Abstract
Volumetric muscle loss (VML) is a loss of skeletal muscle that results in a sustained impairment of function and is often accompanied by physical deformity. To address the need for more innovative repair options, our laboratory has developed scaffold-free, multiphasic tissue-engineered skeletal muscle units (SMUs) to treat VML injuries. In our previous work, using the concept of the “body as a bioreactor”, we have shown that implantation promotes the maturation of our SMUs beyond what is possible in vitro. Thus, in this study we sought to better understand the effect of implantation on the maturation of our SMUs, including the effects of implantation on SMU force production and cellular remodeling. We used an ectopic implantation so that we could more easily dissect the implanted tissues post-recovery and measure the force contribution of the SMU alone and compare it to pre-implantation values. This study also aimed to scale up the size of our SMUs to enable the replacement of larger volumes of muscle in our future VML studies. Overall, implantation resulted in extensive maturation of the SMUs, as characterized by an increase in force production, substantial integration with native tissue, innervation, vascularization, and the development of structural organization similar to native tissue.
Keywords: satellite cell, scaffold-free, tissue engineering, skeletal muscle, implantation
Lay Summary
To address the need for more innovative repair options for severe muscle injuries, our laboratory has developed a lab-grown, living muscle tissue for implantation. In our previous work, we have shown that implantation of our engineered tissue promotes its maturation beyond what is possible in the lab. Thus, in this study we sought to scale-up the size of our engineered muscle and to better understand the effect of implantation on the maturation of our engineered muscle, including the effects on the force production. Overall, implantation resulted in extensive maturation, including an increase in force production, innervation, and vascularization.
In an effort to make this technology more clinically relevant, future work will involve the development and implantation of larger quantities of engineered tissue to replace a larger percentage of the lost muscle. We believe this will help restore muscle function to that of pre-injury levels.
Introduction
Following skeletal muscle injury, skeletal muscle stem cells, known as satellite cells, become activated to assist in the repair of damaged muscle. Satellite cells proliferate and differentiate into myoblasts which fuse to form multinucleated myofibers as part of the normal physiological response to both trauma and microinjury [1]. However, following severe injury to skeletal muscle, including acute trauma or post-operative trauma involving more than 30% tissue loss, patients experience volumetric muscle loss (VML), which is the acute traumatic loss of skeletal muscle that overwhelms the body’s natural repair mechanisms and results in a loss of muscle function that is often accompanied by physical deformity [2, 3]. In these events, the myogenic potential of satellite cells is insufficient to fully restore damaged skeletal muscle force production and such injuries require surgical intervention to regain significant function [4].
Due to the large volume of skeletal muscle lost at the injury site, current VML treatments focus on replacing the lost tissue via reflected muscle grafts or autologous muscle transfer. These treatments, however, are limited by donor site morbidity and tissue availability [2]. In response, researchers are developing new methods to treat VML and combat the limitations of traditional surgical approaches. Specifically, our group has developed scaffold-free, multi-phasic, tissue-engineered skeletal muscle units (SMUs) composed of engineered skeletal muscle constructs fused to engineered bone-tendon anchors. In a previous study, the efficacy of our SMUs was tested in a rat model with an acute 30% VML deficit in the tibialis anterior (TA) muscle [5]. We replaced approximately 10% of the lost TA muscle volume with an SMU and allowed 28 days for recovery. After 28 days in vivo, the SMUs demonstrated structural advancement towards a native muscle phenotype through the formation of distinct and uniaxially aligned muscle fibers encased in an extensive extracellular matrix, as well as the presence of neovascularization and neuromuscular junctions. Furthermore, VMLs repaired with SMUs demonstrated a 24% ± 3.0% maximum force production deficit while the acute VML group suffered a significantly higher 38% ± 3.5% force production deficit compared to uninjured controls [5].
