distribution for Swedish-born adoptees at first-time diagnosis of HF (median [range], 55 [20-72] years).
Figure. Age Distribution for Swedish-Born Adoptees at First-Time Diagnosis of Heart Failure.
Of 194 individuals with HF born between 1942 and 1990, the median age at onset was 55 (range, 20-72) years; the curve had a mean (SD) value of 53 (10) years.
Table 1 shows descriptive statistics for adopted offspring, their biological parents, and their adoptive parents. The prevalence of HF among biological parents was 8.5% (n = 3657 of 43 286), while among the adoptive parents it was 11.3% (n = 3972 of 35 016), which constituted a statistically significant difference between these groups (P < .001).
Table 1. Descriptive Statistics of Adoptees (Born 1942-1990) and Their Adoptive and Biological Parents.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Adoptees (n = 21 643) |
Adoptive Parents (n = 35 016) |
Biological Parents (n = 43 286) |
|
| Female | 10 626 (49.1) | 14 872 (42.5) | 21 643 (50.0) |
| High education level (≥12 y) | 6206 (28.7) | 5587 (16.0) | 380 (9.2) |
| High socioeconomic statusa | 5375 (24.8) | 6238 (17.8) | 5814 (13.4) |
| Occupational positionb | 3412 (15.8) | 1346 (3.8) | 1051 (2.4) |
| Age at end of study period, median (IQR), y | 52 (45-58) | 77 (68-84) | 70 (62-77) |
| Birth year, median (IQR) | 1963 (1955-1968) | 1928 (1919-1940) | 1938 (1929-1945) |
| Birth year, range | 1942-1990 | 1886-1972 | 1877-1974 |
| Birth, mean (SD), y | 1963 (10) | 1929 (14) | 1937 (12) |
| Patients with heart failure | |||
| Heart failure | 194 (0.9) | 3972c (11.3) | 3657d (8.5) |
| Female | 59 (0.3) | 1 700 (4.9) | 1482 (3.4) |
| Age at diagnosis, median (IQR), y | 55 (47-61) | 79 (73-85) | 72 (65-79) |
| Cardiomyopathy patients | |||
| Cardiomyopathy | 118 (0.6) | 386 (1.1) | 526 (1.2) |
| Female | 33 (0.2) | 119 (0.4) | 176 (0.4) |
| Age at diagnosis, median (IQR), y | 50 (43-57) | 69 (61-77) | 63 (55-70) |
Abbreviation: IQR, interquartile range.
Per neighborhood deprivation index.
Manager position or occupation with a requirement for in-depth university competence according to the Swedish Standard Classification of Occupations 2012 by Statistics Sweden (https://www.scb.se).
This includes 216 families with both adoptive parents affected.
This includes 206 families with both biological parents affected.
In Table 2, nonaffected adoptees are descriptively compared with affected adoptees. Affected adoptees were significantly more likely to be male (those with no heart failure: females, 10 564 of 21 449 [49.3%]; those with heart failure: 62 of 194 [40.0%]; P < .001), had lower mean educational attainment (those with no heart failure: 6177 of 21 449 [28.8%]; those with heart failure: 29 of 194 [14.9%]; P < .001), had higher mean Neighborhood Deprivation Index scores (those with no heart failure: 5343 of 21 449 [24.9%]; those with heart failure: 32 of 194 [16.5%]; P < .007), were older at the end of study period (those with no heart failure: median [interquartile range], 52 [45-58] years; those with heart failure: 60 [52-65] years; P < .001), and were less likely to be managers or employed in a position with a requirement for in-depth university competence (those with no heart failure: 3403 of 21 449 [15.9%]; those with heart failure: 9 of 194 [4.6%]; P < .001). Adoptees with HF also more often had HF comorbidities, including CHD (those with no heart failure: 758 of 21 449 [3.5%]; those with heart failure: 68 of 194 [35.1%]; P < .001), hypertension (those with no heart failure: 2311 of 21 449 [10.8%]; those with heart failure: 100 of 194 [51.5%]; P < .001), valvular heart disease (those with no heart failure: 194 of 21 449 [0.9%]; those with heart failure: 39 of 194 [20.1%]; P < .001), diabetes mellitus (those with no heart failure: 1103 of 21 449 [5.1%]; those with heart failure: 63 of 194 [32.5%]; P < .001), chronic obstructive pulmonary disease (those with no heart failure: 1480 of 21 449 [6.9%]; those with heart failure: 50 of 194 [25.8%]; P < .001), and cardiomyopathy (those with no heart failure: 48 of 21 449 [0.2%]; those with heart failure: 70 of 194 [36.1%]; P < .001). eTable 2 in the Supplement shows the same pattern, with overrepresentation of comorbidities in both biological and adoptive parents with HF compared with parents without HF.
