Abstract
This randomized clinical trial examines the use of transcranial magnetic stimulation targeting the left dorsal-lateral prefrontal cortex to modulate withdrawal symptoms in men addicted to methamphetamine.
Drug withdrawal is associated with aversive experiences, which promotes relapse.1 Different neurotransmitters, neuropeptides, signal transduction pathways, and brain regions (especially the nucleus accumbens) have been implicated in the occurrence of withdrawal syndrome during abstinence from addictive drugs.2 Withdrawal from methamphetamine results in fatigue, irritability, disturbed sleep, exhaustion, and symptoms of depression and anxiety, which might last for months. Currently, limited pharmaceutical tools are available for detoxification from methamphetamine; vitamins, antidepressants, and antipsychotics have been used to ameliorate withdrawal symptoms in clinical practices.3
In an animal study, optogenetic stimulation of the thalamic-accumbens dopamine D2 medium spiny neuron pathway alleviated somatic signs induced by opiate withdrawal.4 However, it is unknown if noninvasive brain stimulation could facilitate detoxification during the withdrawal period in humans. In this study, we used repetitive transcranial magnetic stimulation (rTMS) targeting the left dorsal-lateral prefrontal cortex (DLPFC) to modulate symptoms of withdrawal from methamphetamine.
Methods
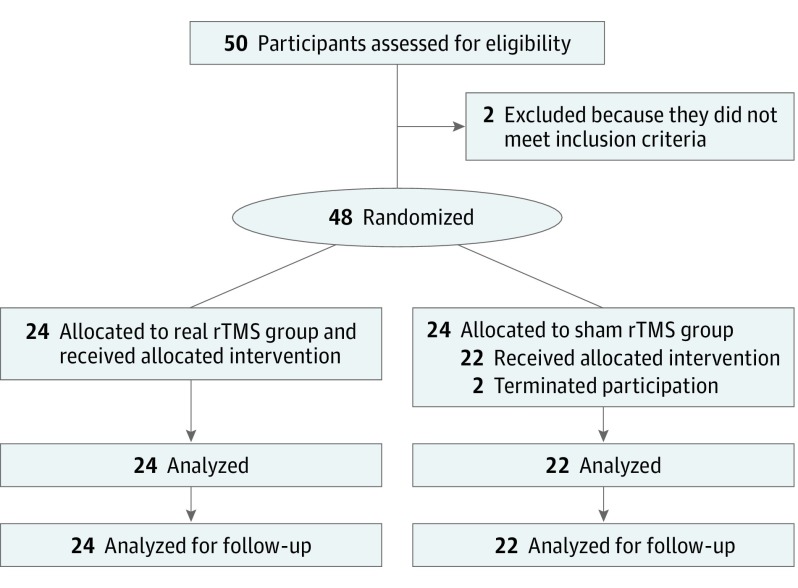
This double-blind, randomized, clinical, parallel-group intervention trial, conducted from August 1, 2017, to February 15, 2018, recruited 50 men with methamphetamine addiction (positive results of urine test; abstinence length, 1-15 days) at Nanjing Shifosi Addiction Rehabilitation Center, Nanjing, China (Figure 1). One man was excluded owing to use of heroin, and another man was excluded owing to use of ketamine. The remaining 48 men (mean [SD] age, 33.3 [9.8] years; range, 18-54 years) were randomly assigned to the real rTMS group (24 men) or sham rTMS group (24 men) by another researcher who did not directly participate in the study. Participants reported no history of head trauma, epilepsy, heart diseases, or metal implants in the body. The study was approved by the ethics committee of Nanjing Normal University and all experimental procedures followed the guidelines of human medical research (Declaration of Helsinki5). All participants provided written informed consent. The clinical trial registration was ChiCTR-IOR-16008060. The trial protocol is in the Supplement.
Figure 1. Study Flowchart and Design.
rTMS indicates repetitive transcranial magnetic stimulation.