Overall, this study aimed to better understand the maturation process that occurs within the SMU during implantation, including the effects of implantation on SMU force production and cellular remodeling. While our previous study showed promising force regeneration of the entire TA muscle, we were unable to isolate and measure SMU force production alone. Following 28 days, our SMUs were thoroughly integrated into the host TA muscles, making separation and force testing of the SMU alone infeasible without causing damage to the construct itself. Thus, one purpose of this study was to allow our SMUs to mature in an ectopic site adjacent to the host TA and perform in situ force testing of the construct alone after a 28-day recovery. We hypothesized that the ectopic site would allow us to more easily dissect and recover our construct tissue to conduct force testing. Additionally, we hypothesized that the SMUs would produce significantly higher forces after remodeling for 28 days in vivo when compared to the pre-implantation forces, as the result of host-driven construct maturation. Furthermore, this study also aimed to scale-up the size of our SMUs. Previous results showed that replacing 10% of lost tissue significantly increased the force restoration of damaged TAs [5]. We suspect that the scale-up method described herein can be used to fabricate constructs of larger sizes in order to replace a larger percentage of lost tissue in future VML studies.
Furthermore, we hypothesized that host cells infiltrate the SMU to promote vascularization, innervation, and myofiber development within the site of implantation. In our previous study, SMUs were implanted into a volumetric muscle loss injury site. We wanted to observe the degree of vascularization, innervation, and construct development that occurs in the absence of a VML injury when the SMU is implanted ectopically. Overall, this study aimed to better understand the effect of an ectopic implantation on the development and maturation of an engineered tissue.
Methods
Animal Model and Animal Care
Cells used in this study were obtained from the soleus muscles and bone marrow from 120 to 220g female Fischer 344 rats, obtained from Charles River Laboratories, Inc. (Wilmington, MA) and Harlan Laboratories (Haslett, MI). All animals were acclimated to colony conditions for at least 1 week prior to any procedure. Animals were fed Purina Rodent Chow 5001 laboratory chow and water ad libitum. These animals were used for either cell harvest or implantations. The harvested tissues were used as an allogenic cell source of muscle precursor cells and bone marrow-derived stem cells for production of muscle and bone-tendon tissues that were later implanted into the host animals. All surgical procedures were performed in an aseptic environment, with animals in a deep plane of anesthesia induced by isoflurane or intraperitoneal injections of sodium pentobarbital (65 mg/kg). Supplemental doses of pentobarbital were administered as required to maintain an adequate depth of anesthesia. All animal care and animal surgery procedures were in accordance with The Guide for Care and Use of Laboratory Animals [6] and the protocol was approved by the University Committee for the Use and Care of Animals.
SMU Fabrication
Cells were isolated from the dissected tissues and used for SMU fabrication as described previously [5, 7-9]. Briefly, SMUs were fabricated on 100mm plates layered with 5mL of Sylgard substrate (type 184 silicon elastomer; Dow ChemicalCorp., Midland, MI) and coated with laminin protein (NaturalMouse Laminin, cat. No. 23017-015; Gibco BRL, Carlsbad, CA) at a concentration of 1 μg/cm2. Following enzymatic digestion of tissues, the muscle cell isolates were plated at a seeding density of 1.5 × 106 cells per 100mm plate. The cells were sustained on Muscle Growth Media (MGM) until the cells became confluent and began fusing to form myotubes, approximately 6-7 days after seeding. MGM is composed of 60% F-12 Kaighn’s modification nutrient mixture (Cat. No. 21127-022; Gibco BRL), 24% Dulbecco’s modified Eagle’s medium (DMEM; Cat. No. 11995-065; Gibco BRL), 15% fetal bovine serum (FBS; Cat. No. 10437-028; Gibco BRL), and 1% antibiotic-antimycotic (ABAM; Cat. No. 15240-062; Gibco BRL) supplemented with 2.4 ng/mL basic fibroblast growth factor (Cat. No. 100-18B; Peprotech, Rocky Hill. NJ). On day 7, once the cells had reached confluency and began to form myotubes, the cells were switched to Muscle Differentiation Media (MDM) to promote further differentiation and the formation of integrated myotube networks (Figure 1A). MDM is composed of 70% M199 (Cat. No. 11150-059; Gibco BRL), 23% DMEM, 6% FBS, and 1% ABAM supplemented with 1 μL/mL insulin-transferrin selenium-X (Cat. No. I1884; Sigma-Aldrich, St. Louis, MO) and 14.4 μg/mL ascorbic acid 2-phosphate. Prior to spontaneous delamination of the monolayers, 5mm long engineered bone-tendon anchors were pinned to the confluent monolayer 5cm apart, approximately 14 days after initial seeding. Engineered bone-tendon anchors were fabricated as described previously, without modification [5, 7, 10-13]. Continued development of the monolayer between days 14-20 resulted in its spontaneous delamination from the plate around the bone-tendon anchors to form a 3D cylindrical construct. At this point, the final constructs measured 5cm long and approximately 2-3mm wide (Figure 1B). These SMUs were allowed to fuse overnight before in vitro force testing was conducted. After force testing, the bone anchors were re-pinned 3.5cm apart and the SMUs were allowed to sit undisturbed for at least 24hrs in order to shorten the SMU for implantation (Figure 1C).
Figure 1. Fabrication of Skeletal Muscle Units (SMUs).

(A) Over time in culture, the cells grown on 100mm tissue culture plates formed extensive myotube networks, similar to those in our previous study. (B) Engineered bone-tendon anchors were pinned to the skeletal muscle monolayer 5cm apart after the cells reached confluence. Prolonged development of the monolayer resulted in its spontaneous delamination around the bone-tendon anchors to form a multiphasic, three-dimensional SMU that was 5cm in length. (C) After biomechanical testing, the distant between constraint pins was reduced to 3.5cm to produce an SMU that better matched the anatomy of the host animal.
Pre-Implantation and Post-Implantation SMU Force Production Measurements
Prior to implantation, biomechanical testing was conducted on individual SMUs approximately 24 hours after their spontaneous delamination into 3D constructs. The protocol for measuring contractility of engineered muscle constructs has been described previously [5, 14, 15]. Briefly, the pin on one end of the construct was released from the Sylgard substrate and attached to an optical force transducer with canning wax. For field stimulation of the entire construct, platinum wire electrodes were placed longitudinally along each side of the SMU. The temperature was maintained at 37°C throughout the duration of testing using a heated aluminum platform. Passive baseline force was measured as the average baseline passive force preceding the onset of stimulation. Maximum tetanic force was determined using a 1s train of 5ms pulses at 90 mA and 10, 20, 40, 60, and 80 Hz. Data for each peak tetanic force was recorded and subsequently analyzed using a custom program written on LabVIEW 2013 (National Instruments, Austin, TX).
Surgical Implantation Procedures
Each host rat was anesthetized using isoflurane. An incision was made along the left lower hindlimb exposing the TA muscle. A single SMU (Figure 2) was then placed ectopically along the host’s TA, with each of the bone-tendon ends attached to the host’s TA tendons with 7-0 prolene suture. The peroneal nerve distal to the innervation of the extensor digitorum longus and its associated vasculature was transected and re-routed to the mid-belly of the construct and sutured in place with 9-0 prolene suture. The surgical site was then closed with surgical staples and Carprofen was administered following each procedure at a dose of 5mg/kg every 12hrs for 48hrs post-surgery. The staples were removed after 10 days. A total of n=11 SMUs were fabricated and implanted into host rats for 28 days in vivo.
Figure 2. Ectopic Implantation Site.

A single SMU (black arrow) was placed ectopically along the lateral side of the host’s TA muscle, with each of its bone-tendon ends attached to the host’s TA tendons with 7-0 prolene suture. A branch of the peroneal nerve and its associated vasculature was transected and routed to the mid-belly of the construct and sutured in place with 9-0 prolene suture. Re-routing the nerve and associated vasculature was performed to accelerate the innervation and vascularization of the implanted tissue.
Post-Implantation SMU Force Production Measurements
Following 28 days of recovery, contractile properties were measured as described above. Briefly, the host rats were anesthetized with an injection of sodium pentobarbital (65 mg/kg) with supplemental injections given to maintain an adequate level of anesthesia throughout the procedures. In anesthetized rats, the SMU was isolated from surrounding tissue using great care not to damage nerve or blood vessels during the dissection. Despite the ectopic implantation site of our SMUs, some of the constructs became so well integrated with the host tissue that dissection of the construct alone was not possible without damaging the construct. In these instances, the SMUs were not biomechanically tested, but the native TA and well-integrated SMU were frozen for histology. While force measurements of SMUs were being conducted, TA muscles and constructs were fully dissected, and deeply anesthetized rats were euthanized by administration of a bilateral pneumothorax. The TA muscles were trimmed of their tendons, blotted, and weighed. Immediately after, tissues were coated in tissue-freezing medium (Triangle Biomedical Sciences, Durham, NC), frozen in dry ice-chilled isopentane, and stored at −80°C until histological analysis was conducted.
Histological Analyses of SMUs
Following measures of mechanical function, the frozen samples of both the dissected constructs and TA muscles with well-integrated constructs were sectioned at 12mm, mounted on Superfrost Plus microscopy slides, and stained for histological analysis. Sections were stained to observe general morphology with hematoxylin and eosin (H&E). For immunohistochemical analysis, frozen sections were fixed in −20°C methanol for 10 min and subsequently rinsed with DPBS at room temperature. The sections were submerged for 15 minutes in 0.1% Triton X-100 (Sigma-Aldrich) in DPBS (PBST) and blocked with PBST containing 3% bovine serum albumin (PBST-S, cat. No. A2153-10g; Sigma-Aldrich) at room temperature for 30 minutes. The sections were then incubated overnight with primary antibodies diluted in PBST-S at 4°C. Immunofluorescent staining with specific antibodies was performed to detect the presence of myosin heavy chain (MF-20 mouse monoclonal antibody, 1:20 dilution, cat. No. ALD-58, Developmental Studies Hybridoma Bank), laminin (rabbit polyclonal antibody, 1:200 dilution, cat. No. ab7463; Abcam), pan-axonal neurofilament (mouse monoclonal antibody, 1:300 dilution, cat. No. SMI-312R; Covance, Inc.), CD-31 (mouse monoclonal antibody, 1:100 dilution, cat. No. ab24590; Abcam), α-bungarotoxin (1:20 dilution, cat. No. B1601; Molecular Probes) and Pax7 (mouse monoclonal antibody, 1:200 dilution, cat. No. ab199010; Abcam). Following three washes in PBST, the sections were incubated in 1:500 dilutions of Alexa Fluor anti-mouse or anti-rabbit antibodies (Life Technologies, Carlsbad, CA) for 3hr at room temperature. Following three washes in PBST, the sections were fixed in Prolong Gold with DAPI and cover-slipped. The sections were examined and photographed with an Olympus BX-51 microscope and cross-sections of the samples were analyzed using the Image J software package.
Statistical Analysis
All values described in this study are presented as mean ± standard error, unless otherwise noted. Measurements of significant differences between pre- and post-implantation forces were performed using a paired T-test. Differences were considered significant at p < 0.05.
Results
Scaled-Up SMUs
SMUs were successfully scaled-up through fabrication on 100mm tissue culture plates, as opposed to the 60mm plates used in our previous study. Prior to 3D construct formation, muscle monolayers showed advanced myotube networking characteristic of our past studies (Figure 1A). After spontaneous delamination of the monolayer and subsequent 3D construct formation, the dimensions of the SMUs fabricated on 100mm plates were 5cm long and 2-3mm wide (Figure 1B), as opposed to the constructs fabricated in our previous study which were 3cm long and approximately 1-2mm wide. Assuming the SMUs are cylindrical in shape, this size difference amounts to more than 350% increase in volume, on average. By subsequently reducing the distance between constraint pins after biomechanical testing to 3.5cm, we were able to successfully manipulate the length of the SMUs so that they matched the anatomy of the host animal. These resultant SMUs were approximately 3mm wide (Figure 1C) and weighed 395.2 ± 2.6 mg on average (n=14 SMUs). Despite the increase in size, the SMUs grown on 100mm plates generated forces similar, on average, to SMUs grown on 60 mm plates. Typically, the constructs fabricated on 60mm plates generate a maximum tetanic force between 100 and 300μN [5, 7]. In this study, pre-implantation forces measured 152.2 ± 20.65μN (n=14 SMUs).
Pre- and Post-Implantation SMU Force Data
Before implantation, SMUs were forced tested in vitro to establish their baseline force production capabilities. Pre-implantation force production was measured for n=14 SMUs and the average maximum tetanic force was 152.2 ± 20.65μN. However, despite the ectopic implantation site of our SMUs, some of the constructs became so well integrated with the host tissue that dissection of the construct alone was not possible without damaging the construct. Thus, only n=4 SMUs were able to be measured for force production post-implantation, and the average pre-implantation tetanic force of these four SMUs was 232.3 ± 28.4μN. After 28 days in vivo, these four SMUs were carefully dissected and isolated from host muscle, and tetanic forces were measured to determine the functional maturation of SMUs. After development in vivo, these SMUs produced an average force of 1011.9 ± 376.2 μN (Figure 3A). A paired T-test comparing pre- and post-implantation forces for individual SMUs did not reveal significant differences in force production (p=0.1221); however, it should be noted that all SMUs experienced an increase in maximum force production as the result of implantation (Figure 3B).
Figure 3. The Effect of Implantation on SMU Force Production.

A total of n=4 constructs were able to be force tested both pre- and post-implantation. (A) The average pre-implantation force production for these constructs was 232.3 ± 28.4μN while the average post-implantation force production was 1011.9 ± 376.2 μN. While this difference was not statistically significant (p=0.1221), each of these constructs did experience an increase in maximum tetanic force production as the result of implantation (B). Bars indicate mean ± standard error.
Development of Myotube Structure after 28 Days in Vivo
Upon explant, SMUs were observed within the ectopic surgical site, suggesting that the construct was largely intact after 28 days in vivo. However, in some instances, the construct was so well integrated with the host tissue that it could not be visualized through gross observation alone (Figure 4A). In these cases, the sutures that were used to attach the construct to the host tissue were used as landmarks to determine the location of the implanted SMU. This high degree of construct assimilation was further noted by the integration between the re-routed nerve and the SMU, which was obvious through gross observation (Figures 4B-C). H&E staining of the implantation site revealed naïve myotube-like structures within the construct region (Figure 5A-B). This structure was also visualized through immunostaining for myosin heavy chain (MF20) and laminin, which further verified that these structures were skeletal muscle fibers (Figure 5C). On average, there were 232 ± 23 fibers in each graft site. These myofibers were notably smaller than the adjacent native fibers; however, they were characterized by an otherwise nearly identical structure of myosin heavy chain surrounded by laminin protein. The average cross-sectional area of these smaller myofibers was 113.7 ± 2.6 μm2 which is on par with the myofiber sizes observed in our previous study (118 ± 6 μm2) [5].
Figure 4. SMU Integration with Host Tissue.
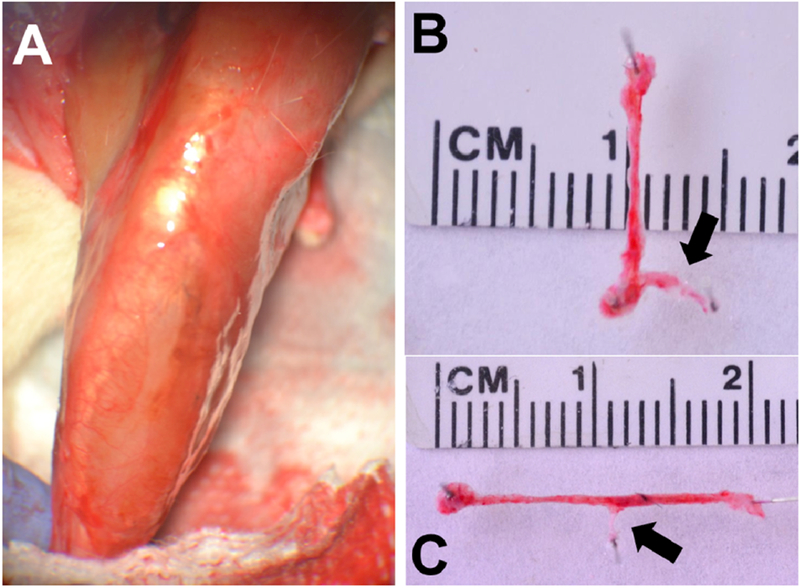
(A) After 28-days in vivo the SMU became so well integrated with the host tissue that the construct was nearly indistinguishable through gross observation. A lack of scar tissue at the implantation site was also noted. In some instances, the SMU was so well integrated with the host tissue that force production measurements of the SMU alone were not possible. (B, C) In other instances, the SMU and the accompanying neurovascular bundle was able to be dissected from the surrounding tissue. The branch of the peroneal nerve (black arrows) was well integrated with our implanted tissue.
Figure 5. Development of Myotube Structure after 28 Days in Vivo.

(A,B) H&E staining of a cross-section of the implantation site revealed hyper-cellularity, vascularization, and small myofibers (black arrows) within the site of implantation. (C) Immunostaining for myosin heavy chain (MF20, red) and laminin (green) confirmed that these structures (white outline) were in fact myofibers and that they had developed a nearly identical to that of the native myofibers.
Vascularization, Innervation, and Satellite Cells within SMUs
After 28 days in vivo the implanted tissues underwent extensive cellular remodeling, including the infiltration of native nerve and vasculature. Vascularization is the primary factor governing long-term viability of our implanted tissues, as nutrient delivery to the construct tissue is vital in preventing necrosis. Thus, viability of the SMUs can largely be attributed to the presence of an extensive vascular network within the construct (Figure 6A). Additional immunostaining of the explanted SMU revealed substantial innervation, along with the presence of neuromuscular junctions in the location where the peroneal nerve had been re-routed (Figure 5B-C). Re-innervation of our constructs was also evidenced by force production during contractile testing. In fact, one explanted SMU produced 580.0 μN of force after direct electrical stimulation of the re-routed peroneal nerve. Additionally, we conducted immunostaining for Pax7+ satellite cells in the explanted construct to determine if the tissue contained a population of these myogenic precursor cells (Figure 7B-C). The presence of satellite cells within the construct region further emphasizes implantation-driven maturation of SMUs, as satellite cells were found in greater number than what was found in in vitro constructs. Although we have noted the presence of satellite cells in our constructs in vitro (Figure 7A), the exact origin of the satellite cells present in the construct at the time of explant could not be determined in this study.
Figure 6. Vascularization and Innervation of the SMU after 28 Days in Vivo.

(A) Immunostaining for vasculature (CD31, red), cell nuclei (DAPI, blue), and collagen (green) in a longitudinal section of the explanted tissue revealed the presence of an extensive vascular network within the construct. (B) Immunostaining for pan-axonal neurofilament (green) in a cross-section of the peroneal nerve (white arrow) and a longitudinal section of an explanted SMU revealed that the construct was well innervated and formed (C) neuromuscular junctions which were visualized through staining with α-bungarotoxin (red), pan-axonal neurofilament (green), and DAPI (blue).
Figure 7. Satellite Cells within SMUs.

(A) Satellite cells are present in SMUs prior to implantation, as noted by the positive Pax7 staining (red) which signifies the presence of a satellite cell (white arrow) surrounded by cell nuclei (DAPI, blue) and collagen (green). (B, C) Pax7+ satellite cells (red, white arrows), were found in greater numbers in both (B) cross-sectional and (C) longitudinal sections of explanted constructs (collagen, green).
Discussion
Overall, the study aimed to utilize the concept of the “body as a bioreactor” and observe the effects of an ectopic implantation on our tissue-engineered skeletal muscle units (SMUs). This idea has been implemented before to promote the maturation of engineered bone [16–20], trachea [21, 22], and cardiac tissue [23], among others. In these studies and others, implantation has been shown to promote vascularization, innervation, and progenitor cell recruitment. In this study specifically, we aimed to scale up the size of our tissue-engineered SMUs, elucidate the effect of implantation on the force production and structure of individual SMUs, and assess the cell migration and cellular remodeling that occurs within the site of implantation.
Notably, although the constructs were fabricated on larger 100mm plates instead of the 60mm plates used in our previous study, they produced pre-implantation forces that were on par with constructs fabricated on the smaller plates. We suspect that this is because although the constructs were 2cm longer, they were not necessarily thicker, and an increased number of sarcomeres in parallel (greater cross-sectional area) is what contributes to increased force production. To date, our lab has successfully modified our fabrication protocol to produce constructs of even greater volumes. These constructs will be used in our future VML studies to replace an even larger percentage of the lost tissue. We suspect that replacing a larger volume of muscle in the future will result in a functional recovery beyond what was observed in our previous VML study.
Histologically, we noted that the construct fibers (as identified by suture landmarks) were smaller than the adjacent native fibers. We suspect that the small fiber size is the result of immaturity and that prolonged implantation would result in the hypertrophy of these construct myofibers. Notably, these fibers, albeit smaller than native, possessed nearly identical structure and were characterized by the presence of myosin heavy chain within the fiber and a laminin-rich extracellular matrix surrounding each fiber. Furthermore, the host-driven cellular remodeling was apparent with the infiltration of nerve and vasculature throughout the construct. The development of vasculature, neuromuscular junctions, and immature myotubes within the SMUs after 28 days in vivo suggests that the construct was becoming well-integrated with surrounding host tissue. After surgery, the inflammation and wound healing processes associated with the surgical implantation likely promoted angiogenesis within the SMU; the increased blood perfusion within the construct tissue would have exposed the SMU to nutrients, growth factors, and hormones that promote tissue development beyond what was possible in vitro. Similarly, neurofilament and neuromuscular junction staining showed host-driven innervation within ectopic SMUs. The presence of neurofilament and neuromuscular junctions within the construct tissue likely contributed to the increased force production of the SMUs, as innervation is necessary for proper skeletal muscle development and force production.
Biomechanically, implantation increased the force production of all SMUs that were able to be dissected from surrounding tissue without damage. This increase in force generating capacity of our SMUs suggests that the host’s physiological environment promotes functional advancement within the implanted tissue. However, there was no statistically significant difference between the pre- and postimplantation force production, potentially due to a combination of small n-number and high standard deviation. We suspect that prolonged implantation would result in an even greater increase in force production due to hypertrophy of the construct myofibers; however, prolonged implantation would likely make it even more difficult to isolate the construct from surrounding tissue. Even after 28 days in vivo, many of the constructs were so well integrated with surrounding tissue that they could not be dissected without damage to the construct. This degree of integration is further emphasized by the clear junction between the re-routed host nerve branch and the SMU, which allowed us to directly stimulate the nerve to produce a muscle contraction.
Conclusion
In conclusion, our SMUs experienced a significant advancement in phenotype after 28 days in vivo, as evidenced by extensive innervation, vascularization, structural maturation, and an increase in force production. It should be noted that while force production was not significantly different after the 28-day implantation, the environment was sufficient to maintain the structure and function of highly metabolic skeletal muscle tissue. In an effort to determine the source of the regenerative cells and the relative roles of both the implanted cells and host cells in the regeneration process, our lab has recently developed a model to track cell migration and cell origin in tissue implantation studies. We have created a ubiquitous tdTomato rat for use in conjunction with GFP markers to track the origins and migration of cells involved in the regeneration process [24]. Future studies will utilize this combination of fluorescent markers to better determine the origin of the cells involved in the cellular remodeling process. Furthermore, modifications to our SMU fabrication protocol will be made to replace an even larger percentage of lost muscle tissue in our future VML studies.
Acknowledgements
The authors would like to acknowledge the support of the NIH/NIAMS R01 grant: 1R01AR067744-01, as well as NIH Research Supplement to Promote Diversity in Health Related Research: 3R01AR067744-02W1. The authors have no conflicts of interest to disclose.
