Key Points
Question
Does neoadjuvant treatment affect muscle mass and adipose tissue in patients with borderline resectable and locally advanced pancreatic cancer, and are there any differences in those changes between patients who eventually undergo resection and those who do not?
Findings
During a 3-year cohort study, 193 patients were analyzed at 4 institutions. Adipose tissue significantly decreased during neoadjuvant treatment, while muscle mass slightly increased. Patients who underwent resection experienced a higher increase in muscular tissue during neoadjuvant treatment compared with those who did not undergo resection.
Meaning
Lean tissue gain during neoadjuvant treatment for borderline resectable and locally advanced pancreatic cancer is associated with a higher likelihood of resection.
Abstract
Importance
Sarcopenia and sarcopenic obesity have been associated with poor outcomes in unresectable pancreatic cancer (PC). Neoadjuvant treatment (NT) is used increasingly to improve resectability; however, its effects on fat and muscle body composition have not been characterized.
Objectives
To evaluate whether NT affects muscle mass and adipose tissue in patients with borderline resectable PC (BRPC) and locally advanced PC (LAPC) and determine whether there were potential differences between patients who ultimately underwent resection and those who did not.
Design, Setting, and Participants
In this retrospective cohort study conducted at 4 academic medical centers, 193 patients with BRPC and LAPC undergoing surgical exploration after NT who had available computed tomographic scans (both at diagnosis and preoperatively) and confirmed pancreatic ductal adenocarcinoma were evaluated. The study was conducted from January 2013 to December 2015. Data analysis was performed from September 2016 to May 2017. Measurement of body compartments was evaluated with volume assessment software before and after NT. A radiologist blinded to the patient outcome assessed the areas of skeletal muscle, total adipose tissue, and visceral adipose tissue through a standardized protocol.
Exposures
Receipt of NT.
Main Outcomes and Measures
Achievement of pancreatic resection at surgical exploration after the receipt of NT.
Results
Of the 193 patients with complete radiologic imaging available after NT, 96 (49.7%) were women; mean (SD) age at diagnosis was 64 (11) years. Most patients received combined therapy with fluorouracil, irinotecan, oxaliplatin, leucovorin, and folic acid (124 [64.2%]) and 86 (44.6%) received chemoradiotherapy as well. The median interval between pre-NT and post-NT imaging was 6 months (interquartile range [IQR], 4-7 months). All body compartments significantly changed. The adipose compound decreased (median total adipose tissue area from 284.0 cm2; IQR, 171.0-414.0 to 250.0 cm2; IQR, 139.0-363.0; P < .001; median visceral adipose tissue area from 115.2 cm2; IQR, 59.9-191.0 to 97.7 cm2; IQR, 48.0-149.0 cm2; P < .001), whereas the lean mass slightly improved (median skeletal muscle from 122.1 cm2; IQR, 99.3-142.0 to 123 cm2; IQR 104.8-152.5 cm2; P = .001). Surgical resection was achievable in 136 (70.5%) patients. Patients who underwent resection had experienced a 5.9% skeletal muscle area increase during NT treatment, whereas those who did not undergo resection had a 1.7% decrease (P < .001).
Conclusions and Relevance
Patients with PC experience a significant loss of adipose tissue during neoadjuvant chemotherapy, but no muscle wasting. An increase in muscle tissue during NT is associated with resectability.
This cohort study evaluates the changes in the amount of muscular and adipose tissue before and after neoadjuvant therapy in patients with pancreatic cancer.
