Key Points
Question
Does the phenotype of Graves orbitopathy, especially its activity, reflect the extent and features of the lymphocytic infiltrate of orbital tissues?
Findings
In this cohort study conducted in 20 consecutive patients with Graves orbitopathy, an association between the clinical activity score and orbital lymphocytes was found, analyzed as total number and main lymphoid subsets, both in a simple and multiple regression model.
Meaning
These findings suggest a correlation between T and B lymphocytes infiltrating orbital tissue and Graves orbitopathy activity, possibly enhancing understanding of the association between Graves orbitopathy immunologic features and clinical expression.
Abstract
Importance
Graves orbitopathy (GO) responds to immunosuppressive treatments when clinically active but poorly when inactive. In other autoimmune diseases, response has been ascribed to a reduction in lymphocytes infiltrating the target organ. It is not known whether active vs inactive GO differs in this regard, which would help in understanding the link between GO immunologic features and clinical behavior.
Objective
To investigate the association between orbital lymphocytic infiltrate and GO clinical features.
Design, Setting, and Participants
A cohort study aimed at assessing the extent and immunohistochemical phenotype of orbital lymphocytes and associating it with the ophthalmologic features of GO, especially its clinical activity score (CAS), was conducted at a tertiary referral center. Twenty consecutive patients with GO who underwent orbital decompression were included. The study was conducted from January 1 to May 31, 2017.
Exposures
Orbital tissue histology and immunohistochemistry testing as well as ophthalmologic evaluation.
Main Outcomes and Measures
Association between CAS and orbital lymphocytes, analyzed as total number of lymphocytes and main lymphoid subsets.
Results
The patient population included 8 men and 12 women, all of white race, with a mean (SD) age of 46 (13) years. With an established cutoff value of 300 lymphoid cells per tissue sample, lymphocytes above this value were found in orbital tissues of 9 of 20 patients (45%), often organized into distinct foci. The lymphocytes comprised a mixture of T (CD3-positive) and B (CD20-positive) cells, suggesting a mature, polyclonal autoimmune response. In a simple linear regression model, the total number of lymphocytes, as well as the number of CD3- and CD20-positive subsets, correlated with CAS (R = 0.63; 95% CI, 0.27-0.84; P = .003; R = 0.59; 95% CI, 0.20-0.82; P = .006; and R = 0.65; 95% CI, 0.30-0.85; P = .002, respectively). In a multiple linear regression model, lymphocytes maintained their effect on CAS when adjusted for 2 additional variables that were correlated with CAS—smoking and GO duration—highlighting even more the important role of orbital lymphocytes in affecting CAS (total number: R = 0.58; 95% CI, 0.18-0.82; P = .01; CD3-positive: R = 0.58; 95% CI, 0.17-0.82; P = .01; and CD20-positive: R = 0.59; 95% CI, 0.19-0.83; P = .01).
Conclusions and Relevance
This study shows a correlation between T and B lymphocytes infiltrating orbital tissues and the activity of GO, possibly enhancing our understanding of the association between GO immunologic features and clinical expression.
This cohort study examines the immunohistochemical phenotype of orbital lymphocytes and clinical features in patients with Graves orbitopathy.
Introduction
Graves orbitopathy (GO) is a chronic inflammatory disease associated with Graves hyperthyroidism in 95% of patients, with hypothyroid autoimmune thyroiditis in 3% to 4% and observed cases in 1% to 2% of patients who have thyroid autoimmunity but without overt thyroid dysfunction.1,2,3 Graves orbitopathy has an autoimmune pathogenesis, whereby the thyrotropin (TSH) receptor is considered the major autoantigen, thus establishing a direct link between the thyroid and orbital tissues.4 Unlike Graves hyperthyroidism, which is a classic example of humoral autoimmunity caused by TSH receptor–stimulating autoantibodies,5 GO is believed to reflect T-cell–mediated autoimmunity, although B-cell– and antibody-mediated autoimmunity, to some extent, may also participate in the pathogenic process.4,6,7 The role of B lymphocytes in the pathogenesis of GO is suggested by the finding that rituximab, a monoclonal antibody that destroys B cells by binding to CD20 on their surface, may ameliorate GO activity.8 The mechanism through which destruction of antibody-producing B cells ameliorates a T-cell–mediated disease is unknown but has been ascribed to the role that B cells have as professional antigen-presenting cells.
Rituximab has been used in other autoimmune diseases, such as type 1 diabetes,9 in which patients are known to be heterogeneous in terms of CD20-positive lymphocytes infiltrating the pancreatic islets.10 We hypothesized that something similar occurs in the orbits of patients with GO, a heterogeneity that may shed light on the link between GO immunologic features and clinical expression and possibly explain the variable response to immunosuppressive drugs. We thus designed this study to characterize in a relatively large cohort of patients the presence and composition of lymphocytes infiltrating orbital tissues and relate them to the clinical features of GO.
Methods
Patient Characteristics and Study Design
We arbitrarily chose a sample size of 20 consecutive patients with GO who underwent orbital decompressive surgery. In all patients, we recorded smoking habits; analyzed thyroid function through measurement of free thyroxine, free triiodothyronine (Vitros Immunodiagnostics), and TSH (Immulite 2000; Siemens Healthcare); measured the presence of anti-TSH receptor antibodies (TRAbs) (Brahms); and performed a detailed ophthalmologic evaluation that included 6 factors: (1) exophthalmometry, (2) clinical activity score (CAS) (range, 0-7; GO active for CAS values ≥3),11 (3) diplopia according to the Gorman score (from absent to constant),11 (4) corneal status, (5) ocular fundus, and (6) visual acuity.
The study was conducted from January 1 to May 31, 2017. Written and signed informed consent was obtained from all patients. The study was approved by the local ethics committee (Comitato Etico Area Vasta Nord Ovest). The participants did not receive financial compensation.
Tissue Assays
Orbital tissues collected at surgery were fixed in formalin and embedded in paraffin for routine hematoxylin and eosin histology and immunohistochemistry (Ventana Benchmark system; Ventana Medical Systems) testing. Archival paraffin-embedded thyroid tissue samples from patients with Graves hyperthyroidism as well as archival orbital and abdominal fibroadipose tissue samples from patients without Graves hyperthyroidism and GO were used as controls. Sections 3- to 5-μm thick were deparaffinized in xylene, dehydrated, and processed using a diaminobenzidine detection system (Ventana), according to the manufacturer’s instructions. CD3, a pan T-lymphocyte marker, was detected using a rabbit monoclonal antibody (immunoglobulin [Ig]G) against the nonglycosylated ε chain of the CD3 molecule expressed by human T lymphocytes (CONFIRM anti-CD3, clone 2GV6; Ventana). CD20, a pan B-lymphocyte marker, was detected using a mouse monoclonal antibody (IgG2a κ) (CONFIRM anti-CD20, clone L26; Ventana). After hematoxylin-eosin immunostaining, the entire orbital adipose tissue was scanned at low power ( × 2.5 magnification) and then analyzed at ×20 magnification to count the total number of infiltrating lymphocytes and T and B cells in 4 representative fields. The final number of lymphoid cells per patient was expressed as the sum of the cells in all 4 fields. More than 300 lymphocytes per tissue sample were found in 9 of 20 patients (45%). The same number of patients had more than 100 CD3-positive cells, and 8 of 20 patients (40%) had more than 200 CD20-positive cells. These cutoff values were obtained using the finite mixture models, assuming the sum of 2 gaussian curves as a model. Thus, the finite mixture model is a convex combination of 2 or more probability density functions, and mixture models are capable of approximating value distributions by combining the properties of the individual probability density functions.12 Once the parameters of the 2 curves (mean [SD]) were established, cutoff levels were calculated as the values on which the probability of the 2 curves was equal. Thus, the reported cutoff values were those that best discriminated the 2 normal distributions identified with the finite mixture model analysis. The pathologists (L.T. and F.B.) who examined the tissue sections and performed the cell counting were masked (ie, they were not aware of the clinical features of the patients).
Statistical Analysis
Data were summarized as mean (SD) or median and interquartile range. Lymphocytes were expressed in a logarithmic scale because their distribution was not gaussian. Groups were compared by simple or multiple linear regression, χ2 test, t test, or Mann-Whitney test, as appropriate. Level of significance was set at P ≤ .05; testing was 2-tailed and unpaired. SPSS, version 25 (IBM Corp) was used for analysis.
Results
Clinical Features of Patients
The patient population included 8 men and 12 women, all of white race, with a mean (SD) age of 46 (13) years. Most of the 20 patients were nonsmokers (Table 1). Graves orbitopathy was associated with Graves hyperthyroidism in 19 patients and occurred alone (euthyroid GO) in the remaining patient. All of the patients with Graves hyperthyroidism had received a form of treatment (radioiodine in 10, thyroidectomy in 6, and methimazole in 3) and were euthyroid at the time of orbital decompression. This surgery was performed for severe, disfiguring proptosis in 17 patients or for optic neuropathy in 3 patients. Four patients with GO were untreated, whereas the remaining patients had received glucocorticoids alone or with orbital radiotherapy, the latter being performed in 5 patients. The median time elapsed since the last glucocorticoid administration was 4 months. Three patients had been treated with glucocorticoids in the 3 months preceding orbital decompression. Most patients had moderately severe GO, and the eye disease was variably active, as shown by CAS values reported in Table 1.
Table 1. Demographic and Clinical Features of the Patient Population.
| Characteristic | Value |
|---|---|
| Sex, No. (%) | |
| Male | 8 (40) |
| Female | 12 (60) |
| Age, mean (SD) [range], y | 46 (13) [16-67] |
| Smoking habits, No. (%) | |
| Smoker | 5 (25) |
| Ex-smoker | 2 (10) |
| Nonsmoker | 13 (65) |
| Thyroid treatment, No. (%) | |
| Methimazole | 3 (15) |
| Radioiodine | 10 (50) |
| Thyroidectomy | 6 (3) |
| None | 1 (5) |
| FT3, mean (SD) [range], pg/dL | 0.38 (0.04) [0.32-0.47] |
| TSH, median (IQR) [range], μU/mL | 0.6 (0.1-1.9) [0.003-4.2] |
| TRAbs, median (IQR) [range], U/L | 3.9 (2.3-5.7) [0.3-9.8] |
| GO duration, median (IQR) [range], mo | 34 (18-51) [2-85] |
| Indication for OD, No. (%) | |
| Optic neuropathy | 3 (15) |
| Severe proptosis | 17 (85) |
| GO treatment, No. (%) | |
| Intravenous GC and radiotherapy | 4 (20) |
| Intravenous GC alone | 11 (55) |
| Oral GC and radiotherapy | 1 (5) |
| None | 4 (20) |
| Months since last GC administration, median (IQR) [range] | 4 (3-18.5) [0-36] |
| Exophthalmometry, mean (SD) [range], mm | 24.6 (2.7) [17-28] |
| Clinical activity score, median (IQR) [range]a | 4 (3-5) [1-7] |
| Diplopia (Gorman score), No. (%) of patients | |
| Absent | 8 (40) |
| Intermittent | 1 (5) |
| Inconstant | 5 (20) |
| Constant | 6 (30) |
| Visual acuity (Snellen), mean (SD) [range] | 18.6/20 (2.4/20) [12/20-20/20] |
Abbreviations: FT3, free triiodothyronine; GC, glucocorticoid; GO, Graves orbitopathy; IQR, interquartile range; TRAbs, anti-thyrotropin (TSH) receptor antibodies.
SI conversion factor: To convert FT3 to picomoles per liter, multiply by
0.0154.
Range, 0 to 7; GO active for clinical activity score values of 3 or higher.
Infiltrating Lymphocytes and Their Correlation With CAS
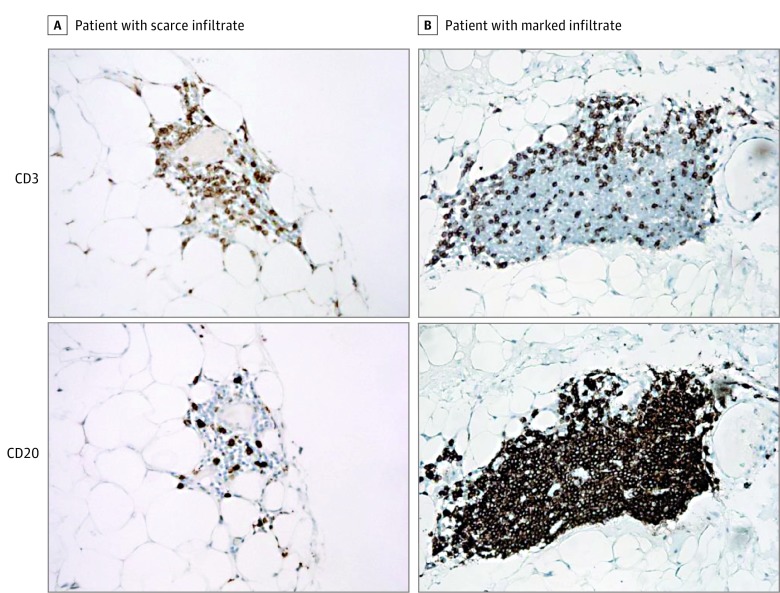
Nine patients (45%) had a total number of orbital infiltrating lymphocytes above the 300 cutoff value. Two representative cases, 1 with a scarce and 1 with a marked infiltrate, are shown in Figure 1. Lymphocytes comprised a mixture of both CD3-positive T cells and CD20-positive B cells, without an overall predominance of 1 subset over another (Figure 1). Lymphocytes that stained positive for either CD3 or CD20 were also detected in thyroid tissue samples from patients with Graves hyperthyroidism, used as positive controls (not shown). In contrast, no lymphocytes and, consequently, no CD3 and CD20 staining were observed in archival orbital and abdominal fibroadipose tissue samples from patients without Graves hyperthyroidism or GO, used as negative controls.
Figure 1. Immunohistochemical Staining for CD3 and CD20 in Orbital Tissues.
CD3 and CD20 staining in orbital tissues of 2 representative patients with Graves orbitopathy with scarce (A) and marked (B) infiltrate (hematoxylin-eosin, original magnification ×112).
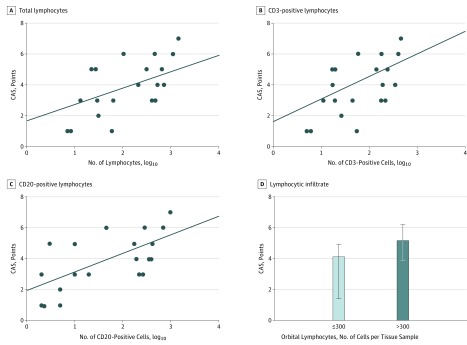
In a simple linear regression model that assessed the overall effect of orbital lymphocytes on CAS, lymphocytes positively correlated with CAS (Table 2 and Figure 2A). For every 10-fold increase in the total number of orbital lymphocytes, CAS increased by 1.52 points (R = 0.63; 95% CI, 0.27-0.84; P = .003). The correlation was similar when lymphocytes were analyzed separately as T cells (Table 2, Figure 2B) or B cells (Table 2, Figure 2C). For every 10-fold increase in CD3-positive cells, CAS increased by 1.68 points (R = 0.59; 95% CI, 0.20-0.82; P = .006). For every 10-fold increase in CD20-positive cells, CAS increased by 1.2 points (R = 0.65; 95% CI, 0.30-0.85; P = .002). Lymphocytes did not correlate with age, sex, smoking habits, GO duration, thyroid treatment, previous GO treatments, thyroid function, levels of TRAbs, exophthalmometry, eyelid aperture, diplopia, and visual acuity, overall suggesting that their number is mainly an indicator of GO activity. Of the various patient features under examination, apart from orbital lymphocytes, smoking and GO duration correlated with CAS (Table 2).
Table 2. Correlation Between Clinical Activity Score of Patients With GO and the Indicated Featuresa.
| Variable | Linear Regression | Multiple Regressionb | ||
|---|---|---|---|---|
| R (95% CI) | P Value | R (95% CI) | P Value | |
| Total No. of lymphocytesc | 0.63 (0.27 to 0.84) | .003 | 0.58 (0.18 to 0.82) | .01 |
| CD3-positive cells | 0.59 (0.20 to 0.82) | .006 | 0.58 (0.17 to 0.82) | .01 |
| CD20-positive cells | 0.65 (0.30 to 0.85) | .002 | 0.59 (0.19 to 0.83) | .01 |
| Smoking habitsd | −0.63 (−0.84 to −0.25) | .003 | NA | NA |
| vs Total No./GO duration | NA | NA | −0.43 (−0.73 to 0.01) | .08 |
| vs CD3/GO duration | NA | NA | −0.43 (−0.73 to 0.01) | .09 |
| vs CD20/GO duration | NA | NA | −0.44 (−0.74 to 0.003) | .08 |
| GO duration | −0.70 (−0.88 to −0.36) | <.001 | NA | NA |
| vs Total No./smoking | NA | NA | 0.68 (−0.86 to −0.34) | .002 |
| vs CD3/smoking | NA | NA | −0.69 (−0.87 to −0.36) | <.001 |
| vs CD20/smoking | NA | NA | −0.68 (−0.86 to 0.34) | <.001 |
Abbreviations: GO, Graves orbitopathy; NA, not applicable.
Population included 20 white patients (8 men, 12 women; mean [SD] age, 46 [13 years]).
Multiple regressions were performed individually.
For lymphocytes, log10 values were used.
Smoking habits were converted into continuous values.
Figure 2. Orbital Lymphocytes and Clinical Activity Score (CAS) in White Patients (8 Men, 12 Women; Mean [SD] Age, 46 [13] Years) With Graves Orbitopathy.
A, Correlation between total lymphocytes and CAS (P = .003). B, Correlation between CD3-positive lymphocytes and CAS (P = .006). C, Correlation between CD20-positive lymphocytes and CAS (P = .002). D, CAS (median and interquartile range) according to the presence a relevant (>300 cells in 4 fields) lymphocytic infiltrate.
As reported in Table 2, in a multiple linear regression model that analyzed the effect on CAS not only of the orbital lymphocytes but also of smoking and GO duration, orbital lymphocytes maintained their action on CAS when adjusted for the other covariates, highlighting even more the important role of orbital lymphocytes in affecting CAS.
Features of Patients According to the Orbital Lymphocytic Infiltrate
In confirmation of the findings reported above, when patients were grouped based on the total number of orbital infiltrating lymphocytes, those with counts above the cutoff value had a greater CAS than those below the cutoff value (Figure 3D). In addition, patients with counts above the cutoff value were more often smokers (4 smokers, 1 ex-smoker, and 4 nonsmokers vs 1 smoker, 1 ex-smoker, and 9 nonsmokers in patients with a number of lymphocytes below the cutoff level; P = .007), which was in line with the correlation between smoking and CAS. The remaining features (age, sex, GO duration, thyroid treatment, previous GO treatments, thyroid function, levels of TRAbs, exophthalmometry, eyelid aperture, diplopia, and visual acuity) did not differ significantly between the 2 groups.