Table 2. Descriptive Statistics of Adoptees With and Without Heart Failure.
| Characteristic | No. (%) | ||
|---|---|---|---|
| No Heart Failure (n = 21 449) |
Heart Failure (n = 194) |
P Value | |
| Female | 10 564 (49.3) | 62 (32.0) | <.001a |
| High education level (≥12 y) | 6177 (28.8) | 29 (14.9) | <.001a |
| High socioeconomic statusb | 5343 (24.9) | 32 (16.5) | .007a |
| Occupational positionc | 3403 (15.9) | 9 (4.6) | <.001a |
| Age at end of study period, median (IQR), y | 52 (45-58) | 60 (52-65) | <.001d |
| Coronary heart disease | 758 (3.5) | 68 (35.1) | <.001a |
| Hypertension | 2311 (10.8) | 100 (51.5) | <.001a |
| Valvular heart disease | 194 (0.9) | 39 (20.1) | <.001a |
| Diabetes mellitus | 1103 (5.1) | 63 (32.5) | <.001a |
| Chronic obstructive pulmonary disease | 1480 (6.9) | 50 (25.8) | <.001a |
| Cardiomyopathy | 48 (0.2) | 70 (36.1) | <.001a |
Abbreviation: IQR, interquartile range.
χ2 Test.
Per neighborhood deprivation index.
Manager position or occupation with a requirement for in-depth university competence according to the Swedish Standard Classification of Occupations 2012 by Statistics Sweden (https://www.scb.se).
Wilcoxon test.
Cohort Study
The estimated risks of HF among adoptees in the cohort design, expressed as ORs with 95% CIs, are presented in Table 3. Calculations were performed both with and without the inclusion of patients with cardiomyopathy. For both these cohorts, with and without cardiomyopathies, the ORs for HF in adoptees with at least 1 affected biological parent were significantly increased in all models. In the full model (model 3), which adjusted for birth year, sex, county, and education, as well as for comorbidities, these biological familial ORs were 1.45 (95% CI, 1.04-2.03) with cardiomyopathies included and 1.58 (95% CI, 1.03-2.42) with cardiomyopathies excluded. Moreover, there was a tendency for a higher OR if both biological parents were affected (eTable 3 in the Supplement); however, this was not significant in the fully adjusted model (OR, 1.97 [95%, CI 0.80-4.87]).
Table 3. Odds Ratioa of Heart Failure in Adoptees, Stratified by Affected Parent (Cohort Study).
| Outcome | No Biological Parental Heart Failure | Biological Parental Heart Failure | No Adoptive Parental Heart Failure | Adoptive Parental Heart Failure |
|---|---|---|---|---|
| Cardiomyopathies included | ||||
| Person-years at risk | 844 228 | 167 010 | 828 375 | 182 863 |
| Cases per persons at risk | 135/18 192 | 59/3451 | 155/17 887 | 39/3756 |
| Incidence rate per 1000 person-years | 0.16 (0.13-0.19) | 0.35 (0.27-0.46) | 0.19 (0.16-0.22) | 0.21 (0.15-0.29) |
| Incidence ratio | 1 [Reference] | 2.21 (1.62-2.99) | 1 [Reference] | 1.14 (0.79-1.61) |
| Odds ratio (95% CI) | ||||
| Model 1c | 1 [Reference] | 2.33 (1.71-3.17) | 1 [Reference] | 1.20 (0.84-1.71) |
| Model 2c | 1 [Reference] | 1.51 (1.10-2.08) | 1 [Reference] | 1.19 (0.83-1.70) |
| Model 3c | 1 [Reference] | 1.45 (1.04-2.03) | 1 [Reference] | 0.83 (0.57-1.20) |
| Cardiomyopathies excludedd | ||||
| Person-years at risk | 825 608 | 150 032 | 807 625 | 168 015 |
| Cases per persons at risk | 79/17 796 | 37/3092 | 93/17 444 | 23/3444 |
| Incidence rate per 1000 person-years | 0.10 (0.08-0.12) | 0.25 (0.17-0.34) | 0.12 (0.09-0.14) | 0.14 (0.09-0.21) |
| Incidence ratio | 1 [Reference] | 2.58 (1.73-3.79) | 1 [Reference] | 1.19 (0.74-1.85) |
| Odds ratio (95% CI) | ||||
| Model 1c | 1 [Reference] | 2.72 (1.84-4.02) | 1 [Reference] | 1.26 (0.79-1.98) |
| Model 2c | 1 [Reference] | 1.62 (1.09-2.43) | 1 [Reference] | 0.82 (0.52-1.31) |
| Model 3c | 1 [Reference] | 1.58 (1.03-2.42) | 1 [Reference] | 0.79 (0.49-1.29) |
Risks are presented as odds ratios and are derived from unconditional logistic regression.