Introduction
Anorexia, weight loss, and depletion of lean body mass are typical presentation hallmarks of pancreatic cancer (PC).1,2 Both the severity of these events and the consequent changes in body composition have been correlated with the stage of the disease and unfavorable oncologic outcomes.2,3 Specifically, sarcopenia and sarcopenic obesity are associated with increased postoperative morbidity,4,5,6,7 impaired adherence to adjuvant chemotherapy, and reduced long-term survival.8
Surgical resection remains the best opportunity for cure in PC; however, resectability is achievable in less than 20% of the patients, and locally advanced or metastatic disease is diagnosed in most patients.9 Evidence suggests that neoadjuvant treatment (NT) may enhance the resectability rate in borderline resectable PC (BRPC) and locally advanced PC (LAPC), but NT might also have significant effects on body composition. These changes, in turn, may affect postoperative outcomes or prevent the possibility of surgery.10,11,12 In esophageal and gastric cancer, it has been observed that patients who experience lean mass and adipose tissue wasting during NT have a higher rate of postoperative complications,13 but literature is lacking on the outcome of NT in patients with PC. To our knowledge, the only published retrospective analysis on patients with PC reported that depletion of skeletal muscle and adipose tissue during NT led to earlier recurrence and worse survival.14 However, this study focused on patients with early-stage, resectable disease, with indications for NT remaining controversial. The goal of this study was to evaluate the association between NT and body composition in patients with BRPC and LAPC and investigate whether body changes could be predictive of response.
Methods
Patient Selection
The institutional, prospectively maintained electronic databases of the Department of Surgery at the Massachusetts General Hospital (Boston), Milano-Bicocca University at San Gerardo Hospital (Monza, Italy), The Pancreas Institute at University of Verona Hospital Trust (Verona, Italy), and the Department of Surgery, Vita-Salute San Raffaele University (Milan, Italy) were queried for patients evaluated for surgery after NT for BRPC or LAPC. The study was conducted from January 2013 to December 2015. Data analysis was performed from September 2016 to May 2017.
Institutional review board approval was obtained from the Massachusetts General Hospital. Italian local ethical committees’ review of the protocol deemed that formal approval was not required owing to the retrospective, observational, and anonymous nature of this study.
Inclusion criteria were surgical exploration for PC after NT, diagnosis of ductal adenocarcinoma confirmed at pathologic examination, availability of 2 computed tomographic scans (1 before the beginning of NT and 1 just before the operation) and diagnosis of BRPC or LAPC at diagnosis, according to the International Study Group of Pancreatic Surgery criteria.9
Imaging Anthropometric Analysis and Tumor Staging
Computed tomography examinations were evaluated by 4 abdominal radiologists (M.P., D.I., M.D., D.S.) experienced in pancreatic diseases who assessed the degree of vascular involvement at the time of diagnosis and estimated the radiologic response after NT as stable disease, partial response, progressive disease, and complete response.15
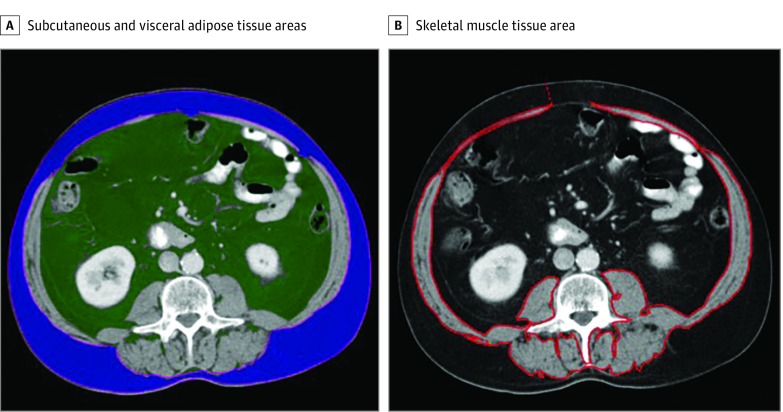
Images were analyzed using volume assessment software (Aquarius iNtuition, version 4.4.12; TeraRecon). One radiologist (C.A.A.-P.) blinded to the patient information, manually segmented the total area of skeletal muscle using an axial computed tomographic image in the venous phase of contrast enhancement at the level of the third lumbar vertebra. The total area of abdominal wall, paraspinal, and psoas muscles was included. Care was taken to avoid perimuscular adipose tissue. These measures have been validated as an accurate estimate of the total muscular mass (Figure).16
Figure. Example of Body Composition Analysis for Measurement of Tissue Areas.
A, Subcutaneous (blue) and visceral adipose (green) tissue areas. B, Skeletal muscle area.
