Abstract
Objective
To examine the test performance of careHPV, Hybrid Capture2 (HC2) and visual inspection with acetic acid (VIA) for detection of cytologically diagnosed high-grade cervical lesions or cancer (HSIL+).
Design
Cross-sectional study.
Setting
Ocean Road Cancer Institute (ORCI) and Kilimanjaro Christian Medical Center (KCMC), Tanzania.
Population
Women attending routine cervical cancer screening.
Method
We enrolled 4080 women (25–60 years) in the study. The women were interviewed on lifestyle habits, and tested for HIV. A cervical specimen for careHPV testing (performed at ORCI and KCMC), and a liquid-based cytology sample for HPV DNA detection using HC2 (performed at Tuebingen University Hospital, Germany) and for cytology assessment (performed at Vejle Hospital, Denmark) were obtained at a gynecological examination. Subsequently, VIA was performed. With cytology as gold standard, the sensitivity and specificity of careHPV, HC2, and VIA for detection of HSIL+ were calculated.
Results
Altogether, 23.6% had a positive careHPV test, 19.1% had positive HC2 test, and 6.3% had a positive VIA test. The sensitivity/specificity was 88.9%/78.9% for careHPV and 91.1%/83.7%, for HC2. VIA showed a low sensitivity of 31.1% but a high specificity (94.6%) for detection of HSIL+. The sensitivity of careHPV, HC2 and VIA was higher among younger women, and among HIV positive women. VIA triage of careHPV positive women improved specificity, but sensitivity dropped to 27%.
Conclusion
Our results confirm the low sensitivity of VIA for detection of HSIL+ and further document that careHPV test is promising as a primary screening method for cervical-cancer prevention in low-resource regions. A suitable triage test has to be identified.
Introduction
Cervical cancer is the fourth most common cancer among women in the world and the second most common in low income countries (LICs) [1]. In 2012, it was estimated that 580,000 new cases were diagnosed globally, of which 85% were from LICs [1,2]. With an annual incidence rate of 34.8 per 100.000 women and a mortality rate of 22 per 100.000 women, sub-Saharan Africa is the region with the highest burden of cervical cancer [3]. The corresponding figures for North America are 7.5 per 100,000 being diagnosed and 2.3 per 100.000 dying from the disease [4].
Cervical high-grade lesions and cervical cancer are caused by persistent infection with human papillomavirus (HPV). It has been shown that immunocompromised individuals who are positive to human immunodeficiency virus (HIV), have an increased risk of HPV infection [5]. Furthermore, a higher HPV prevalence has been documented in women with decreasing CD4 count [6]. Finally, studies have shown that HIV positive women are at increased risk of cervical cancer. Cervical cancer is preventable, and curable if detected at an early stages. In high income countries (HICs), screening against cervical cancer using cervical cytology has reduced the incidence of cervical cancer with more than 50% during the past 40 years [7]. In contrast, there has been observed no decline or only little decline in LICs [8]. Moreover in sub-Saharan Africa, it appears as the incidence has increased during the past years [9,10]. The high incidence of cervical cancer in sub Saharan Africa reflects a low coverage of cervical cancer screening as well as high prevalence of HPV and HIV [11].
Visual inspection with acetic acid (VIA) is used as a primary screening test in many LICs since it is cheap and easily accessible. One of the drawbacks of VIA is the subjectivity of the diagnosis. This is reflected in, a meta-analysis that reported a sensitivity range between 41% and 92% for the detection of high-grade cervical lesions [12]. In an increasing number of HICs, HPV DNA testing is used as the primary screening method followed by e.g. cytology as a triage test. HPV DNA testing has good sensitivity but the specificity is not as optimal due to the occurrence and detection of transient infections with no concomitant cervical lesions. However, most HPV DNA tests such as Hybrid Capture 2 (HC2) are expensive and require sophisticated infrastructure and qualified laboratory staff, which makes them unsuitable for most LICs. As a response, efforts have been made to manufacture less expensive and simple HPV DNA tests such as the careHPV test, which can be used at low costs, with minimal expertise and at the point of care [13]. The performance of careHPV has been evaluated in a recent meta-analysis and the test was found to have good sensitivity for the detection of CIN2+ and CIN3+ [14]. Based on these encouraging findings, leading health agencies, including the WHO, are increasingly recommending that simple and cheap HPV testing is used as primary screening modality in LICs followed by VIA [15,16]. While the performance of careHPV has been evaluated in specific focused studies in a few selected countries, it is not yet established how the test perform in a routine screening setting in a Tanzanian context.
The aim of this study was to examine the performance of careHPV, HC2, and VIA, the standard screening method in Tanzania, for detection of cytologically diagnosed cervical high grade lesions or cancer, overall and according to age and HIV status. Furthermore, we examined the value of VIA as a triage strategy following careHPV testing.
Materials and method
Enrolment and data collection
This study is a part of the CONCEPT project (Comprehensive prevention of cervical cancer in Tanzania), which is based on a collaboration between Ocean Road Cancer Institute (ORCI), Kilimanjaro Christian Medical Centre (KCMC), the Danish Cancer Society Research Center (DCS) and Southern University of Denmark (SDU).
Women were enrolled from the cervical cancer screening clinics at ORCI, KCMC and Kilimanjaro Regional Hospital (Mawenzi) in Tanzania. ORCI is the national designated institute for cancer care and treatment in Tanzania. It is located in Dar es Salaam region which has a population of 4,364,541 inhabitants based on National census data for 2012 [17]. KCMC and Mawenzi hospital are situated in Kilimanjaro region, which has a population of 1,640,087 according to the 2012 census report [17]. Both hospitals serve as referral hospitals for people living in the northern zone of Tanzania.