Reference group.
Model 1 was univariate model; model 2 was adjusted for adoptee birth year, sex, county, and education; model 3 was adjusted for adoptee birth year, sex, county, education, coronary heart disease, hypertension, valvular heart disease, diabetes mellitus, and chronic obstructive pulmonary disease.
All adoptees who were diagnosed with cardiomyopathy or had a biological or adoptive parent diagnosed with cardiomyopathy were excluded.
The estimated ORs for HF in adoptees with an affected adoptive parent were not statistically significant in any model in the cohort study. Cox regression, which estimated the risk of HF by also taking into account the follow-up time, showed similar hazard ratios (eTable 4 in the Supplement) associated with adoptive parental HF and biological parental HF.
Case-Control Study
The results of the case-control study are shown in Table 4. Heart failure in the adoptees was significantly associated with HF in biological parents, with an OR of 1.49 (95% CI, 1.05-2.12) with cardiomyopathies included and an OR of 1.64 (95% CI, 1.05-2.56) with cardiomyopathies excluded. For both cases, after age stratification, the familial OR was only increased in the group of adoptees who were aged 46 to 59 years, the subgroup in which most cases were found. Heart failure in an adoptive parent was not significantly associated with HF in adoptees in any model in the case-control study.
Table 4. Odds Ratioa of Heart Failure (HF) in Adoptees, Stratified by Affected Parent (Case-Control Study)b.
| Parental Disease Status | Cases, No. | Controls, No. | Odds Ratio (95% CI) | |||
|---|---|---|---|---|---|---|
| All Agesc | Age ≤45 yd | Age 46-59 ye | Age ≥60 yf | |||
| Cardiomyopathies included | ||||||
| Biological parental HF | 58 | 220 | 1.49 (1.05-2.12) | 1.39 (0.49-3.49) | 1.81 (1.11-2.94) | 1.16 (0.63-2.15) |
| No biological parental HF | 133 | 735 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Adoptive parental HF | 37 | 235 | 0.74 (0.50-1.09) | 0.37 (0.10-1.39) | 0.64 (0.37-1.10) | 1.11 (0.60-2.07) |
| No adoptive parental HF | 154 | 720 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Cardiomyopathies excludedg | ||||||
| Biological parental HF | 37 | 130 | 1.64 (1.05-2.56) | 1.08 (0.28-4.19) | 2.09 (1.13-3.87) | 1.34 (0.64-2.81) |
| No biological parental HF | 77 | 440 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Adoptive parental HF | 22 | 138 | 0.75 (0.45-1.24) | 0.28 (0.03-2.37) | 0.81 (0.40-1.65) | 0.82 (0.38-1.77) |
| No adoptive parental HF | 92 | 432 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
Abbreviation: HF, heart failure.
Odds ratios derived from unconditional logistic regression.
Cases and controls were matched by sex, birth year (±5 years), county of birth, and level of education and were stratified by adoptee age at diagnosis.
Numbers of adoptee cases and controls per category of affected parents as specified in left columns.
Thirty-seven cases and 185 controls; 17 cases and 102 controls with cardiomyopathies excluded.
Ninety-nine cases and 495 controls; 58 cases and 348 controls with cardiomyopathies excluded.
Fifty-five cases and 275 controls; 39 cases and 234 controls with cardiomyopathies excluded.