Using the same computed tomographic image, the total subcutaneous adipose tissue area, defined as fat between the skin surface and abdominal wall musculature, and the visceral adipose tissue area, defined as fat within the abdominal cavity, were measured automatically by using default pixel density thresholds (between −30 and −190 Hounsfield U for subcutaneous adipose tissue and −50 to −150 Hounsfield U for visceral adipose tissue). Both skeletal muscle area and adipose tissue area were measured at the time of diagnosis and after NT.
To identify the prevalence of sarcopenia in the study cohort, for each patient, the total abdominal muscle area (TAMA) was normalized for height and reported as centimeters squared per meters squared. Cutoff levels for sarcopenia were set at TAMA less than 41 cm2/m2 for women and TAMA less than 43 cm2/m2 (if body mass index is <25 [calculated as weight in kilograms divided by height in meters squared]) or less than 53 cm2/m2 (if body mass index is ≥25) for men, according to Martin et al.17
Clinical Response
The final decision regarding surgical exploration was made after a multidisciplinary meeting (surgeon, radiologist, oncologist, and radiation oncologist), based on the clinical response to NT, critical appraisal of radiologic images, and patient performance status. Surgery was conducted by experienced pancreatic surgeons, 3 from very high-volume referral centers and 1 from a moderate-volume center.18
Statistical Analysis
Continuous variables were investigated with the Shapiro-Wilk test for normality. Data are described as absolute numbers (percentages), means (SDs) for normally distributed variables, or medians and interquartile ranges (IQRs) for nonparametric variables. Differences between groups were assessed with the Fisher exact χ2 test for dichotomous variables and paired t test or Wilcoxon signed rank test for continuous variables (size of the neoplasm, total adipose tissue, visceral adipose tissue, and skeletal muscle). A 2-sided P value <.05 was considered significant.
The predictive ability of significant factors was investigated using the receiver operating characteristic curve method. We computed the area under the curve index and evaluated the optimal cutoff point predictive of resection (as the one closest to the upper-left corner of the receiver operating characteristic plot),19 together with other diagnostic measures (sensitivity and specificity).
The variables dichotomized according to these thresholds were then used as predictors in a multivariate logistic regression model. Using an Akaike information criterion–based stepwise selection method,20 we computed the best predictive model.
All statistical computations were performed using IBM SPSS software, version 24 (IBM Corp).
Results
Table 1 summarizes the characteristics of the study population. During the study period, 193 patients with complete radiologic images available were evaluated after receipt of NT. The mean age at diagnosis was 64 (11) years and the median BMI was 23.8 (IQR, 21.9-26.0). At first presentation, 63 (32.6%) patients had a borderline resectable cancer and the remaining 130 (67.4%) patients had locally advanced PC. The overall rate of sarcopenia was 43.5% (84 of 193 patients). The most commonly used NT regimen was combined fluorouracil, irinotecan, oxaliplatin, leucovorin, and folic acid (FOLFIRINOX) (124 [64.2%] patients), followed by combined cisplatin, capecitabine, gemcitabine, and nanoparticle albumin–bound paclitaxel (PAXG) plus combined cisplatin, epirubicin, capecitabine, and gemcitabine (PEXG) (54 [28.0%]) and gemcitabine-based schemes (15 [7.8%]). Eighty-six patients (44.6%) also underwent chemoradiotherapy as part of the NT.
Table 1. General Characteristics of Patients.