References
- 1.Zouraq F, et al. , Skeletal Muscle Regeneration for Clinical Application. Regenerative Medicine and Tissue Engineering, 2013: p. 679–712. [Google Scholar]
- 2.Mertens JP, et al. , Engineering muscle constructs for the creation of functional engineered musculoskeletal tissue. Regen Med, 2014. 9(1): p. 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grogan BF, Hsu JR, and Consortium STR, Volumetric muscle loss. J Am Acad Orthop Surg, 2011. 19 Suppl 1: p. S35–7. [DOI] [PubMed] [Google Scholar]
- 4.Tedesco FS, et al. , Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest, 2010. 120(1): p. 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.VanDusen KW, et al. , Engineered skeletal muscle units for repair of volumetric muscle loss in the tibialis anterior muscle of a rat. Tissue Eng Part A, 2014. 20(21-22): p. 2920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Council NR, Guide for the Care and Use of Laboratory Animals 2011, The National Academies Press; Washington D.C. [PubMed] [Google Scholar]
- 7.Syverud BC, VanDusen KW, and Larkin LM, Effects of Dexamethasone on Satellite Cells and Tissue Engineered Skeletal Muscle Units. Tissue Eng Part A, 2016. 22(5–6): p. 480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams ML, et al. , Effect of implantation on engineered skeletal muscle constructs. J Tissue Eng Regen Med, 2013. 7(6): p. 434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larkin LM, et al. , Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng, 2006. 12(11): p. 3149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smietana MJ, et al. , The effect of implantation on scaffoldless three-dimensional engineered bone constructs. In Vitro Cell Dev Biol Anim, 2009. 45(9): p. 512–22. [DOI] [PubMed] [Google Scholar]
- 11.Mahalingam VD, et al. , Fresh versus frozen engineered bone-ligament-bone grafts for sheep anterior cruciate ligament repair. Tissue Eng Part C Methods, 2015. 21(6): p. 548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florida SE, et al. , In vivo structural and cellular remodeling of engineered bone-ligament-bone constructs used for anterior cruciate ligament reconstruction in sheep. Connect Tissue Res, 2016: p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J, et al. , Morphological and functional characteristics of three-dimensional engineered bone-ligament-bone constructs following implantation. J Biomech Eng, 2009. 131(10): p.101017. [DOI] [PubMed] [Google Scholar]
- 14.Larkin LM, et al. , Effect of age and neurovascular grafting on the mechanical function of medial gastrocnemius muscles of Fischer 344 rats. J Gerontol A Biol Sci Med Sci, 1998. 53(4): p. B252–8. [DOI] [PubMed] [Google Scholar]
- 15. Dennis RG and Kosnik PE, Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol Anim, 2000. 36(5): p. 327–35. [DOI] [PubMed] [Google Scholar]
- 16.Service RF, Tissue engineering. Technique uses body as ‘bioreactor’ to grow new bone. Science, 2005. 309(5735): p. 683. [DOI] [PubMed] [Google Scholar]
- 17.Stevens MM, et al. , In vivo engineering of organs: the bone bioreactor. Proc Natl Acad Sci U S A, 2005. 102(32): p. 11450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holt GE, et al. , Evolution of an in vivo bioreactor. J Orthop Res, 2005. 23(4): p. 916–23. [DOI] [PubMed] [Google Scholar]
- 19.Warnke PH, et al. , Growth and transplantation of a custom vascularised bone graft in a man. Lancet, 2004. 364(9436): p. 766–70. [DOI] [PubMed] [Google Scholar]
- 20.Warnke PH, et al. , Man as living bioreactor: fate of an exogenously prepared customized tissue-engineered mandible. Biomaterials, 2006. 27(17): p. 3163–7. [DOI] [PubMed] [Google Scholar]
- 21.Jana T, et al. , The body as a living bioreactor: a feasibility study of pedicle flaps for tracheal transplantation. Eur Arch Otorhinolaryngol, 2013. 270(1): p. 181–6. [DOI] [PubMed] [Google Scholar]
- 22.Laurance J, British boy receives trachea transplant built with his own stem cells. BMJ, 2010. 340:p.c1633. [DOI] [PubMed] [Google Scholar]
- 23.Dvir T, et al. , Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc Natl Acad Sci U S A, 2009. 106(35): p. 14990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syverud BC, et al. , A Transgenic tdTomato Rat for Cell Migration and Tissue Engineering Applications. Tissue Eng Part C Methods, 2018. 24(5): p. 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]