Withdrawal symptoms from methamphetamine were evaluated with a Chinese version of the methamphetamine withdrawal symptom scale, quality of sleep was measured with Pittsburgh Sleep Quality Index, depression was evaluated with the self-rating depression scale, and anxiety was evaluated with the self-rating anxiety scale. Evaluation of cue-induced craving was performed with a visual analog scale as previously described.6 High (10 Hz for 10 minutes) rTMS targeting the left DLPFC was performed as previously described.6 The intervention lasts for 10 days, with 2 days rest after the first 5 days (total, 12 days). The data were analyzed with SPSS, version 23.0 (SPSS Inc). Preprotocol analysis was conducted for assessments. Intergroup differences were examined with an independent sample t test or χ2 test. A 2-way repeated-measure analysis of variance was used to assess the main effects of the groups (real rTMS vs sham rTMS) and time (before stimulation, mid-stimulation, and after stimulation). Post hoc t tests used Bonferroni adjustments for multiple comparisons; all post hoc tests’ level of significance were designated at P < .05/5 = .01. All other P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Results
There were no significant differences between the real rTMS and sham rTMS groups in mean (SD) age (31.8 [1.9] vs 34.4 [2.3] years), educational level (mean [SD], 9.9 [2.9] vs 11.3 [2.1] years), race (ratio of Han Chinese race to other races, 21:3 vs 21:1), mean (SD) body mass index (23.9 [2.9] vs 23.1 [3.9]; calculated as weight in kilograms divided by height in meters squared), mean (SD) length of abstinence (6.5 [4.4] vs 8.5 [4.2] days), mean (SD) duration of methamphetamine use (4.6 [3.0] vs 5.6 [3.3] years), and mean (SD) dose of methamphetamine per day (0.6 [0.3] vs 0.4 [0.3] g).
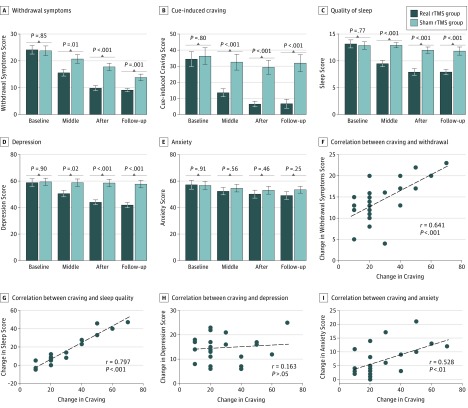
The results demonstrated significant changes in withdrawal symptoms, craving, quality of sleep, and mood status (depression and anxiety) after 10 days of rTMS treatments (Figure 2). Withdrawal symptoms showed a significant difference for time (F3,32 = 198.18; P < .001; ηp2 = 0.81) and the descriptive data showed that withdrawal symptoms decreased. There was also a significant time × group interaction effect (F3,132 = 20.27; P < .001; ηp2 = 0.31) and post hoc t tests (with Bonferroni correction for multiple comparisons) showed that withdrawal symptoms were significantly reduced for both the rTMS group (t23 = 13.21; P < .001) and for the sham group (t21 = 9.53; P < .001). For craving, the analysis indicated a significant difference for time (F3,132 = 50.52; P < .001; ηp2 = 0.53) and the descriptive data showed that craving decreased. There was also a significant time × group interaction effect (F3,132 = 22.93; P < .001; ηp2 = 0.34) and post hoc t tests (with Bonferroni correction for multiple comparisons) showed that the craving score was significantly reduced for the real rTMS group (t23 = 8.59; P < .001), but not for the sham rTMS group (t21 = 2.40; P = .046) after applying Bonferroni correction for multiple comparisons. Quality of sleep showed a significant difference for time (F3,132 = 32.76; P < .001; ηp2 = 0.42) and the descriptive data showed that sleep difficulties decreased. There was also a significant time × group interaction effect (F3,132 = 22.59; P < .01; ηp2 = 0.33) and post hoc t tests (with Bonferroni correction for multiple comparisons) showed that sleep difficulties were significantly reduced for the real rTMS group (t23 = 8.85; P < .001) but not for the sham rTMS group (t21 = 1.08; P = .29).
Figure 2. Repetitive Transcranial Magnetic Stimulation (rTMS) Intervention Effects on Withdrawal Symptoms, Craving, Quality of Sleep, and Depression and Anxiety Scores .