| Characteristic | Overall Cohort (N = 193) |
|---|---|
| Age, mean (SD), y | 64 (11) |
| BMI, median (IQR) | 24.0 (21.5-27.0) |
| <25 | 131 (67.9) |
| ≥25 | 62 (32.1) |
| Sarcopenia, No. (%) | 84 (43.5) |
| Sex, No. (%) | |
| Male | 97 (50.3) |
| Female | 96 (49.7) |
| Stage at diagnosis, No. (%) | |
| Borderline resectable | 63 (32.6) |
| Locally advanced | 130 (67.4) |
| NT scheme, No. (%) | |
| FOLFIRINOX | 124 (64.2) |
| PAXG/PEXG | 54 (28.0) |
| Gemcitabine based | 15 (7.8) |
| Tumor size, mean (SD), mm | |
| Pre-NT | 33.4 (13.4) |
| Post-NT | 25.8 (13.51) |
| Radiologic response, No. (%) | |
| Stable disease | 127 (65.8) |
| Partial or complete | 51 (26.4) |
| Progressive disease | 15 (7.8) |
| Months from diagnosis to surgery, median (IQR) | 6 (4-7) |
| Resection, No. (%) | |
| No. | 57 (29.5) |
| Yes | 136 (70.5) |
| Vascular resection, No. (%) | 19 (14.0) |
| Category at pathologic examination, No. (%) | |
| Tx | 7 (5.1) |
| T1-T2 | 25 (18.4) |
| T3 | 100 (73.5) |
| T4 | 4 (2.9) |
| Nodal involvement, No. (%) | |
| N0 | 82 (60.3) |
| N1 | 54 (39.7) |
| Negative resection margins (R0), No. (%) | 92 (67.6) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FOLFIRINOX, combined fluorouracil, irinotecan, oxaliplatin, leucovorin, and folic acid; PEXG, combined cisplatin, epirubicin, capecitabine, and gemcitabine; IQR, interquartile range; NT, neoadjuvant treatment; PAXG, combined cisplatin, capecitabine, gemcitabine, and nanoparticle albumin–bound paclitaxel.
At restaging after NT, 127 (65.8%) patients had stable disease, 51 (26.4%) patients had partial or complete response, and 15 (7.8%) patients had progressive disease. All patients underwent surgical exploration after being reviewed at a multidisciplinary conference and 136 (70.5%) patients underwent surgical resection. Conversely, 57 (29.5%) patients did not receive the procedure owing to major vascular involvement at surgical exploration (31 patients) and unexpected metastatic disease to the liver or peritoneum (26 patients). No significant differences in the resection rate were observed among the institutions (P = .07). Detailed information on patients who underwent resection is provided in Table 1.
Anthropometric Measures
As reported in Table 2, all of the body compartments significantly changed during neoadjuvant treatment. Specifically, the amount of adipose tissue decreased for total adipose tissue area (median pretreatment: 284.0 cm2; IQR, 171.0-414.0 vs posttreatment: 250.0 cm2; IQR, 139.0-363.0; P < .001) and visceral adipose tissue area (median pretreatment: 115.2 cm2; IQR, 59.9-191.0 cm2 vs posttreatment: 97.7 cm2; IQR, 48.0-149.0 cm2; P < .001). The lean mass increased (median skeletal muscle pretreatment: 122.1 cm2; IQR, 99.3-142.0 cm2 vs posttreatment: 123.7 cm2; IQR 104.8-152.5 cm2; P = .001).
Table 2. Body Composition Changes During Neoadjuvant Treatment.
| Outcome | Median (IQR) | Pearson Correlation | P Valuea |
|---|---|---|---|
| Total adipose tissue, cm2 | 0.687 | ||
| Pretreatment | 284.0 (171.0-414.0) | <.001 | |
| Posttreatment | 250.0 (139.0-363.0) | ||
| Visceral adipose tissue, cm2 | 0.789 | ||
| Pretreatment | 115.2 (59.9-191.0) | <.001 | |
| Posttreatment | 97.7 (48.0-149.0) | ||
| Skeletal muscle, cm2 | 0.553 | ||
| Pretreatment | 122.1 (99.3-142.0) | .001 | |
| Posttreatment | 123.7 (104.8-152.5) |
Abbreviation: IQR, interquartile range.
P values are related to the differences between median values before and after treatment (related-samples Wilcoxon signed-rank test).
Patients in the FOLFIRINOX group experienced the highest muscle gain (median skeletal muscle area difference between pre-NT and post-NT: 6.8 cm2; IQR, −3.9 to 19.0 cm2; P = .04). No significant differences were observed in adipose tissue wasting or in the resection rate among the different NT groups. At radiologic restaging, patients in the gemcitabine-based therapy group experienced the highest rate of stable disease after NT (80.0%), while patients in the FOLFIRINOX group had the highest rate of partial response (19.4%) (eTable in the Supplement).