All adoptees diagnosed with cardiomyopathy or who had a biological or adoptee parent diagnosed with cardiomyopathy were excluded.
Heritability
Heritability was determined in the case-control study with different estimates of the prevalence of HF. The prevalence in the particular source population is unknown, but a range of likely estimates were selected based on previous studies.1,4,29 The corresponding range of heritability estimates is presented in eTables 5 and 6 in the Supplement.
Using the method of tetrachoric correlation with cardiomyopathies included, heritability was found to vary from 14% (SE, 6%) in a population with 0.1% prevalence to a heritability of 25% (SE, 10%) in a population with 12% prevalence (eTable 5 in the Supplement). With assuming a prevalence of 7.83%, as in the present population (Table 1), the heritability was estimated to be 24% (SE, 10%) with inclusion of cardiomyopathies. The heritability determined by using Falconer regression was 26% (SE, 14%) with cardiomyopathies included.
With exclusion of cardiomyopathies, heritability was found to be higher (eTable 6 in the Supplement); it varied from 18% (SE, 8%) in a population with 0.1% prevalence to 32% (SE, 14%) in a population with 12% prevalence. With an assumed prevalence of 7.29% without cardiomyopathies, as observed in the present study population, the heritability determined by tetrachoric correlation was calculated to 29% (SE, 12%). For the study population with cardiomyopathies excluded, the heritability determined by using Falconer regression was 34% (SE, 18%).
Sensitivity Analyses
To determine the robustness of results, we performed a sensitivity analysis including only adoptees that could be linked to both adoptive parents. These results, both with and without exclusion of cardiomyopathies, for the cohort study (eTable 7 in the Supplement) and for the case-control study (eTable 8 in the Supplement) were similar to the main analyses (Table 3 and 4).
Coronary Heart Disease and Heart Failure
Heritability for CHD in this study population with cardiomyopathies included was determined to 31% (SE, 6%) using Falconer regression and 29% (SE, 6%) using tetrachoric correlation with a prevalence of CHD of 20.0%. We performed a stratified analysis of heart failure according to the presence or absence of CHD in adoptees and biological parents (eTable 9 in the Supplement). Only 1 significant association was found; this was between HF in biological parents without CHD and HF in adoptees without CHD (fully adjusted OR, 1.93 [95% CI, 1.12-3.34]). Heart failure with CHD in biological parents was not associated with HF in adoptees.
Sex-Specific Risks
More males than females were affected by HF (patients with HF: female: 62 of 194 [32.0%]; male: 132 of 194 [68.0%]; Table 1; Table 2). Sex-specific biological familial transmission was determined (eTable 10 in the Supplement). Only maternal HF was significantly associated with HF in adoptees in the adjusted models (model 2: OR, 1.81 [95% CI, 1.19–2.74]; model 3: OR, 1.77 [95% CI, 1.14-2.77]. Paternal biological HF was not significantly associated with HF in adoptees (model 2: OR, 1.20 [95% CI, 0.77-1.87]; model 3: OR, 1.14 [95% CI, 0.71-1.81]).
Discussion
To our knowledge, this is the first adoption study of HF and also the first study to estimate heritability for HF. We found a moderate heritability, which suggests that it could be meaningful to search for genetic variants that cause HF. The present study results concur with those from 2 previously published studies, which found increased familial risks of HF in offspring5 and siblings6 of affected probands. Importantly, the present study also extends the previous 2 studies on familial risks of HF through use of an adoption design, which thereby facilitates the discernment of environmental and genetic contributions to HF and the estimation of heritability.
In this study, heritability was found to be lower with the inclusion of cardiomyopathies, which might be because especially dilated cardiomyopathy could be brought on by nongenetic causes, such as myocarditis and alcohol cardiomyopathy, but also to recessive or X-linked inheritance (although dominant inheritance is known to be most common in familial cases).30 Our results indicate that familial history of HF in a biological parent is an important risk factor for HF in adoptees. The magnitude of this association suggests that a family history in a biological parent should be considered clinically as a risk factor for HF. The moderately increased heritability of HF indicates that genetic factors are important for HF and that genome-wide surveys of susceptibility variants for HF may yield important insights. Although the adoptees with HF in this study represent early-onset HF (with a median age at diagnosis of 55 years), their biological parents represent late-onset HF (with a median age at diagnosis of 72 years).
A common feature of complex traits is that familial risks are higher at younger ages.7 The finding of higher familial risks with an affected biological mother compared with an affected biological father is consistent with the Carter effect.31 The Carter effect predicts higher familial risks in the sex with the lowest incidence and is typical for a complex trait. The present results suggest that familial and genetic factors may also be present in late-onset HF (ie, in biological parents), although probably being stronger in early-onset HF (ie, in adoptees). A number of mechanisms for this association of are possible. Although familial risks remained significant after adjustments were made for the covariance of HF comorbidities, heritability for the severity of any these comorbidities as well for an inherent liability for subsequent development of HF (eg, via maladaptive neurohormonal responses and remodeling) are plausible explanatory mechanisms.32 To our knowledge, no previous study has determined the heritability for HF. It could be of interest in the future to confirm these findings in twin studies and studies in other countries.
Among the strengths of this study are that the registers used in our study are almost complete and have successfully been used to estimate familial risks for a number of diseases.13,14,15,16,17 Furthermore, with an all-embracing health care system, the risks of selection bias because of factors such as socioeconomic inequality and sex are reduced.
Limitations
Limitations include the use of register-based data. We had no data on specific blood pressures, body mass index, smoking, lipids, glucose levels, and similar clinical data. Information on medication has only been available for a short time of the study period and was therefore not included. Individual case-specific information of the basis for the diagnosis of HF was also unavailable, including that of current ejection fraction. The study design does not enable complete distinction of HF type (eg, nonischemic cardiomyopathy, ischemic cardiomyopathy, left ventricular phenotype, preserved ejection fraction, and secondary cardiomyopathies). However, we performed subanalyses of HF according to the presence of CHD and cardiomyopathy. In addition, the Swedish hospital register carries high diagnostic validity (85%-95%), especially regarding many cardiovascular diseases (which range from 90% to 95%).13,16 Moreover, HF registered as a main diagnosis is of high validity (around 95%),21 and, with the National Patient Register having a nationwide coverage since 1987, we had complete ascertainment of all hospitalized cases since that time. To account for the possible influence of differential follow-up time among adoptees, we performed a Cox regression analysis, which also showed a significant association between biological parent history of HF and HF risk in adoptees.
Another limitation is that we had no information about the age at which children were adopted, although it is likely that most adoptions occurred in early childhood. In previous studies, most children were adopted before 12 months of age.33,34 However, there was no significant association between heart failure among adoptive parents and their adopted children, arguing against a strong shared environmental effect in the present cohort. Regardless, as with any adoption study, the study design inherently includes an initial time of shared environment with the biological parent. The present study thus cannot rule out any effects of early shared environmental factors. To minimize possible bias from adoption (ie, that adoption may be predisposed by early HF), we excluded those adoptees that died before age of 16 years or were affected by heart failure before the age of 10 years. Moreover, the multivariable adjustments in models examining associations between adopted heart failure and parental heart failure may be further confounded. As in all epidemiological and clinical studies, residual confounding is likely to exist.
Conclusion
In conclusion, a history of HF in a biological parent is an important risk factor for HF. The heritability of HF is significant in the Swedish population, which indicates that the further studies search for the genetic variants causing liability for HF are warranted.
eFigure. Schematics of Exclusions in the Enrollment of Swedish Born Adoptees.
eTable 1. International Classification of Diseases and Related Health Problems (ICD) Codes Used for Studied Comorbidities.
eTable 2. Comorbidities in Biological and Adoptive Parents With and Without Heart Failure.
eTable 3. Odds Ratio of Heart Failure (HF) in Adoptees, Stratified by Number of Affected Biological Parents (Cohort Study). Individuals With Cardiomyopathies Included.
eTable 4. Hazard Ratio of Heart Failure in Adoptees, Stratified by Affected Parent.
eTable 5. Heritability of Heart Failure Based on Estimated Population Prevalence and Tetrachoric Correlation in the Case-Control Study. Individuals With Cardiomyopathies Included.
eTable 6. Heritability of Heart Failure Based on Estimated Population Prevalence and Tetrachoric Correlation in the Case-Control study. Individuals With Cardiomyopathy Excluded.
eTable 7. Odds Ratio of Heart Failure in Adoptees, Stratified by Affected Parent (Cohort Study). Only Adoptees With Both Biological and Both Adoptive Parents Identified Included.