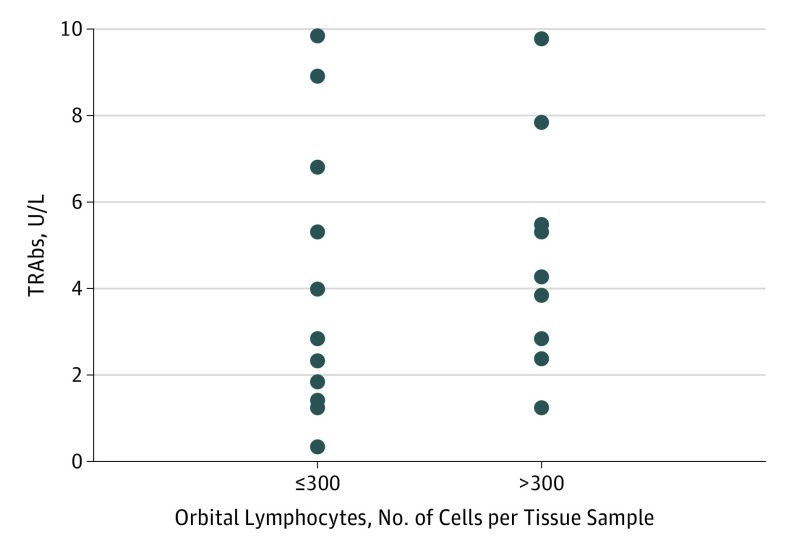
Figure 3. Individual Levels of Serum Anti–Thyrotropin Receptor Antibodies (TRAbs) in White Patients (8 Men, 12 Women; Mean [SD] Age, 46 [13] Years).
Levels of serum TRAbs according to presence of a relevant (>300 cells in 4 fields) orbital lymphocytic infiltrate.
The absence of a correlation between the lymphocytic infiltrate in orbital tissues and TRAbs was surprising in view of the knowledge that TRAbs are correlated with GO activity and severity.13 Individual levels of TRAbs according to the presence of a relevant orbital lymphocytic infiltrate are shown in Figure 3. The findings probably reflect the relatively long GO duration (median, 34 months) and especially the fact that most patients had undergone an ablative thyroid treatment that, with the exception of a known transient increase in TRAbs after radioiodine,14 is generally followed in the long term by a reduction of these autoantibodies.15 This reduction may explain the relatively low levels and, consequently, the lack of correlation with orbital lymphocytes. Overall, our findings do not exclude with certainty a general correlation between orbital infiltrating lymphocytes and antibodies to the TSH receptor; some studies have shown that thyroid-stimulating immunoglobulins may be more sensitive than TRAbs in correlating with GO features.16
Discussion
In the present study, we show that the lymphocytic infiltrate of orbital tissues in patients with GO correlates with GO activity, thereby shedding light on an association between GO immunologic features and clinical expression and possibly posing an immunopathologic basis for future studies aimed at investigating the association between the orbital lymphocytic infiltrate and the response of GO to immunosuppressive treatments.17,18 Results supporting our conclusions can be summarized as follows.
We examined orbital tissue samples from 20 patients with GO who underwent orbital decompression. At histology examination in 45% of the patients, several focal areas of distinct lymphocytic infiltration could be seen in orbital tissues. On immunohistochemistry testing, lymphocytes stained positive for both CD3 and CD20, indicating that both T and B cells were represented, with variable predominance. The total number of infiltrating lymphocytes as well as the number of CD3- and CD20-positive cells correlated with CAS, which in turn correlated with smoking and GO duration. Multivariate analyses confirmed the correlation between CAS and the total number of infiltrating lymphocytes as well as CD3-positive and CD20-positive infiltrating cells, indicating that orbital infiltrating lymphocytes correlate with GO activity independently. In confirmation of these findings, CAS was greater in patients with a total number of orbital infiltrating lymphocytes above a cutoff level of 300 in 4 microscopic fields, established using a finite mixture model, compared with patients who had levels of orbital infiltrating lymphocytes equal to or below the cutoff value. Patients with a relevant orbital lymphocytic infiltrate were more often smokers, thereby indirectly confirming the correlation between smoking and CAS observed here and in agreement with the notion that smoking is a GO risk factor.11
Apart from studies in which the phenotype of orbital T cells in culture was investigated,19,20 to our knowledge, 1 previous study on the same issue was conducted in a smaller number of patients (n = 14).21 In that study, orbital T cells were detected to a greater extent in patients with GO of recent onset compared with those with long-standing GO, which, in view of the knowledge that GO is more active in its early phases,22 is indirectly in line with our findings. However, in the same study, the extent of B cell involvement was negligible, both in early and late GO.21 This apparent discrepancy with our findings may reflect the fact that, in that previous investigation, patients were selected and not consecutive, the number (5) of subjects with early and presumably active GO was limited, and 2 of these 5 patients had undergone immunosuppressive treatment (glucocorticoids and, in 1 patient, also azathioprine) in the month preceding tissue harvesting.21