A, Withdrawal symptoms showed a significant difference for time (F3,32 = 198.18; P < .001; ηp2 = 0.81) and for a time × group interaction effect (F3,132 = 20.27; P < .001; ηp2 = 0.31). Post hoc t tests (with Bonferroni correction for multiple comparisons) showed that withdrawal symptoms were significantly reduced for both the real rTMS group (t23 = 13.21; P < .001) and the sham rTMS group (t21 = 9.53, P < .001). B, Cue-induced craving showed a significant difference for time (F3,132 = 50.52; P < .001; ηp2 = 0.53) and for a time × group interaction effect (F3,132 = 22.93; P < .001; ηp2 = 0.34). Post hoc t tests (with Bonferroni correction for multiple comparisons) showed that the craving score was significantly reduced for the real rTMS group (t23 = 8.59; P < .001) but not for the sham rTMS group (t21 = 2.40; P = .046) after applying Bonferroni correction for multiple comparisons. C, Quality of sleep showed a significant difference for time (F3,132 = 32.76; P < .001; ηp2 = 0.42) and for a time × group interaction effect (F3,132 = 22.59; P < .001; ηp2 = 0.33). Post hoc t tests (with Bonferroni correction for multiple comparisons) showed that sleep difficulties were significantly reduced for the real rTMS group (t23 = 8.85; P < .001) but not for the sham rTMS group (t21 = 1.08; P = .290). D, Depression scores showed a significant difference for time (F3,132 = 83.43; P < .001; ηp2 = 0.65) and for a time × group interaction effect (F3,132 = 63.77; P < .001; ηp2 = 0.59). Post hoc t tests showed that depression was significantly reduced for the real rTMS group (t23 = 11.97; P < .001) but not for the sham group (t21 = 1.86; P = .076). E, Anxiety scores showed a significant difference for time (F3,132 = 25.59; P < .001; ηp2 = 0.36) and for a time × group interaction effect (F3,132 = 4.560; P = .01; ηp2 = 0.03). Further analyses (paired-samples t tests, 5% level) showed that anxiety was significantly reduced for the real rTMS group (t23 = 5.28; P < .001) but not for the sham rTMS group (t21 = 2.35; P = .03) after applying Bonferroni correction for multiple comparisons. F, Correlation between reduced craving was positively associated with reductions in withdrawal symptoms (P < .001). G, Correlation between reduced craving was positively associated with improvements in sleep (P < .001). H, Correlation between reduced craving was not positively associated with the depression score (P = .45). I, Correlation between reduced craving was positively associated with decreased severity of anxiety (P = .007).
For depression, a significant difference was seen for time (F3,132 = 83.43; P < .001; ηp2 = 0.65) and the descriptive data showed that the depression score decreased (Figure 2). There was also a significant time × group interaction effect (F3,132 = 63.77; P < .001; ηp2 = 0.59) and post hoc t tests showed that depression was significantly reduced for the real rTMS group (t23 = 11.97; P < .001) but not for the sham rTMS group (t21 = 1.86; P = .08). For anxiety, a significant difference was seen for time (F3,132 = 25.59; P < .001; ηp2 = 0.36) and there was a significant time × group interaction effect (F3,132 = 4.560; P = .01; ηp2 = 0.03); further analyses (paired-samples t tests, 5% level) showed that anxiety was significantly reduced for the real rTMS group (t23 = 5.28; P < .001) but not for the sham rTMS group (t21 = 2.35; P = .03) after applying Bonferroni correction for multiple comparisons. The reduced craving score was correlated positively with reductions in withdrawal symptoms (r = 0.641; P < .001), improvements in sleep (r = 0.797; P < .001), and decreased severity of anxiety (r = 0.528; P < .01), but not the depression score (r = 0.163; P = .44).
Discussion
Repetitive transcranial magnetic stimulation treatments have been shown to reduce craving to drug-associated cues in individuals who are addicted to different substances, including heroin, cocaine, methamphetamine, and nicotine.6,7 This study further proved that rTMS acts as a prospective tool against withdrawal syndrome; therefore, the application of rTMS to patients in different stages of addiction may be expanded. Stimulation targeting the left DLPFC leads to increased dopamine release, enhanced cortical activity, and reorganized cortical networks, which might trigger substantial changes in the nucleus accumbens and alleviate withdrawal symptoms. Further mechanistic and longer follow-up studies are required to elucidate the long-term effects of rTMS on addiction.
In conclusion, high-frequency rTMS targeting the left DLPFC could facilitate methamphetamine detoxification. The potential value of the procedure should be tested in larger clinical trials and to prevent relapse from addiction.
Trial Protocol
References
- 1.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52-58. doi: 10.1126/science.278.5335.52 [DOI] [PubMed] [Google Scholar]
- 2.Harris GC, Aston-Jones G. Involvement of D2 dopamine receptors in the nucleus accumbens in the opiate withdrawal syndrome. Nature. 1994;371(6493):155-157. doi: 10.1038/371155a0 [DOI] [PubMed] [Google Scholar]
- 3.Morley KC, Cornish JL, Faingold A, Wood K, Haber PS. Pharmacotherapeutic agents in the treatment of methamphetamine dependence. Expert Opin Investig Drugs. 2017;26(5):563-578. doi: 10.1080/13543784.2017.1313229 [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Wienecke CF, Nachtrab G, Chen X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature. 2016;530(7589):219-222. doi: 10.1038/nature16954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 6.Shen Y, Cao X, Tan T, et al. . 10-Hz repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex reduces heroin cue craving in long-term addicts. Biol Psychiatry. 2016;80(3):e13-e14. doi: 10.1016/j.biopsych.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 7.Gorelick DA, Zangen A, George MS. Transcranial magnetic stimulation in the treatment of substance addiction. Ann N Y Acad Sci. 2014;1327:79-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol