Elevated plasma lipoprotein(a) [Lp(a)] is an independent risk factor for cardiovascular disease (CVD). Although Lp(a) consists of an apolipoprotein(a) molecule bound to an ApoB-containing LDL-like particle, most conventional lipid-lowering agents have minimal effects on Lp(a) level.
Bariatric surgery produces substantial weight loss and improves many CVD risk factors. Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG), the two most common bariatric procedures, reduce both plasma LDL-cholesterol and ApoB.1 However, there are scant data on the effects of bariatric surgery on Lp(a). This study aimed to prospectively investigate the effects of RYGB and SG on Lp(a).
Premenopausal women undergoing RYGB or SG at Bellevue Hospital (NY, NY) were recruited for an IRB-approved study. Patients taking antihyperglycemic agents or medications known to influence lipid levels were excluded. Subjects were examined within one month before surgery, and at one and six months post-operatively.
Pre-operative comparisons were performed with t-tests or Mann-Whitney U tests. Interval changes between surgical groups were compared with independent samples t-tests. Pearson’s correlations and multivariable linear regression were performed to assess associations of post-operative changes in Lp(a) and ApoB.
Sixty-two subjects underwent surgery and testing (n=27, RYGB; n=35, SG). There were no significant differences in pre-operative characteristics between groups, including Lp(a) (RYGB = 17[8 – 38] mg/dL, SG = 27 [17 – 43]mg/dL; NS). Post-operatively, both groups (RYGB vs SG, respectively) experienced improvements in body weight (−28.7% ± 3.8 vs –26.3% ± 5.3; p=0.04), hsCRP (−81.7% [−88.5% - −72.1] vs −76.7% −86.7% - −48.5]; NS) and HOMA-IR (−58.4% ± 22.8 vs −59.7% ± 23.4; NS). Notably, changes in Lp(a) and ApoB differed greatly by procedure (Figure 1.). Whereas Lp(a) was reduced at one month after both procedures, it was persistently reduced only after RYGB. In contrast, ApoB was significantly, but more modestly than Lp(a), reduced only at six months following RYGB.
Figure 1.
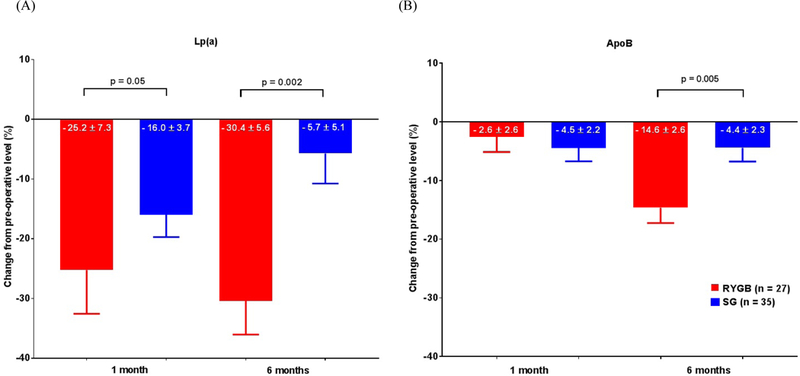
Percent change in (A) Lp(a) and (B) ApoB from pre-operative levels at 1 and 6 months post-operatively.
Data represented as mean ± SEM.
Changes in ApoB were predictive of changes in Lp(a) after SG, but not RYGB, in both correlative (RYGB, r=0.15, p=0.46; SG, r=0.48, p=0.003) and multivariable analyses correcting for age, pre-operative Lp(a), 6-month weight loss and changes in LDL-cholesterol (RYGB, β=−0.06, p=0.92; SG, β=−1.49, p<0.001).
In this study of obese women undergoing bariatric surgery, we found that only RYGB elicited sustained reductions in Lp(a) out to six months following surgery. Further, reductions in Lp(a) after RYGB were proportionally greater than, and not associated with, reductions in ApoB. Conversely, changes in Lp(a) and ApoB were strongly positively associated after SG.