Univariate Analysis
The amount of skeletal mass normalized for height (TAMA) after NT was significantly higher in patients who underwent resection compared with those in whom resection could not be achieved (median TAMA posttreatment: 45.1 cm2/m2; IQR, 40.3 to 50.8 vs 42.0 cm2/m2; IQR, 35.8 to 48.8 cm2/m2, respectively; P = .004). Similarly, patients who underwent resection demonstrated a gain in muscular mass, while the unresected group experienced muscle wasting during neoadjuvant treatment (median TAMA difference: 2.3 cm2/m2; IQR, 1.3 to 6.7 vs −1.2 cm2/m2; IQR −5.8 to 1.2; P < .001, respectively). Patients who underwent resection had experienced a 5.9% skeletal muscle area increase during NT treatment, whereas those who did not undergo resection had a 1.7% decrease (P < .001).
Moreover, in the unresected group, sarcopenia increased from 36.8% at baseline to 52.8% after NT, while in the resected group, sarcopenia dropped from 46.3% to 36.8% (P = .27 and P = .054 pre-NT and post-NT, respectively). We built a logistic regression model comparing 4 groups of patients: (1) nonsarcopenic at both times (considered as the reference group), (2) sarcopenic at baseline and nonsarcopenic after NT, (3) nonsarcopenic at baseline and sarcopenic after NT, and (4) sarcopenic at both times. The likelihood of resection was significantly lower only for patients who developed sarcopenia in the NT time frame (group 3) (odds ratio [OR], 0.35; 95% CI, 0.14-0.87; P = .02) (eFigure in the Supplement). Finally, the mean size of the tumor on computed tomographic scans after treatment was significantly smaller in patients who underwent resection compared with those who did not (23.3 [13.3] vs 31.7 [21.2] mm, respectively; P < .001). The type of NT regimen, the time frame from diagnosis to surgical exploration, or the radiologic assessment at restaging had no association with the likelihood of resection (Table 3).
Table 3. Changes in Body Composition During Neoadjuvant Treatment.
| Outcome | No Resection (n = 57) | Resection (n = 136) | P Value |
|---|---|---|---|
| TAT, median (IQR), cm2 | |||
| Pretreatment | 251.0 (165.6 to 392.0) | 301.0 (183.4 to 414.0) | .30 |
| Posttreatment | 244.9 (128.2 to 331.2) | 255.0 (144.0 to 370.2) | .30 |
| Δ TAT | −25.4 (−92.8 to 32.0) | −44.9 (−109.5 to 30.0) | .56 |
| VAT, median (IQR), cm2 | |||
| Pretreatment | 91.8 (50.0 to 168.3) | 123.3 (70.0 to 192.0) | .18 |
| Posttreatment | 67.7 (33.5 to 146.4) | 100.0 (53.0 to 149.0) | .08 |
| Δ VAT | −9.47 (−53.0 to 9.0) | −14.7 (−52.6 to 11.35) | .73 |
| SM, median (IQR), cm2 | |||
| Pretreatment | 126.0 (104.3 to 160.0) | 119.7 (99.0 to 138.6) | .14 |
| Posttreatment | 113.7 (93.2 to 150.0) | 129.0 (106.8 to 153.2) | .06 |
| Δ SM | −2.5 (−16.0 to 3.3) | 7.2 (−2.3 to 19.8) | <.001 |
| TAMA, median (IQR), cm2/m2 | |||
| Pretreatment | 44.4 (37.9 to 52.2) | 42.1 36.4 to 47.7) | .11 |
| Posttreatment | 42.0 (35.8 to 48.8) | 45.1 (40.3 to 50.8) | .049 |
| Δ TAMA | −1.23 (−5.84 to 1.18) | 2.32 (1.25 to 6.72) | <.001 |
| Sarcopenia, No. (%) | |||
| Pretreatment | 21 (36.8) | 63 (46.3) | .27 |
| Posttreatment | 30 (52.8) | 50 (36.8) | .054 |
| Sarcopenic obesity, No. (%) | |||
| Pretreatment | 5 (8.8) | 21 (15.4) | .26 |
| Posttreatment | 8 (14.0) | 17 (12.5) | .82 |
| Tumor size, mean (SD), mm | |||
| Pre-NT | 34.5 (14.2) | 32.1 (12.4) | .38 |
| Post-NT | 31.7 (21.2) | 23.3 (13.3) | <.001 |
Abbreviations: IQR, interquartile range; NT, neoadjuvant treatment; SM, skeletal muscle; TAMA, total abdominal muscle area; TAT, total adipose tissue area; VAT, visceral adipose tissue.