eTable 8. Odds Ratio of Heart Failure (HF) in Adoptees, Stratified by Affected Parent (Case-Control Study). Only Adoptees With Both Biological and Both Adoptive Parents Identified included.
eTable 9. Odds Ratio of Heart Failure (HF) in Adoptees, Stratified by Affected Biological Parents, in Relation to Coronary Heart Disease (CHD) (Cohort Design). Individuals With Cardiomyopathies Included.
eTable 10. Odds Ratio of Heart Failure (HF) in Adoptees, Stratified by Affected Biological Parents and by Sex (Cohort Study). Individuals With Cardiomyopathies Included.
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. ; Authors/Task Force Members; Document Reviewers . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891-975. doi: 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 2.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113(6):646-659. doi: 10.1161/CIRCRESAHA.113.300268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368-378. doi: 10.1038/nrcardio.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DS, Pencina MJ, Benjamin EJ, et al. Association of parental heart failure with risk of heart failure in offspring. N Engl J Med. 2006;355(2):138-147. doi: 10.1056/NEJMoa052948 [DOI] [PubMed] [Google Scholar]
- 6.Lindgren MP, Smith JG, Li X, Sundquist J, Sundquist K, Zöller B. Sibling risk of hospitalization for heart failure—a nationwide study. Int J Cardiol. 2016;223:379-384. doi: 10.1016/j.ijcard.2016.08.067 [DOI] [PubMed] [Google Scholar]
- 7.Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265(5181):2037-2048. doi: 10.1126/science.8091226 [DOI] [PubMed] [Google Scholar]
- 8.Cahill TJ, Ashrafian H, Watkins H. Genetic cardiomyopathies causing heart failure. Circ Res. 2013;113(6):660-675. doi: 10.1161/CIRCRESAHA.113.300282 [DOI] [PubMed] [Google Scholar]
- 9.Lopes LR, Elliott PM. Genetics of heart failure. Biochim Biophys Acta. 2013;1832(12):2451-2461. doi: 10.1016/j.bbadis.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 10.Dorn GW., II The genomic architecture of sporadic heart failure. Circ Res. 2011;108(10):1270-1283. doi: 10.1161/CIRCRESAHA.110.229260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cappola TP, Matkovich SJ, Wang W, et al. Loss-of-function DNA sequence variant in the CLCNKA chloride channel implicates the cardio-renal axis in interindividual heart failure risk variation. Proc Natl Acad Sci U S A. 2011;108(6):2456-2461. doi: 10.1073/pnas.1017494108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Risch N. The genetic epidemiology of cancer: interpreting family and twin studies and their implications for molecular genetic approaches. Cancer Epidemiol Biomarkers Prev. 2001;10(7):733-741. [PubMed] [Google Scholar]
- 13.Centrum SE. Värdering av diagnoskvaliteten för akut hjärtinfarkt i patientregistret 1987 och 1995. http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/17891/2000-0-100.pdf. Published April 2000. Accessed June 4, 2018.
- 14.Ahrens W, Pigeot I. Handbook of Epidemiology. Berlin, Germany: Springer Berlin Heidelberg; 2007. [Google Scholar]
- 15.Ekbom A. The Swedish Multigeneration Register. Methods Mol Biol. 2011;675:215-220. doi: 10.1007/978-1-59745-423-0_10 [DOI] [PubMed] [Google Scholar]
- 16.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zöller B. Nationwide family studies of cardiovascular diseases-clinical and genetic implications of family history. EMJ Cardiology. 2013;1:102-113. [Google Scholar]
- 18.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659-667. doi: 10.1007/s10654-009-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statistics Sweden Longitudinell integrationsdatabas för Sjukförsäkrings- och Arbetsmarknadsstudier (LISA): Arbetsmarknad och utbildning bakgrundsfakta 2016:1. https://www.scb.se/Statistik/AM/AM9901/_dokument/AM9901_1990I13_BR_AM76BR1601.pdf. Published 2016. Accessed June 6, 2018.