Limitations
Most patients (16 [80%]) from whom tissues were taken had been treated with glucocorticoids before orbital decompression, and it could be argued that this intervention may have affected the results. However, the median time that elapsed since the last glucocorticoid administration was longer than 3 months, suggesting that glucocorticoids unlikely affected the lymphocytic infiltration of orbital tissues. There was no correlation between previous glucocorticoid treatment or dosage and the lymphocytic infiltration in orbital tissues. On the same line, 5 patients had received orbital radiotherapy, but again there was no correlation between orbital radiotherapy and the findings reported above. Our results may suggest a possible role of infiltrating lymphocytes in the pathogenesis of GO, although data are not sufficient to prove it, which is a limitation of our study. In this regard, it should be taken into account that lymphocytes likely represent only 1 of the many causative cell elements involved, in addition to orbital fibroblasts and fibrocytes.4 We observed a persistent lymphocytic infiltration that correlates with CAS even in chronic, long-standing cases of GO. The mechanism underlying this observation is unknown. It remains to be established whether lymphocytes are somehow “trapped” in orbital tissues or whether they continue to marginate; further studies are needed. Another limitation of our study is that CAS was not originally intended as a long-term factor for GO evaluation (although it is widely used in this manner), instead being intended as an indicator for the early stages of the disease.11
As mentioned above, our findings may pose the basis for future studies aimed at investigating the association between response to immunosuppressive treatments, in particular glucocorticoids, GO activity, and immunohistologic features of GO.8 In this regard, we considered the possibility that the variable presence of orbital infiltrating lymphocytes and, in particular, CD20-positive cells explains the discrepant findings on the action of rituximab in patients with GO.8 Thus, rituximab was shown to determine an improvement of GO in patients with an eye disease of recent onset but not in those with long-standing GO.8 However, we did not find a correlation between CD20-positive lymphocytes infiltrating orbital tissue and disease duration, suggesting that the variable response to rituximab unlikely reflects a different expression of these cells in orbital tissues. However, Salvi et al23 showed that CD20-positive lymphocytes infiltrating orbital tissues were depleted after administration of rituximab in a small number (2) of patients with active GO who responded to the treatment, which suggests that an association between CD20-positive lymphocytes and response to rituximab may exist. In any case, our investigation may not be relevant to treatment with rituximab considering that most of our patients had long-standing GO, which may not be the proper population to be treated with the anti-CD20 monoclonal antibody.
Conclusions
Our study provides evidence for a correlation between both T and B cells in the activity of GO and establishes a basis for understanding the association between the immune system and clinical features of GO, as well as for future studies aimed at the comprehension of the response of GO to immunosuppressive treatment based on its activity.
References
- 1.Leo M, Menconi F, Rocchi R, et al. Role of the underlying thyroid disease on the phenotype of Graves’ orbitopathy in a tertiary referral center. Thyroid. 2015;25(3):347-351. [DOI] [PubMed] [Google Scholar]
- 2.Piantanida E, Tanda ML, Lai A, Sassi L, Bartalena L. Prevalence and natural history of Graves’ orbitopathy in the XXI century. J Endocrinol Invest. 2013;36(6):444-449. [DOI] [PubMed] [Google Scholar]
- 3.Bartalena L, Masiello E, Magri F, et al. The phenotype of newly diagnosed Graves’ disease in Italy in recent years is milder than in the past: results of a large observational longitudinal study. J Endocrinol Invest. 2016;39(12):1445-1451. [DOI] [PubMed] [Google Scholar]