Multivariate Analysis
The diagnostic performance measures of the continuous variables that showed statistical significance at univariate analysis were investigated according to the receiver operating characteristic curve method. The area under the curve values and optimal cutoff points are reported in Table 4. The difference in TAMA between pre-NT and post-NT times and the preoperative size of the tumor for each patient (dichotomized on the calculated cutoff points) were determined in a logistic regression model for multivariate analysis. A Δ TAMA larger than 4 cm2/m2 (adjusted OR, 3.7; 95% CI, 1.5-9.6) and preoperative tumor size smaller than 30 mm (adjusted OR, 4.7; 95% CI, 2.4-37.1) were independently associated with the likelihood of resection (Table 4).
Table 4. Diagnostic Performance of Variables Independently Associated With Resection at Multivariate Analysis and Accuracy of Calculated Cutoff Points.
| Predictor | AUC | Optimal Cutoff Point | Sensitivity, % | Specificity, % | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Δ TAMA | 0.711 | +4 cm2/m2 | 63.2 | 75.9 | 3.7 (1.5-9.6) | .006 |
| Tumor size post-NT | 0.697 | 30 mm | 70.4 | 64.2 | 4.7 (2.4-37.1) | <.001 |
Abbreviations: AUC, area under the curve using receiver operating characteristic curve method; NT, neoadjuvant treatment; OR, odds ratio; TAMA, total abdominal muscle area.
Discussion
Recent and growing evidence indicates that body composition can predict the outcome of patients undergoing medical therapies or surgical procedures for PC and that these assessments are more accurate than gross changes in body weight or body mass index. Particularly, loss of muscle mass, which may be underestimated in normal-weight or obese patients, is predictive of a complicated postoperative course, reduced tolerance to adjuvant chemotherapy, and worse long-term survival.5,6,8,21,22,23 The combination of sarcopenia with visceral obesity appears to be even more deleterious for surgical morbidity and oncologic outcomes because the endocrine activity of visceral adipose tissue may be synergistic with cancer hormone–like mechanisms, promoting inflammation and protein wasting.24
Detrimental effects of oncologic treatments on body composition, muscle mass, and functional reserve have been described, although most of the evidence comes from cohorts of patients with advanced-stage disease.25
Unexpectedly, patients in our cohort experienced positive changes of body composition during NT, characterized by loss of adipose tissue, with no reduction in the lean compartment. Even though surgical resections after NT are longer and more challenging than up-front resections, postoperative complications do not appear to be increased.11,26 It is well known that excess visceral fat and decreased muscle mass are predictive of poor outcome after pancreatic operations.4,5,6,7,8 Therefore, it could be argued that advantageous modifications in the body compartments may be partially responsible for the favorable postoperative course in patients receiving NT.