- 20.Winkleby M, Sundquist K, Cubbin C. Inequities in CHD incidence and case fatality by neighborhood deprivation. Am J Prev Med. 2007;32(2):97-106. doi: 10.1016/j.amepre.2006.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingelsson E, Arnlöv J, Sundström J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7(5):787-791. doi: 10.1016/j.ejheart.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 22.de Faire U, Friberg L, Lorich U, Lundman T. A validation of cause-of-death certification in 1,156 deaths. Acta Med Scand. 1976;200(3):223-228. [DOI] [PubMed] [Google Scholar]
- 23.Johansson LA, Westerling R. Comparing Swedish hospital discharge records with death certificates: implications for mortality statistics. Int J Epidemiol. 2000;29(3):495-502. doi: 10.1093/intjepid/29.3.495 [DOI] [PubMed] [Google Scholar]
- 24.Thomas DC. Statistical Methods in Genetic Epidemiology. Oxford, England: Oxford University Press; 2004. [Google Scholar]
- 25.Yates WR. Adoption Studies. eLS. New York, NY: John Wiley & Sons, Ltd; 2001. [Google Scholar]
- 26.Tenesa A, Haley CS. The heritability of human disease: estimation, uses and abuses. Nat Rev Genet. 2013;14(2):139-149. doi: 10.1038/nrg3377 [DOI] [PubMed] [Google Scholar]
- 27.Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. New York, NY: Pearson; 1996. [Google Scholar]
- 28.Falconer DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet. 1965;29(1):51-76. doi: 10.1111/j.1469-1809.1965.tb00500.x [DOI] [Google Scholar]
- 29.Zarrinkoub R, Wettermark B, Wändell P, et al. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail. 2013;15(9):995-1002. doi: 10.1093/eurjhf/hft064 [DOI] [PubMed] [Google Scholar]
- 30.Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet. 2017;390(10092):400-414. doi: 10.1016/S0140-6736(16)31713-5 [DOI] [PubMed] [Google Scholar]
- 31.Carter CO, Evans KA. Inheritance of congenital pyloric stenosis. J Med Genet. 1969;6(3):233-254. doi: 10.1136/jmg.6.3.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krum H, Abraham WT. Heart failure. Lancet. 2009;373(9667):941-955. doi: 10.1016/S0140-6736(09)60236-1 [DOI] [PubMed] [Google Scholar]
- 33.Bohman M. Adopted Children and Their Families: A Follow-Up Study of Adopted Children, Their Background, Environment and Adjustment. Stockholm, Sweden: Proprius [Solna, Seelig]; 1970. [Google Scholar]
- 34.Nordlöf B. Svenska Adoptioner i Stockholm 1918-1973. Stockholm, Sweden: Socialtjänstförvaltningen, Forsknings-och utvecklingsenheten; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Schematics of Exclusions in the Enrollment of Swedish Born Adoptees.
eTable 1. International Classification of Diseases and Related Health Problems (ICD) Codes Used for Studied Comorbidities.
eTable 2. Comorbidities in Biological and Adoptive Parents With and Without Heart Failure.
eTable 3. Odds Ratio of Heart Failure (HF) in Adoptees, Stratified by Number of Affected Biological Parents (Cohort Study). Individuals With Cardiomyopathies Included.
eTable 4. Hazard Ratio of Heart Failure in Adoptees, Stratified by Affected Parent.
eTable 5. Heritability of Heart Failure Based on Estimated Population Prevalence and Tetrachoric Correlation in the Case-Control Study. Individuals With Cardiomyopathies Included.
eTable 6. Heritability of Heart Failure Based on Estimated Population Prevalence and Tetrachoric Correlation in the Case-Control study. Individuals With Cardiomyopathy Excluded.
eTable 7. Odds Ratio of Heart Failure in Adoptees, Stratified by Affected Parent (Cohort Study). Only Adoptees With Both Biological and Both Adoptive Parents Identified Included.
eTable 8. Odds Ratio of Heart Failure (HF) in Adoptees, Stratified by Affected Parent (Case-Control Study). Only Adoptees With Both Biological and Both Adoptive Parents Identified included.
eTable 9. Odds Ratio of Heart Failure (HF) in Adoptees, Stratified by Affected Biological Parents, in Relation to Coronary Heart Disease (CHD) (Cohort Design). Individuals With Cardiomyopathies Included.
eTable 10. Odds Ratio of Heart Failure (HF) in Adoptees, Stratified by Affected Biological Parents and by Sex (Cohort Study). Individuals With Cardiomyopathies Included.