- 4.Bahn RS. Current Insights into the pathogenesis of Graves’ ophthalmopathy. Horm Metab Res. 2015;47(10):773-778. [DOI] [PubMed] [Google Scholar]
- 5.Marinò M, Latrofa F, Menconi F, Chiovato L, Vitti P. Role of genetic and non-genetic factors in the etiology of Graves’ disease. J Endocrinol Invest. 2015;38(3):283-294. [DOI] [PubMed] [Google Scholar]
- 6.de Carli M, D’Elios MM, Mariotti S, et al. Cytolytic T cells with Th1-like cytokine profile predominate in retroorbital lymphocytic infiltrates of Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1993;77(5):1120-1124. [DOI] [PubMed] [Google Scholar]
- 7.Aniszewski JP, Valyasevi RW, Bahn RS. Relationship between disease duration and predominant orbital T cell subset in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2000;85(2):776-780. [DOI] [PubMed] [Google Scholar]
- 8.Stan MN, Salvi M. Management of endocrine disease: rituximab therapy for Graves’ orbitopathy—lessons from randomized control trials. Eur J Endocrinol. 2017;176(2):R101-R109. [DOI] [PubMed] [Google Scholar]
- 9.Hamad AR, Ahmed R, Donner T, Fousteri G. B cell–targeted immunotherapy for type 1 diabetes: what can make it work? Discov Med. 2016;21(115):213-219. [PMC free article] [PubMed] [Google Scholar]
- 10.Arif S, Leete P, Nguyen V, et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes. 2014;63(11):3835-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartalena L, Baldeschi L, Boboridis K, et al. ; European Group on Graves’ Orbitopathy (EUGOGO) . The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J. 2016;5(1):9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everitt BS, Hand DJ, eds. Finite Mixture Distributions. London: Chapman and Hall; 1981. [Google Scholar]
- 13.Eckstein AK, Plicht M, Lax H, et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91(9):3464-3470. [DOI] [PubMed] [Google Scholar]
- 14.Laurberg P, Wallin G, Tallstedt L, Abraham-Nordling M, Lundell G, Tørring O. TSH-receptor autoimmunity in Graves’ disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. Eur J Endocrinol. 2008;158(1):69-75. [DOI] [PubMed] [Google Scholar]
- 15.Menconi F, Leo M, Vitti P, Marcocci C, Marinò M. Total thyroid ablation in Graves’ orbitopathy. J Endocrinol Invest. 2015;38(8):809-815. [DOI] [PubMed] [Google Scholar]
- 16.Diana T, Krause J, Olivo PD, et al. Prevalence and clinical relevance of thyroid stimulating hormone receptor–blocking antibodies in autoimmune thyroid disease. Clin Exp Immunol. 2017;189(3):304-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartalena L. Diagnosis and management of Graves disease: a global overview. Nat Rev Endocrinol. 2013;9(12):724-734. [DOI] [PubMed] [Google Scholar]
- 18.Bartalena L, Veronesi G, Krassas GE, et al. ; European Group on Graves’ Orbitopathy (EUGOGO) . Does early response to intravenous glucocorticoids predict the final outcome in patients with moderate-to-severe and active Graves’ orbitopathy? J Endocrinol Invest. 2017;40(5):547-553. [DOI] [PubMed] [Google Scholar]
- 19.Förster G, Otto E, Hansen C, Ochs K, Kahaly G. Analysis of orbital T cells in thyroid-associated ophthalmopathy. Clin Exp Immunol. 1998;112(3):427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckstein AK, Quadbeck B, Tews S, et al. Thyroid associated ophthalmopathy: evidence for CD4+ γδ T cells; de novo differentiation of RFD7+ macrophages, but not of RFD1+ dendritic cells; and loss of γδ and αβ T cell receptor expression. Br J Ophthalmol. 2004;88(6):803-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pappa A, Lawson JM, Calder V, Fells P, Lightman S. T cells and fibroblasts in affected extraocular muscles in early and late thyroid associated ophthalmopathy. Br J Ophthalmol. 2000;84(5):517-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menconi F, Profilo MA, Leo M, et al. Spontaneous improvement of untreated mild Graves’ ophthalmopathy: Rundle’s curve revisited. Thyroid. 2014;24(1):60-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvi M, Vannucchi G, Currò N, et al. Small dose of rituximab for Graves orbitopathy: new insights into the mechanism of action. Arch Ophthalmol. 2012;130(1):122-124. [DOI] [PubMed] [Google Scholar]