In esophageal and gastric cancer, the progressive depletion of lean mass and adipose tissue wasting during NT have been associated with poor postoperative outcomes. Yet, in these patients the loss of muscle mass and adipose tissue is often due to the location of the tumor significantly affecting food intake independently from the response to chemotherapy.12,13 The indirect demonstration of this factor is that, when adequate nutritional support maintains lean mass during NT, the outcome may improve.27
A potential explanation of our results could be the reduction of cancer-related inflammatory factors secondary to chemotherapy. Decreased hormone-like activity of the tumor may translate into a downregulation in mechanisms implied in glucose-fat metabolism and protein wasting.28 Supporting this hypothesis, it has been observed that approximately 60% of patients with PC with new-onset diabetes experienced an improvement in glucose metabolism after radical resection. Furthermore, the disappearance of cancer-induced diabetes after an operation correlated with better oncologic outcomes.29
Because NT is becoming the standard of care for patients with BRPC and LAPC, we wanted to explore its association with body composition and clinical response. In this context, it may be proposed that screening changes in body composition during NT might be more useful than body weight monitoring to establish the early need for nutritional counseling or intervention. It has been shown that timely nutritional and metabolic support can increase adherence and improve response to chemotherapy.30
The main findings of the present study are that muscle gain during NT was independently associated with the likelihood of resection and patients who underwent surgical exploration but not resection had experienced a decrease in lean mass during NT. In addition, patients determined to be unsuitable for resection showed a higher prevalence of sarcopenia after NT, while patients who developed sarcopenia during NT showed the lowest likelihood of resection.
The effects of NT in BRPC and LAPC on body compartments have not been thoroughly investigated and, to our knowledge, this is the first study that addresses the issue in patients who are not suitable for resection and instead undergo NT. Another study reported that patients with initially resectable PC who did not receive NT showed depletion of both the muscular and adipose compartments and that those features were associated with shorter disease-free and overall survival rates.14 As the resection could be successfully performed in patients who had no disease progression in the NT time frame, we can assume that the muscular gain can be consistent with the response to NT or at least with an indolent biological behavior of the tumor. Preservation of lean mass may suggest that a cytoreductive effect in chemosensitive neoplasms may be associated with decreased tumor-related inflammatory activity and, consequently, protein sparing.30
The radiologic restaging after NT for BRPC and LAPC for determination of surgical resectability remains inaccurate, because, during computed tomographic scans, images at restaging the fibrosis resulting from a response to the tumor are barely distinguishable from viable tumor.11,31 Because of this inaccuracy, additional metrics for assessment of resectability after NT are needed.31 The different characteristics we found between patients in the resection and nonresection groups at surgical exploration could potentially provide additional information to help clinicians in reevaluation of PC-bearing patients after NT.
Limitations
This study has some limitations. First, its retrospective nature does not allow avoidance of the risk of selection bias. Second, we included patients who underwent various schemes of NT; however, we observed no differences in the resectability rate according to the type of chemotherapy used. Finally, related to the retrospective design, we lacked information on standardized nutritional counseling during NT.
Conclusions
Even with the study’s limitations, we observed that NT had an overall positive association with body composition in patients with PC. The determination of body compartments is an easy and quick tool that can be added, without the need for additional examinations, to the radiologic and clinical re-evaluation of patients after NT.
eTable. Changes in Body Compartments, Radiologic Response and Resection Rate According to the Neoadjuvant Regimen
eFigure. Likelihood of Resection According to the Presence of Sarcopenia
References
- 1.Ozola Zalite I, Zykus R, Francisco Gonzalez M, et al. Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: a systematic review. Pancreatology. 2015;15(1):19-24. [DOI] [PubMed] [Google Scholar]
- 2.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629-635. [DOI] [PubMed] [Google Scholar]
- 3.Carrara G, Pecorelli N, De Cobelli F, et al. Preoperative sarcopenia determinants in pancreatic cancer patients. Clin Nutr. 2017;36(6):1649-1653. [DOI] [PubMed] [Google Scholar]
