Abstract
TRC105 is a chimeric monoclonal antibody that targets CD105 (endoglin). Heavily pretreated patients with metastatic urothelial carcinoma received TRC105 at 15 mg/m2 every 2 weeks on a 28-day cycle. Treatment was not associated with significant toxicities, but did not improve 6-month progression-free survival. Exploratory analyses suggest interplay between immunosuppressive subsets and TRC105, which warrants further study.
Background:
In this trial we assessed the efficacy and tolerability of TRC105, a chimeric monoclonal antibody that targets CD105 (endoglin) in patients with advanced, previously treated urothelial carcinoma (UC).
Patients and Methods:
Patients received TRC105 15 mg/kg every 2 weeks on days 1 and 15 of each 28-day cycle. The primary end point was progression-free survival (PFS) at 6 months. Secondary end points included safety, toxicity, and overall survival (OS). CD105 expression was evaluated using immunohistochemistry (IHC) in a separate cohort of 50 UC patients. Biomarker studies included immune subsets, circulating tumor cells (CTCs), circulating endothelial cells (CECs), circulating endothelial progenitor cells (CEPs), and osteopontin.
Results:
Of 13 patients enrolled, 12 were evaluable for OS and PFS. The 3-month PFS probability was 18.2% (median PFS, 1.9 months [95% confidence interval (CI), 1.8–2.1 months). This met the criterion for ending accrual on the basis of the 2-stage design. Median OS was 8.3 months (95% CI, 3.3–17.0 months). IHC for CD105 scores was not associated with T stage (P = .26) or presence of lymph nodes (P = .64). Baseline levels of regulatory T and B cells, CEPs, and changes in CEC level after TRC105 exhibited trends toward an association with PFS or OS. CTCs pre- and post-TRC105 were detected in 4 of 4 patients.
Conclusion:
Although TRC105 was well tolerated, it did not improve 6-month PFS in heavily pretreated patients with advanced UC. CD105 staining was present in 50% of UC tumors at different intensities. Our observations on the pharmacodynamic significance of immune subsets, CECs, and CTCs warrant further study.
Keywords: Advanced urothelial cancer, Antiangiogenic therapy, CD105, Immune subsets, Metastatic urothelial cancer, Urothelial cancer
Introduction
Urothelial carcinoma (UC), a common malignancy worldwide,1 is sensitive to chemotherapy. In the first-line metastatic setting, responses to standard combination cisplatin therapy are approximately 50%2 and approximately 30% to 36% for noncisplatin combination therapy in cisplatin-unfit patients.3 However, duration of response is short, and median survival in patients with metastatic disease is approximately 14 months.2 Currently there are no effective therapies for patients whose disease relapses after first-line combination chemotherapy. Therapies tested in this setting have shown discouraging response rates, with a median progression-free survival (PFS) of 2 to 3 months and a median overall survival (OS) of 6 to 9 months.4–6 Clinical trials of immune checkpoint blockade with monoclonal antibodies to programmed death-1 (PD-1) and programmed death-ligand in patients with UC have yielded promising early results, but mature survival data from these trials are still pending.7–10
Endoglin/CD105 is overexpressed in vascular endothelial cells of soft tissues undergoing angiogenesis and in tumors.11,12 Levels of CD105 correlate with endothelial cell proliferation, and CD105 is often used as a marker of tumor angiogenesis.12 CD105 is therefore a potential therapeutic vascular target in oncology.
Urothelial carcinoma is a highly vascular malignancy that produces high levels of proangiogenic factors such as vascular endothelial growth factor, basic fibroblast growth factor, and interleukin-8.13 Microvessel density, a histologic measure of angiogenesis, has been correlated with stage, recurrence, and survival in UC.14–21 Clinical studies in UC with antiangiogenic agents have shown antitumor activity.22–26 Although these studies validate the potential for an antiangiogenic approach to UC, they also indicate the need to investigate alternative ways to target the tumor vasculature. Directly targeting proliferating endothelial cells, a major component of tumor vasculature, by modifying CD105 signaling is a unique mechanism of targeting angiogenesis.
TRC105 is a human/murine chimeric anti-CD105 immunoglobulin (Ig)G1-k monoclonal antibody with an approximate molecular weight of 148 kDa.24 It is composed of 2 light chains of 213 amino acids and 2 heavy chains of 448 amino acids. TRC105 binds with high avidity to human CD105, thus inhibiting angiogenesis and tumor growth.24,25 TRC105 dosing has been established.26
We conducted a phase II trial of TRC105 to determine safety and toxicity and assess PFS in patients with advanced/metastatic UC. CD105 protein is expressed on the surface of CD4-positive (CD4+) T cells, activated monocytes, and macrophages.27,28 Thus, TRC105, a monoclonal antibody targeting CD105, might modulate immune subsets, which might correlate with clinical outcome in patients with UC. In addition to assessing the clinical activity of TRC105, we also assessed its effect on immune subsets, mature circulating endothelial cells (CECs) and circulating endothelial progenitor cells (CEPs), circulating tumor cells (CTCs), and osteopontin.
Patients and Methods
Patient Selection
For this phase II study of TRC105 in advanced/metastatic UC that had progressed despite previous cytotoxic chemotherapy, eligible patients must have received ≥ 1 previous treatment with cisplatin, carboplatin, paclitaxel, docetaxel, or gemcitabine. The primary study objective was to determine the activity of TRC105 using Response Evaluation Criteria in Solid Tumors (RECIST). TRC105 was administered at a dose of 15 mg/kg every 2 weeks on days 1 and 15 of each 28-day cycle. Patients continued in the study as long as they tolerated therapy and showed no disease progression.
Patients were considered eligible if they were ≥ 18 years of age and had: (1) a diagnosis of UC of the bladder, urethra, ureter, or renal pelvis, with histologic confirmation by the National Cancer Institute (NCI) Laboratory of Pathology; and (2) progressive metastatic disease defined as new or progressive lesions on cross-sectional imaging. Patients had to have ≥ 1 measurable site of disease (according to RECIST) that had not been previously irradiated. If marker lesions had been previously irradiated, there had to be evidence of progression after irradiation or the appearance of 1 new bone lesion; (3) previous treatment with ≥ 1 previous cytotoxic agent (including cisplatin, carboplatin, paclitaxel, docetaxel, or gemcitabine), which might have been administered in the perioperative or metastatic setting, either sequentially (eg, first-line treatment followed by second-line treatment at time of disease progression) or as part of a single regimen; (4) Eastern Cooperative Oncology Group performance status of < 2 or Karnofsky Performance Status of≥ 60%; (5) resolution of all acute toxic effects of previous chemotherapy, radiotherapy, or surgical procedures; and (5) adequate organ function. Patients were excluded if they had: (1) received an investigational agent within 4 weeks of the first dose of TRC105; (2) major surgery (including open biopsy) or systemic therapy within 4 weeks of the first dose ofTRC105; (3) radiation therapy (except small field) within 3 weeks of the first dose of TRC105; (4) small field radiation therapy within 2 weeks of the first dose ofTRC105; (5) minor surgical procedures within 2 weeks of the first dose of TRC105; (6) uncontrolled chronic hypertension (systolic > 140 or diastolic > 90 mm Hg despite optimal therapy); (7) brain metastasis or lep- tomeningeal disease; (8) unstable angina, myocardial infarction, symptomatic congestive heart failure, cerebrovascular accident, transient ischemic attack, arterial embolism, pulmonary embolism, deep vein thrombosis, percutaneous transluminal coronary angioplasty, coronary artery bypass grafting within the past 6 months; (9) a serious, nonhealing wound, ulcer, or bone fracture; ( 10) known active hepatitis; or (11) hemorrhage within 30 days of dosing or history of persistent gross hematuria. All patients gave written informed consent in accordance with federal, state, and institutional guidelines. The NCI’s institutional review board approved the study.
Study Design
This was a single-institution, single-arm, open-label, phase II clinical trial, using a 2-stage optimal design. On the basis of previous phase II trials with similar eligibility, the median PFS was ordinarily 2 to 3 months.22 The objective of the present trial was to determine if TRC105 could produce outcomes consistent with 30% of patients being progression-free according to radiographic criteria at around 6 months (P1 = .30) while ruling out 10% of patients being progression-free at around 6 months (P0 = .10), using standard acceptable error probabilities: α = 0.10; β = 0.10. Initially, 13 patients were enrolled, and 12 were evaluable and followed for disease progression. According to the design, if ≤ 1 of the initial 12 patients were progression-free at 6 months, no more patients would be enrolled, and if≥ 2 ofthe initial 12 patients were progression-free at 6 months, enrollment would increase to a total of 35 evaluable patients. If only 2 to 5 of those 35 patients were progression-free at 6 months, this would be deemed an inadequate treatment response, and ≥ 6 of the 35 patients progression-free at 6 months would indicate a PFS probability worthy of further investigation. Under the null hypothesis (≤ 10% of patients were progression-free at 6 months), the probability of early termination after the 6-month evaluation of the initial 12 evaluable patients was 66%.
The safety and toxicity of TRC105 in this patient population were also primary considerations of this study. All Grade 3/4 toxicities were reported according to the NCI Common Terminology Criteria for Adverse Events version 4.0.
Treatment Plan and Evaluation of Toxicities
TRC105 was administered on an outpatient basis at a dose of 15 mg/kg every 2 weeks on days 1 and 15 of each 28-day cycle. Thirty minutes to 2 hours before the start of each TRC105 infusion, patients were premedicated with 1 dose of acetaminophen 650 mg orally (p.o.), 1 dose of dexamethasone 20 mg intravenously (I.V.), 1 dose of famotidine (or similar H2 blocker) 20 mg I.V., and 1 dose of cetirizine (or similar oral or I.V. antihistamine) 10 mg I.V. or p.o. TRC105 was administered I.V. using an infusion pump. On cycle 1 day 1,TRC105 was infused over a period of 4 hours. If patients completed one 4-hour infusion without developing an infusion reaction, subsequent TRC105 infusions were reduced to 2 hours. If patients completed one 2-hour infusion without developing an infusion reaction, subsequent TRC105 infusions were reduced to a minimum of 1 hour. If this infusion rate proved to be safe, the dexamethasone dosage was gradually tapered, at the discretion of the investigator, with each subsequent infusion and eventually discontinued, if possible. If a patient experienced a Grade 2, 3, or 4 adverse reaction during infusion, the infusion was stopped and the patient was treated with an antipyretic, antihistamine, or other drug as indicated.
Treatment-limiting toxicities were defined as any Grade ≥ 3 hematologic or nonhematologic toxicity possibly, probably, or definitely related to TRC105. Any Grade 4 pulmonary embolus, including one without significant hypoxia and hemodynamic instability, was considered dose-limiting, and no more TRC105 was administered. If patients experienced a treatment-limiting toxicity at least possibly related to TRC105, the drug was withheld until the toxicity was resolved. For selected toxicities, TRC105 was reinitiated at a lower dose level (albeit not < 5 mg/kg). Patients with a clinical Grade 4 nonhematologic toxicity were removed from the study.