Lp(a) levels are predominantly regulated by apo(a) biosynthesis, which is almost entirely determined by hepatic transcription of the APOA gene, rather than clearance or circulating concentrations of its other components.2 ApoB-lowering therapies, such as mipomersen, lower Lp(a) by 21–33%, presumably by reducing ApoB available for Lp(a) assembly.3 PCSK9 inhibition produces strongly correlated reductions in both Lp(a) (−32%) and ApoB (−56%).4 These and our data suggest that Lp(a) reductions following RYGB are not primarily ApoB-dependent.
We hypothesize that the RYGB-specific reductions in Lp(a) are related to differences in post-surgical anatomy and physiology between the two procedures that affect bile acid levels, and are distinct from mechanisms of existing therapies. RYGB creates a roux limb that bypasses the duodenum, which may explain increased circulating bile acids after RYGB.5 SG reduces stomach volume without bypassing gastrointestinal anatomy. The few small studies of the effect of SG on circulating bile acids in humans have demonstrated modest (relative to RYGB) and inconsistent results across varied durations of follow-up. 5 Some data point to an early post-operative increase in bile acids following SG, which is transient. Although the causes underlying a transient increase is uncertain, this observation supports our hypothesized mechanism related to bile acids to explain the differing changes in Lp(a) following SG and RYGB in this study, given that circulating bile acids suppress apo(a) transcription in mice via negative regulation by Farnesoid X receptor (FXR), a bile acid-activated nuclear receptor.2 This is complemented by human data showing that FXR agonist use in cholestatic patients reduced Lp(a).6
We acknowledge that our study is limited by a homogeneous population that may limit generalizability. Additionally, a minority of our subjects exhibited pathologically high Lp(a) pre-operatively. Nonetheless, the degree of reduction in Lp(a) was comparable between RYGB subjects with Lp(a) greater and less than 30mg/dL.
In summary, in the first prospective comparison of Lp(a) and ApoB in the two most common bariatric procedures, we demonstrate procedure-dependent effects on Lp(a). Future translational studies measuring bile acids and Lp(a) in humans undergoing bariatric surgery and direct tissue FXR and apo(a) expression assessments in animal models will begin to definitively elucidate the mechanism underlying RYGB-specific reductions in Lp(a) and may provide insight into novel therapies for this CVD risk factor.
Acknowledgments
Sources of funding: This work was supported by AHA 14 CRP18850107. Dr. Heffron was supported in part by T32 HL 098129 and KL2 TR001446.
Footnotes
Conflicts of Interest/Financial disclosures: None
References
- 1.Padilla N, Maraninchi M, Beliard S, et al. Effects of bariatric surgery on hepatic and intestinal lipoprotein particle metabolism in obese, nondiabetic humans - significance. Arterioscler Thromb Vasc Biol 2014;34:2330–7. [DOI] [PubMed] [Google Scholar]
- 2.Chennamsetty I, Claudel T, Kostner KM. et al. Farnesoid X receptor represses hepatic human APOA gene expression. J Clin Invest 2011;121:3724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norata GD, Ballantyne CM, Catapano AL. New therapeutic principles in dyslipidaemia: focus on LDL and Lp(a) lowering drugs. Eur Heart J 2013;34:1783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai NR, Kohli P, Giugliano RP, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation 2013;128:962–9. [DOI] [PubMed] [Google Scholar]
- 5.Albaugh VL, Banan B, Ajouz H, Abumrad NN, Flynn CR. Bile acids and bariatric surgery. Mol Aspects Med 2017;56:75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calmarza P, Bajador E, Lapresta C, Garcia Castanon S, de Castro I, Civeira F. Efecto de la obstrucción de la vía biliar en la concentración de lipoproteína(a). Clínica E Investig En Arterioscler 2014;26:218–23. [DOI] [PubMed] [Google Scholar]