- 4.Nishida Y, Kato Y, Kudo M, et al. Preoperative sarcopenia strongly influences the risk of postoperative pancreatic fistula formation after pancreaticoduodenectomy. J Gastrointest Surg. 2016;20(9):1586-1594. [DOI] [PubMed] [Google Scholar]
- 5.Amini N, Spolverato G, Gupta R, et al. Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: a new tool to assess sarcopenia. J Gastrointest Surg. 2015;19(9):1593-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16(8):1478-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandini M, Bernasconi DP, Fior D, et al. A high visceral adipose tissue-to-skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer. Nutrition. 2016;32(11-12):1231-1237. [DOI] [PubMed] [Google Scholar]
- 8.Pecorelli N, Carrara G, De Cobelli F, et al. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg. 2016;103(4):434-442. [DOI] [PubMed] [Google Scholar]
- 9.Bockhorn M, Uzunoglu FG, Adham M, et al. ; International Study Group of Pancreatic Surgery . Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014;155(6):977-988. [DOI] [PubMed] [Google Scholar]
- 10.Petrelli F, Coinu A, Borgonovo K, et al. ; Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente (GISCAD) . FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas. 2015;44(4):515-521. [DOI] [PubMed] [Google Scholar]
- 11.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261(1):12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ida S, Watanabe M, Karashima R, et al. Changes in body composition secondary to neoadjuvant chemotherapy for advanced esophageal cancer are related to the occurrence of postoperative complications after esophagectomy. Ann Surg Oncol. 2014;21(11):3675-3679. [DOI] [PubMed] [Google Scholar]
- 13.Awad S, Tan BH, Cui H, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr. 2012;31(1):74-77. [DOI] [PubMed] [Google Scholar]
- 14.Cooper AB, Slack R, Fogelman D, et al. Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol. 2015;22(7):2416-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang GS, Kim MJ, Ha HI, et al. Comparison of RECIST version 1.0 and 1.1 in assessment of tumor response by computed tomography in advanced gastric cancer. Chin J Cancer Res. 2013;25(6):689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 2004;97(6):2333-2338. [DOI] [PubMed] [Google Scholar]
- 17.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539-1547. [DOI] [PubMed] [Google Scholar]
- 18.Bassi C, Balzano G, Zerbi A, Ramera M. Pancreatic surgery in Italy: criteria to identify the hospital units and the tertiary referral centers entitled to perform it. Updates Surg. 2016;68(2):117-122. [DOI] [PubMed] [Google Scholar]
- 19.Rota M, Antolini L. Finding the optimal cut-point for gaussian and gamma distributed biomarkers. Comput Stat Data Anal. 2014;69(1):1-14. [Google Scholar]
- 20.Yamashita T, Yamashita K, Kamimura R. A stepwise AIC method for variable selection in linear regression. Commun Stat Theory Methods. 2007;36(13):2395-2403. [Google Scholar]
- 21.Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15(22):6973-6979. [DOI] [PubMed] [Google Scholar]
- 22.Tan BH, Brammer K, Randhawa N, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015;41(3):333-338. [DOI] [PubMed] [Google Scholar]
- 23.Rollins KE, Tewari N, Ackner A, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr. 2016;35(5):1103-1109. [DOI] [PubMed] [Google Scholar]
- 24.Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100-106. [DOI] [PubMed] [Google Scholar]
- 25.Choi Y, Oh DY, Kim TY, et al. Skeletal muscle depletion predicts the prognosis of patients with advanced pancreatic cancer undergoing palliative chemotherapy, independent of body mass index. PLoS One. 2015;10(10):e0139749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatterjee D, Katz MH, Rashid A, et al. Pancreatic intraepithelial neoplasia and histological changes in non-neoplastic pancreas associated with neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma. Histopathology. 2013;63(6):841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavazzi C, Colatruglio S, Valoriani F, et al. Impact of home enteral nutrition in malnourished patients with upper gastrointestinal cancer: a multicentre randomised clinical trial. Eur J Cancer. 2016;64:107-112. [DOI] [PubMed] [Google Scholar]
- 28.Johns N, Stephens NA, Fearon KC. Muscle wasting in cancer. Int J Biochem Cell Biol. 2013;45(10):2215-2229. [DOI] [PubMed] [Google Scholar]
- 29.Wu JM, Kuo TC, Yang CY, et al. Resolution of diabetes after pancreaticoduodenectomy in patients with and without pancreatic ductal cell adenocarcinoma. Ann Surg Oncol. 2013;20(1):242-249. [DOI] [PubMed] [Google Scholar]
- 30.Petruzzelli M, Wagner EF. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016;30(5):489-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118(23):5749-5756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Changes in Body Compartments, Radiologic Response and Resection Rate According to the Neoadjuvant Regimen
eFigure. Likelihood of Resection According to the Presence of Sarcopenia