Response Evaluation
Restaging bone scans and computed tomography scans of the chest, abdomen, and pelvis were scheduled every 2 months for the first 4 months of the study (after cycles 2 and 4), then after every 3 cycles of treatment. Confirmatory scans were obtained 4 weeks after initial documentation of objective response, complete response (CR), or partial response (PR). Objective response and progression were evaluated using the new international criteria proposed by the RECIST version 1.1 Committee.29
Biomarker Evaluations
Whole blood samples were collected in cell preparation tubes with sodium citrate (BD Vacutainer CPT Tubes; BD Biosciences, San Jose, CA) at baseline and after 6 treatments of TRC105 (C3D1). Peripheral blood mononuclear cells (PBMCs) were obtained using centrifugation and viably frozen until analysis. All analyses were performed using multiparametric flow cytometry (MACSQuant; Miltenyi Biotec, Bergisch Gladbach, Germany) and data were analyzed using FlowJo software version 10.0.7 (FlowJo, LLC, Ashland, OR). PBMCs were analyzed for T regulatory (Treg) cells (CD4+CD25hiFoxp3+ T cells), T cells (CD3+CD14-CD19-), B cells (CD19+CD3-CD14-), natural killer cells (CD56+CD3-CD14- CD19), and monocytes (CD45+CD14+). Only viable CD45+ cells were analyzed. All antibodies were purchased from BioLegend (San Diego, CA). CECs (CD146+CD133-CD31+CD45-) and CEPs (CD146-CD133+CD31+CD45dim/-) were analyzed as described previously.30,31 CTCs were detected from 8 mL of peripheral blood drawn into BD Vacutainer CPT Tubes (BD Biosciences). Epithelial cell adhesion molecule (EpCAM)-positive CTCs were isolated using magnetic pre-enrichment and quantified using multiparameter flow cytometry, which is a novel method we recently developed and validated in cancer patients.31,32 CTCs were identified as viable, nucleated, EpCAM+ cells that did not express the common leukocyte antigen CD45, as described previously.31,32 After enumeration of viable nucleated, CD45-, EpCAM+ cells, CTCs were further characterized for expression of Mucin 1, cell surface associated (MUC1), which plays a critical role in tumor growth and is being tested in clinical trials as a possible cancer vaccine target, and the stem cell marker CD133.
CD105 is part of the transforming growth factor beta (TGF-β) receptor complex, and osteopontin gene expression is upregulated by TGF-β. Because circulating levels of osteopontin can be assessed using an enzyme-linked immunosorbent assay,33 we used this platform to measure circulating osteopontin as a potential indicator of the effect of TRC105 on TGF-β signaling. Plasma osteopontin was measured using an enzyme-linked immunosorbent assay (Human Osteopontin Assay Kit; IBL-America, Minneapolis, MN).
Statistical Analysis
Time to disease progression was defined as from the first date of study inclusion until the date of first observation of disease progression, death during the study, or removal from the study at the principal investigator’s discretion. Other reasons for removal from the study, such as adverse events, patient decision, or concurrent illness, were used to censor time to disease progression. Patients remaining in the study or alive at time of analyses were censored at date of last follow-up. The probability of PFS or OS as a function of time was determined using the Kaplan–Meier method, with the statistical significance of the difference between Kaplan–Meier curves determined using an exact log rank test because of the small number of patients. The significance of the difference between 2 dichotomous parameters was determined using Fisher exact test, and the difference in continuous parameters between 2 time points was determined using a Wilcoxon signed rank test. Evaluations of laboratory and correlative parameters were performed as exploratory analyses and would require independent confirmation to be considered potentially definitive.
All P values are 2-tailed and reported without formal adjustment for multiple comparisons.
Immunohistochemistry
Protein expression of CD105 (endoglin) was evaluated using immunohistochemistry with monoclonal mouse antihuman antibody, Clone SN6h (Dako, Dakocytomation; Carpinteria, CA) in a separate cohort of 50 UC patients with muscle-invasive disease. First, paraffin-embedded sections (5 μm) were deparaffinized in xylene and rehydrated in graded alcohol. Tissue sections were microwaved in 10 mM sodium citrate pH 6.0 for 15 minutes, then allowed to cool. A high-sensitivity detection system (Catalyzed Signal Amplification, Dako) was used for antibody detection. Endogenous peroxidase was blocked with 0.3% hydrogen peroxide in phosphate-buffered saline, followed by protein blocking. Sections were incubated for 1 hour at room temperature with the CD105 antibody (1:2000 dilution). Biotinylated link antibody was added, followed by streptavidin-biotin complex. Finally, the amplification reagent was visualized with streptavidin-peroxidase and 3,3’-dia-minobenzidine as chromogen. Slides were counterstained with hematoxylin, dehydrated, and mounted. For negative controls, sections were incubated in parallel with their respective matched isotype nonimmune IgG. Vascular endothelial cells present in the tissue were positively stained as positive control. Level of CD105 expression was scored on the basis of intensity of staining in the cytoplasm of tumor cells. Intensity was recorded as 0 (no staining), 1 (weak staining), 2 (moderate staining), or 3 (strong staining), and the percentage of stained cytoplasmic/nuclear area was recorded.
Results
Patient Characteristics
Thirteen patients were enrolled between April and October 2011 (see Table 1 for study demographic and baseline characteristics). One patient enrolled in the study did not receive the study drug because of clinical disease progression and deterioration before the first dose. Patients received a median of 2 cycles (range, 1–4). Of 12 patients evaluable for disease, 11 (92%) discontinued therapy because of disease progression. One patient discontinued therapy because of deep venous thrombosis. No patients required dose reductions secondary to the management ofTRC105-related toxicities.
Table 1.
Baseline Clinical Characteristics (n = 13)
| Characteristic | Value |
|---|---|
| Median Age (Range) | 67 (51–73) |
| Sex | |
| Male | 8 (62) |
| Female | 5 (38) |
| Karnofsky Performance Status | |
| 60 | 1 (8) |
| 70 | 0 (0) |
| 80 | 2 (15) |
| 90 | 10 (77) |
| Primary Tumor Site | |
| Bladder | 11 (85) |
| Upper urinary tract | 2 (15) |
| Metastatic Sites of Disease | |
| Lung | 7 (54) |
| Liver | 2 (15) |
| Bone | 4 (31) |
| Any visceral metastases | 10 (77) |
| Lymph node only | 3 (23) |
| Number of Previous Therapies for Metastatic Disease | |
| 1 | 3 (23) |
| 2 | 4 (31) |
| 3 | 3 (23) |
| 4 | 2 (15) |
| 5 | 1 (8) |
Data are presented as n (%) except where otherwise stated.
Progpession-Free and OS
Of 13 patients enrolled, 12 were evaluable for OS and PFS. The 3-month PFS probability was 18.2%, and all failures took place within 6 months (median PFS, 1.9 months; 95% confidence interval [CI], 1.8–2.1 months; Figure 1A). This met the criterion for ending accrual according to the 2-stage design, and the study was closed at that point. Median OS was 8.3 months (95% CI, 3.3–17.0 months; Figure 1B); 12-month survival probability was 33%. Patients were followed for up to 25 months. Nine patients subsequently received additional treatment after the study. There were no objective responses (PR or CR) according to RECIST version 1.1. Two patients had stable disease lasting 4 months.
Figure 1.
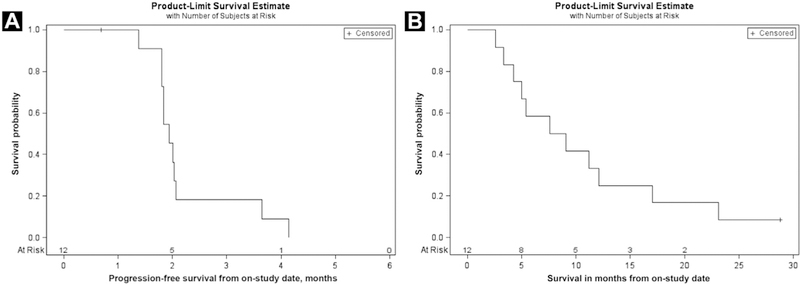
Progression-Free Survival and Overall Survival (n = 12). (A) Progression-Free Survival of Patients Treated With TRC105. (B) Overall Survival of Patients Treated With TRC105
Toxicities
All patients who received treatment were analyzed for toxicity. Interestingly, a common Grade 1 toxicity was asymptomatic telangiectasia. Table 2 shows the most common Grade 2 toxicities and all Grade 3 and 4 toxicities. Significant Grade 2 adverse events included anemia (42%). Grade 3 toxicities included anemia (8%) and skin infection (8%).
Table 2.
Incidence of TRC105-Related Adverse Events (n = 13)
| Adverse Event | All Grades | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Anemia | 6 (46) | 1 (8) | 4 (31) | 1 (8) | – |
| Anorexia | 1 (8) | 1 (8) | – | – | – |
| Cough | 1 (8) | 1 (8) | – | – | – |
| Edema (Limbs) | 1 (8) | 1 (8) | – | – | – |
| Epistaxis | 6 (46) | 6 (46) | – | – | – |
| Fatigue | 1 (8) | 1 (8) | – | – | – |
| Gum Bleeding | 4 (31) | 4 (31) | – | – | – |
| Headache | 9 (69) | 8 (62) | 1 (8) | – | – |
| Hematuria | 1 (8) | 1 (8) | 1 (8) | – | – |
| Hypoalbuminemia | 1 (8) | 1 (8) | – | – | – |
| Hypophosphatemia | 1 (8) | 1 (8) | – | – | |
| Increased Alanine Aminotransferase | 1 (8) | 1 (8) | – | – | – |
|
Infusion-Related Reaction |
9 (69) | 5 (38) | 4 (31) | – | – |
| Maculopapular Rash | 1 (8) | 1 (8) | – | – | – |
| Nasal Congestion | 3 (23) | 3 (23) | – | – | – |
| Nausea | 1 (8) | 1 (8) | – | – | – |
| Proteinuria | 1 (8) | 1 (8) | – | – | – |
| Skin Infection | 1 (8) | – | – | 1 (8) | – |
| Telangiectasia | 6 (46) | 6 (46) | – | – | – |
| Vomiting | 1 (8) | 1 (8) | – | – | – |
| Xerostomia | 1 (8) | 1 (8) | – | – | – |
Biomarker Evaluations
Immunohistochemistry.
Immunohistochemistry for CD105 scores were 0 (50%), 1+ (10%), 2+ (36%), and 3+ (4%). There was no statistical association between CD105 staining (negative vs. positive) and T stage (P = .26) or presence of lymph nodes (P = .64).
Immune Subsets.
In exploratory analyses, the level of Treg cells among CD4+ T cells significantly decreased after treatment compared with baseline (n = 7, P = .016; Figure 2A). Patients whose Treg cell level was lower than the median at baseline showed a trend toward improved OS compared with those whose Treg level was higher than the median at baseline (n = 7; P = .086; Figure 2B). Patients whose B-cell level was lower than the median at baseline had somewhat improved OS compared with those whose B-cell level was higher than the median at baseline (n = 7; P = .057; Figure 2C).
Figure 2.
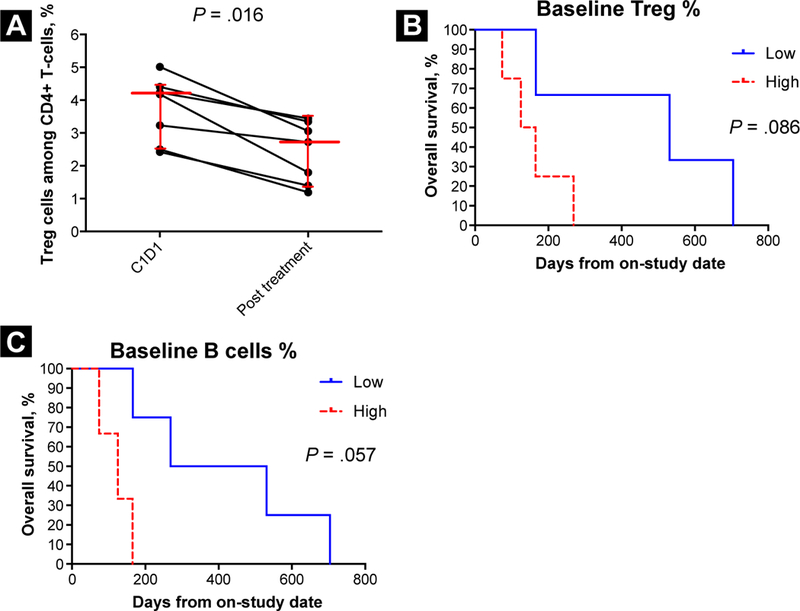
T Regulatory (Treg) and B-Cell Levels in Urothelial Carcinoma Patients Treated With TRC105 and Association With Clinical Outcome. (A) Change of Percentage of Treg Cells Among CD4+ T Cells After TRC105 in Urothelial Carcinoma Patients. Percentage of Treg Cells Decreased After TRC105 (n = 7; Wilcoxon Signed Rank Test, P = .016). Median and Quartiles Are Shown. (B) Kaplan-Meier Curves Showing Overall Survival of Patients With Baseline Percentage of Treg Levels Equal to or Above the Median (High) or Below the Median (Low) (n = 7; Exact Log Rank Test, P = .086). (C) Kaplan-Meier Curves Showing Overall Survival of Patients With Baseline Percentage of B Cells Among CD45+ Cells Above (High) or Equal to or Below the Median (Low) (n = 7; Exact Log Rank Test, P = .057)
Circulating Endothelial Cells and CEPs.
Patients in whom the percentage of apoptotic CECs among nucleated cells decreased after treatment showed a trend toward improved PFS compared with patients in whom the percentage of CECs increased after treatment (n = 7; P = .11; Figure 3A). Patients whose CEP level was lower than the median at baseline had improved PFS compared with those whose CEP level was higher than the median at baseline (n = 7; P = .029; Figure 3B).
Figure 3.
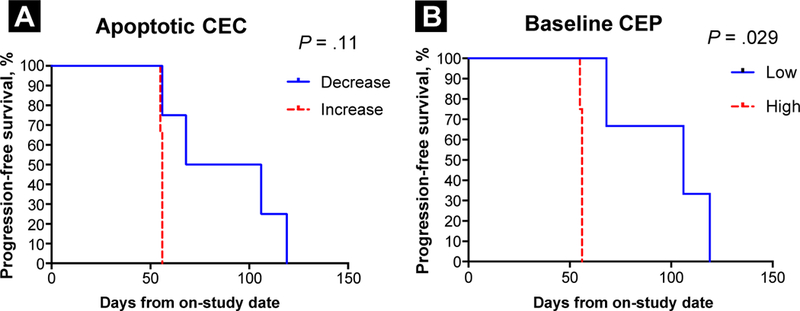
Circulating Endothelial Cell (CEC) and Circulating Endothelial Progenitor Cell (CEP) Levels in Urothelial Carcinoma Patients Treated With TRC105 and Association With Clinical Outcome. (A) Kaplan-Meier Curves Showing Progression-Free Survival of Patients With an Increase or Decrease of Apoptotic CECs From Baseline to Post-Treatment (n = 7; Exact Log-Rank Test, P = .11). (B) Kaplan-Meier Curves Showing Progression-Free Survival of Patients With Baseline CEP Levels Equal to or Above the Median (High) or Below the Median (Low) (n = 7; Exact Log Rank Test, P = .029)
Circulating Tumor Cells.
We measured CTCs in 4 patients (3 with bladder cancer; 1 with ureter cancer) before and after treatment. We detected CTCs in all 4 patients; however, the number of CTCs in 2 patients (1 with bladder cancer; 1 with ureter cancer) decreased after treatment with TRC105 (Figure 4A). Among the 4 patients tested, the 2 patients with bladder cancer who had < 5 CTCs after treatment with TRC105 showed longer OS (> 6 months) compared with those who had > 5 CTCs. One patient with bladder cancer who had < 5 CTCs before and after treatment had the longest OS (704 days; Figure 4A). CD133 and MUC1 expression levels showed dynamic changes before and after treatment. CD133 expression was detected in all bladder cancer patients and the positive ratio increased after treatment, but CTCs in a patient with ureter cancer were negative for CD133 (Figure 4B). MUC1 expression was detected in all 4 patients (Figure 4C).
Figure 4.
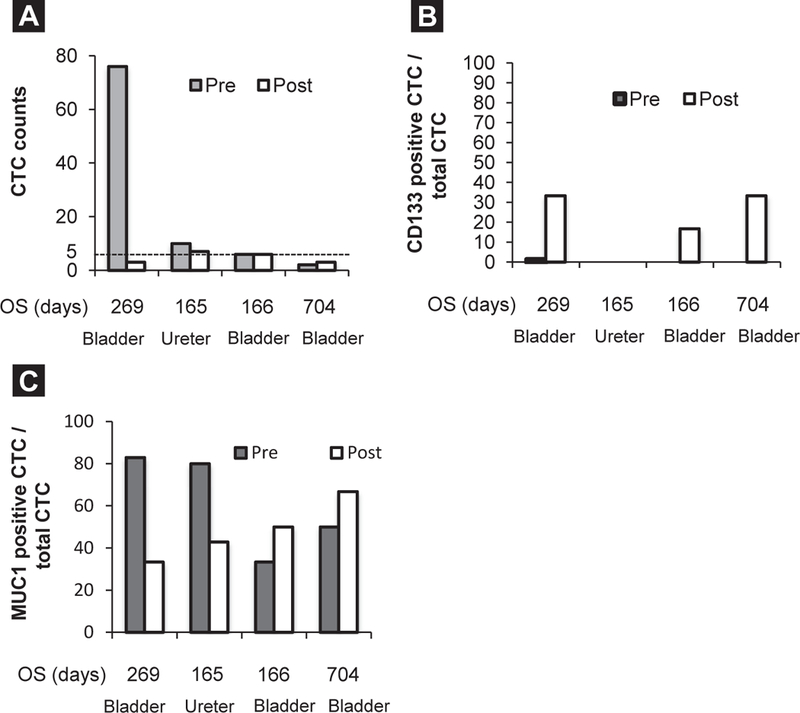
Dynamic Change of Circulating Tumor Cell (CTC) Counts and CTC Phenotypes Pre- and Post-Treatment in Urothelial Carcinoma Patients and Association With Overall Survival (OS). (A) CTC Counts Pre- and Post-Treatment. (B) CD133+ CTC/Total CTC Pre- and Post-Treatment. (C) Mucin 1, cell surface associated (MUC1)+ CTC/Total CTC Pre- and Post-Treatment
Osteopontin.
Osteopontin increased significantly after treatment compared with baseline (n = 7; P = .016; Figure 5).
Figure 5.
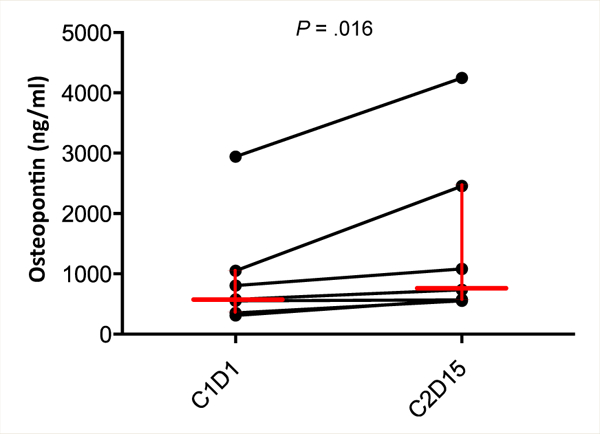
Increase in Plasma Osteopontin Levels After TRC105 Treatment in Urothelial Carcinoma Patients (n = 7; Wilcoxon Signed Rank Test, P = .016). Median and Quartiles Are Shown
Discussion
This study did not meet its primary end point of improving PFS 6 months and closed early for futility. However, correlative studies in this small group of patients suggest that TRC105 has immunomodulatory properties that might be exploited m future combination studies with immunotherapies.
Angiogenesis plays a complex role in tumor development. CD105 is essential in normal vascular development, and high tumor microvessel density assessed using CD105 staining has been correlated with poor prognosis in solid tumors.34,35 In vivo pre-clinical studies revealed that TRC1O5’s parental monoclonal antibody inhibited tumor growth and angiogenesis.36,37 This novel approach to antiangiogenesis identifies CD105 as a potential target in the treatment of solid tumors.
Emerging evidence has revealed the interplay between the host immune system and many anticancer therapies that were not thought to have an immune target or to significantly affect immune cells.38–40 However, how TRC105 might affect immune subsets in patients with UC remains to be shown. The CD105 protein is a TGF-β coreceptor expressed on the surface of CD4+ T cells,27,41 activated monocytes, and macrophages in the tumor microenvi-ronment.28 It has been shown that TGF-β is important in Treg cell induction.42,43 In the current study, TRC105 significantly decreased the level of Treg cells in PBMCs in this small group of patients. Furthermore, patients with high baseline levels of Treg cells and B cells showed a trend toward poor OS. Interestingly, Treg decline in response to TRC105 was also observed in a phase I/II study of TRC105 in metastatic castration-resistant prostate cancer and correlated with prostate-specific antigen decline (NCT01090765).44 These results suggest that TRC105 might regulate Treg cell levels directly or through CD105+ monocytes or macrophages in cancer patients.45,46
Recent studies of immune checkpoint blockade have reported promising results in several cancer types, including UC.7–10 Treg cells express PD-1 and cytotoxic T-lymphocyte-associated protein 4. However, anti–PD-1 and cytotoxic T-lymphocyte-associated protein 4 blockade increased Treg cell levels in patients with melanoma.47–49 Combination therapy with immune checkpoint blockade and TRC105 might, therefore, have a synergistic effect and could potentially improve antitumor responses in UC patients.
CD 19+CD24hiCD38hiCD1dhi and CD19+CD24hiCD27+ regulatory B cells (Bregs) have been identified in humans and have been shown to play a critical role in immune tolerance.50–52 In the current study, a lower baseline CD19+ B-cell level was associated with a trend toward improved OS. Although we used only CD19 for B-cell phenotypic analysis, any CD19+ B cells could differentiate into Bregs in response to environmental stimuli.52 Thus, CD19+ B cells detected in the current study might have an immunosuppressive function similar to Bregs. Although this hypothesis must be confirmed by further phenotypic and functional analyses, our results suggest that peripheral CD19+ B cells might play an important role in disease progression in patients with UC treated with TRC105. Taken together, our data suggest an interplay between immunosuppressive subsets and TRC105.
It has been shown that CECs and CEPs are potential biomarkers of response to antiangiogenic therapies.53,54 In this study, baseline CEP levels and changes in apoptotic CECs by TRC105 showed an association with PFS, although the number of patients analyzed was very small. CECs and CEPs might be predictive, pharmacodynamic markers in UC treated with TRC105.
Circulating tumor cell detection is a noninvasive diagnostic tool that can be used as a liquid biopsy for prognostic and predictive purposes, offering an opportunity for longitudinal, real-time tumor molecular characterization that can guide personalized cancer ther-apy.55,56 It has been reported that CTCs have been detected in localized, high-risk nonmuscle-invasive, and metastatic bladder cancer, and that CTCs have potential prognostic value.56–59 In the current study, we applied an integrated magnetic pre-enrichment and multiparameter flow cytometric analysis, a novel method that we recently developed.31,32 Detection and characterization of CTCs before and after therapy showed dynamic changes in CTC number and expression of MUC1 and CD133 on CTCs, suggesting the presence of intrapatient heterogeneity in UC. CTC analysis might be used to assess response to TRC105; however, larger patient samples will be needed to further elucidate the potential clinical relevance of CTC analysis.
Conclusion
Treatment with TRC105 was not associated with significant toxicities. Grade 3 anemia was noted in 1 of 12 patients, but no dose reductions were required. Single-agent activity in this heavily pretreated population was not significant; however, combination studies are warranted. UC is a highly vascular, chemosensitive malignancy. Recently reported data suggesting UC’s sensitivity to immune-targeted therapy provide a rationale for combining a novel antiangiogenic and immunomodulatory approach with chemotherapy or an immune-targeted therapy.
Clinical Practice Points.
New, more effective therapies are needed in metastatic UC. Median OS in the second-line setting ranges only from 6 to 9 months.
This trial assessed the efficacy and tolerability of single-agent TRC105, a chimeric monoclonal antibody that targets CD105 in patients with metastatic, heavily pretreated UC, a highly vascular malignancy.
CD105 is a protein expressed in endothelial cells, CD4+ T cells, activated monocytes, and macrophages.
Treatment with TRC105 was not associated with significant toxicities.
Although single-agent activity in this heavily pretreated population was not significant, exploratory analyses suggest that TRC105 might affect immune subsets, including Treg cells, in patients with UC.
Recent data suggest that UC is sensitive to immune-targeted therapy, warranting future approaches that combine TRC105 with immunotherapeutic strategies.
Acknowledgments
This work was supported by the Intramural Research Program of the Center for Cancer Research, NCI, National Institutes of Health, and TRACON Pharmaceuticals Inc, San Diego, California.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 2.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000; 18:3068–77. [DOI] [PubMed] [Google Scholar]
- 3.De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012; 30:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 1997; 15: 1853–7. [DOI] [PubMed] [Google Scholar]
- 5.Vaughn DJ, Broome CM, Hussain M, et al. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol 2002; 20: 937–40. [DOI] [PubMed] [Google Scholar]
- 6.Dreicer R, Li S, Manola J, et al. Phase 2 trial of epothilone B analog BMS-247550 (ixabepilone) in advanced carcinoma of the urothelium (E3800): a trial of the Eastern Cooperative Oncology Group. Cancer 2007; 110:759–63. [DOI] [PubMed] [Google Scholar]
- 7.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515: 558–62. [DOI] [PubMed] [Google Scholar]
- 8.Plimack E, Bellmunt J, Gupta S, et al. Pembrolizumab (MK-3475) for advanced urothelial cancer: Updated results and biomarker analysis from KEYN0TE-012 (abstract 4502). J Clin Oncol 2015; 33(suppl). [Google Scholar]
- 9.Apolo AB, Infante J, Hamid O, et al. Safety, clinical activity, and PD-L1 expression of avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with metastatic urothelial carcinoma from the JAVELIN Solid Tumor phase Ib trial (abstract 367). J Clin Oncol 2016; 34(suppl 2S). [Google Scholar]
- 10.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387:1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonsatti E, Jekunen AP, Kairemo KJ, et al. Endoglin is a suitable target for efficient imaging of solid tumors: in vivo evidence in a canine mammary carcinoma model. Clin Cancer Res 2000; 6:2037–43. [PubMed] [Google Scholar]
- 12.Miller DW, Graulich W, Karges B, et al. Elevated expression of endoglin, a component of the TGF-beta-receptor complex, correlates with proliferation of tumor endothelial cells. Int J Cancer 1999; 81:568–72. [DOI] [PubMed] [Google Scholar]
- 13.Chodak GW, Scheiner CJ, Zetter BR. Urine from patients with transitional-cell carcinoma stimulates migration of capillary endothelial cells. N Engl J Med 1981; 305:869–74. [DOI] [PubMed] [Google Scholar]
- 14.Bochner BH, Cote RJ, Weidner N, et al. Angiogenesis in bladder cancer: relationship between microvessel density and tumor prognosis. J Natl Cancer Inst 1995; 87:1603–12. [DOI] [PubMed] [Google Scholar]
- 15.Jaeger TM, Weidner N, Chew K, et al. Tumor angiogenesis correlates with lymph node metastases in invasive bladder cancer. JUrol 1995; 154:69–71. [PubMed] [Google Scholar]
- 16.Dickinson AJ, Fox SB, Persad RA, et al. Quantification of angiogenesis as an independent predictor of prognosis in invasive bladder carcinomas. Br J Urol 1994; 74:762–6. [DOI] [PubMed] [Google Scholar]
- 17.Brown LF, Berse B, Jackman RW, et al. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol 1993; 143:1255–62. [PMC free article] [PubMed] [Google Scholar]
- 18.Chopin DK, Caruelle JP, Colombel M, et al. Increased immunodetection of acidic fibroblast growth factor in bladder cancer, detectable in urine. J Urol 1993; 150: 1126–30. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen M, Watanabe H, Budson AE, et al. Elevated levels of the angiogenic peptide basic fibroblast growth factor in urine of bladder cancer patients. J Natl Cancer Inst 1993; 85:241–2. [DOI] [PubMed] [Google Scholar]
- 20.Inoue K, Slaton JW, Davis DW, et al. Treatment of human metastatic transitional cell carcinoma of the bladder in a murine model with the anti-vascular endothelial growth factor receptor monoclonal antibody DC101 and paclitaxel. Clin Cancer Res 2000; 6:2635–43. [PubMed] [Google Scholar]
- 21.Wu W, Shu X, Hovsepyan H, et al. VEGF receptor expression and signaling in human bladder tumors. Oncogene 2003; 22:3361–70. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher DJ, Milowsky MI, Gerst SR, et al. Phase II study ofsunitinib in patients with metastatic urothelial cancer. J Clin Oncol 2010; 28:1373–9. [DOI] [PubMed] [Google Scholar]
- 23.Hahn N, Stadler W, Zon R, et al. Mature results from Hoosier Oncology Group GU04–75 phase II trial of cisplatin (C), gemcitabine (G), and bevacizumab (B) as first-line chemotherapy for metastatic urothelial carcinoma (UC) (abstract 4541). JClin Oncol 2010; 28(15s). [Google Scholar]
- 24.Balar AV, Apolo AB, Ostrovnaya I, et al. Phase II study of gemcitabine, carboplatin, and bevacizumab in patients with advanced unresectable or metastatic urothelial cancer. J Clin Oncol 2013; 31:724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grivas PD, Daignault S, Tagawa ST, et al. Double-blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma. Cancer 2014; 120:692–701. [DOI] [PubMed] [Google Scholar]
- 26.Sonpavde G, Bellmunt J. Bladder cancer: angiogenesis as a therapeutic target in urothelial carcinoma. Nat Rev Urol, Published online April 13, 2016; 10.1038/nrurol.2016.69. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Weber CB, Letarte M, Kunzmann S, et al. TGF-{beta} signaling of human T cells is modulated by the ancillary TGF-{beta} receptor endoglin. Int Immunol 2005; 17:921–30. [DOI] [PubMed] [Google Scholar]
- 28.Fonsatti E, Nicolay HJ, Altomonte M, et al. Targeting cancer vasculature via endoglin/CD105: a novel antibody-based diagnostic and therapeutic strategy in solid tumours. Cardiovasc Res 2010; 86:12–9. [DOI] [PubMed] [Google Scholar]
- 29.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 30.Park SR, Speranza G, Piekarz R, et al. A multi-histology trial of fostamatinib in patients with advanced colorectal, non-small cell lung, head and neck, thyroid, and renal cell carcinomas, and pheochromocytomas. Cancer Chemother Pharmacol 2013; 71:981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy- refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 2015; 16:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kauffman EC, Lee MJ, Alarcon SV, et al. Lack of impact of robotic assisted laparoscopic radical prostatectomy on intraoperative levels of prostate cancer circulating tumor cells. J Urol 2016; 195(4P1):1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pass HI, Lott D, Lonardo F, et al. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med 2005; 353:1564–73. [DOI] [PubMed] [Google Scholar]
- 34.Li DY, Sorensen LK, Brooke BS, et al. Defective angiogenesis in mice lacking endoglin. Science 1999; 284:1534–7. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S, Ghellal A, Li C, et al. Breast carcinoma: vascular density determined using CD105 antibody correlates with tumor prognosis. Cancer Res 1999; 59:856–61. [PubMed] [Google Scholar]
- 36.Takahashi N, Haba A, Matsuno F, et al. Antiangiogenic therapy of established tumors in human skin/severe combined immunodeficiency mouse chimeras by anti-endoglin (CD105) monoclonal antibodies, and synergy between anti-endoglin antibody and cyclophosphamide. Cancer Res 2001; 61:7846–54. [PubMed] [Google Scholar]
- 37.Uneda S, Toi H, Tsujie T, et al. Anti-endoglin monoclonal antibodies are effective for suppressing metastasis and the primary tumors by targeting tumor vasculature. Int J Cancer 2009; 125:1446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roselli M, Cereda V, di Bari MG, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology 2013; 2:e27025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galluzzi L, Senovilla L, Zitvogel L, et al. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012; 11:215–33. [DOI] [PubMed] [Google Scholar]
- 40.Vacchelli E, Senovilla L, Eggermont A, et al. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology 2013; 2:e23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santner-Nanan B, Peek MJ, Khanam R, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol 2009; 183:7023–30. [DOI] [PubMed] [Google Scholar]
- 42.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell 2008; 134:392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bird L Regulatory T cells: nurtured by TGFbeta. Nat Rev Immunol 2010; 10:466. [DOI] [PubMed] [Google Scholar]
- 44.Karzai FH, Apolo AB, Cao L, et al. A phase I study of TRC105 anti-endoglin (CD105) antibody in metastatic castration-resistant prostate cancer. BJU Int 2015; 116:546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong H, Yazdanbakhsh K. Differential control of Helios(+/−) Treg development by monocyte subsets through disparate inflammatory cytokines. Blood 2013; 121: 2494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010; 236:219–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarhini AA, Butterfield LH, Shuai Y, et al. Differing patterns of circulating regulatory T cells and myeloid-derived suppressor cells in metastatic melanoma patients receiving anti-CTLA4 antibody and interferon-alpha or TLR-9 agonist and GM-CSF with peptide vaccination. J Immunother 2012; 35:702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarhini AA, Edington H, Butterfield LH, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 2014; 9: e87705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibney GT, Kudchadkar RR, DeConti RC, et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res 2015; 21:712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kessel A, Haj T, Peri R, et al. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev 2012; 11:670–7. [DOI] [PubMed] [Google Scholar]
- 51.Lee KM, Stott RT, Zhao G, et al. TGF-beta-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J Immunol 2014; 44: 1728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity 2015; 42:607–12. [DOI] [PubMed] [Google Scholar]
- 53.Fuereder T, Wacheck V, Strommer S, et al. Circulating endothelial progenitor cells in castration resistant prostate cancer: a randomized, controlled, biomarker study. PLoS One 2014; 9:e95310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossi E, Fassan M, Aieta M, et al. Dynamic changes of live/apoptotic circulating tumour cells as predictive marker of response to sunitinib in metastatic renal cancer. Br J Cancer 2012; 107:1286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov 2014; 4:650–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gazzaniga P, de Berardinis E, Raimondi C, et al. Circulating tumor cells detection has independent prognostic impact in high-risk non-muscle invasive bladder cancer. Int J Cancer 2014; 135:1978–82. [DOI] [PubMed] [Google Scholar]
- 57.Flaig TW, Wilson S, van Bokhoven A, et al. Detection of circulating tumor cells in metastatic and clinically localized urothelial carcinoma. Urology 2011; 78:863–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rink M, Chun FK, Dahlem R, et al. Prognostic role and HER2 expression of circulating tumor cells in peripheral blood ofpatients prior to radical cystectomy: a prospective study. Eur Urol 2012; 61:810–7. [DOI] [PubMed] [Google Scholar]
- 59.Alva A, Friedlander T, Clark M, et al. Circulating tumor cells as potential biomarkers in bladder cancer. J Urol 2015; 194:790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