Women aged 25–60 years who attended routine cervical cancer screening were eligible for the study. Exclusion criteria were being pregnant, previous history of cervical precancerous lesion, known allergy to acetic acid and having menstrual period. We aimed at including at least 500 HIV positive women.
The enrolment was done from August 2015 to November 2017 and eligible women underwent face-to-face interview to obtain information on socio-demographic and lifestyle factors followed by voluntarily HIV testing. They were then directed to a special designated room for gynaecologic examination and cervical sample collection. First, a cervical swab for careHPV was taken followed by a swab for LBC and HC2. Subsequently, VIA was performed as routine per Tanzania cervical cancer screening guideline [18].
HIV analysis
All women with unknown HIV status were invited to be tested for HIV according to the Tanzanian HIV protocol [19]. The obtained blood samples were tested by using SD bio-line HIV 1/2 3.0 rapid test (Standard Diagnostics, South Korea) and positive results were confirmed by Uni-gold (RecombigenVR HIV; Trinity Biotech, USA). HIV positive women were linked to the care and treatment clinic at the respective sites for further follow up and treatment of their clinical condition.
HPV DNA detection
Testing with careHPV
The first cervical samples taken were kept in careHPV collection medium (QIAGEN GmbH,D-40724 Hilden, China) and taken to the local laboratory at either ORCI or KCMC where they were stored at room temperature (max 25֯C) for a maximum of two weeks and analysed for high risk (HR) HPV using careHPV machine. The machine enables the detection of at least 13 HR HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68). The principle of the careHPV test is that it targets HPV DNA from lysed cells which is denatured and hybridized by complementary RNA, then captured by antibodies coated on the magnetic beads. The captured hybrids are detected by alkaline phosphatase conjugate, which reacts with an added chemo-luminescent substrate to produce light which is proportional to the number of bound alkaline phosphatase molecules per target [13]
Testing with HC2
This procedure has previously been described in detail [20]. In brief, after cytological assessment in Denmark, aliquot of the samples were sent to Section for Experimental Virology, Tubingen University in Germany for HC2 testing. The samples were tested for HR HPV using the HR HPV probe, which can detect 13 HR HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68). The samples were recorded as positive if attained or exceeded the United States Food and Drug Administration-approved threshold of 1.0 pg HPV DNA/ml, which corresponds to 1.0 relative light unit coefficient (RLU/ CO).
Liquid based cytology
Following cervical sample collection, the samples were kept in PreServCyt solution (Hologic,Inc. 250 Campus Drive Marlborough, MA 01752 USA) and stored at room temperature (max 25֯C) in the laboratories at ORCI and KCMC. The PreServCyt vials were shipped to the Pathology Department at Lillebaelt Hospital, Vejle, Denmark, and processed on the ThinPrep 5000 Autoloader Instrument, Hologic according to manufactures instruction and stained with ThinPrep Stain. The slides were scanned by the ThinPrep Imaging System with selection of 22 Fields of view which was reviewed by cytotechnologist in Review Scopes. The specimens were evaluated for adequacy and for abnormal cells. If abnormal cells were detected the slides were consulted with an expert gyne-pathologist for the final diagnosis. The specimens were all evaluated, without knowledge of clinical or any other information, according to the Bethesda Nomenclature for System for Cervical Cytology 2014 [21]. The adequate samples were categorized as follows: Negative for intraepithelial lesion (NILM), ASCUS, ASCH, LSIL, HSIL and squamous cell carcinoma for squamous lesion and AGC, AIS and adenocarcinoma for glandular lesions. In the inadequate samples, no abnormal cells were detected and for the purpose of this study, they were included in the NILM category. In this paper, HSIL+ includes HSIL, squamous cell carcinoma in situ, AIS, squamous cell carcinoma, and adenocarcinoma.
VIA
This procedure was performed as routinely done in the clinics in Tanzania. A cotton-tipped swab was soaked in 5% acetic acid and then applied at the cervix and left for about 1 minute to allow changes to occur. Women with well-defined and distinct aceto-white lesion were termed as being VIA positive (having signs of cervical precancerous lesion) and those with no aceto-white lesion as VIA negative. VIA positive women were treated by cryotherapy or loop electrosurgical excision procedure (LEEP) according to the extension of the lesion.
Statistical analysis
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for careHPV, HC2, and VIA using cytology as “gold standard” with a threshold of HSIL+. We also calculated sensitivity, specificity and predictive values according to age and HIV status for all three tests, and in addition, we examined the sensitivity, specificity and predictive values for careHPV with VIA as a triage test. All analyses were performed using Stata.
Ethical consideration
Ethical clearance for the CONCEPT project was obtained from the National Institute for Medical Research in Tanzania, reference number NIMR/HQ/R.8a/Vol. IX/1955.
Detailed information about the study was provided to women before they gave informed consent and signed a consent form to take part in the study. All women with a cervical lesion were treated with either cryotherapy or LEEP according to the extent of the lesion. HIV positive women were referred to the nearest care and treatment clinic for further follow up of their clinical condition. In relation to the recruitment process, 39 women with invasive cancer and two women with carcinoma in situ were identified. These women were referred for treatment at ORCI and KCMC, respectively, according to the Tanzanian treatment protocol for cervical cancer.
Results
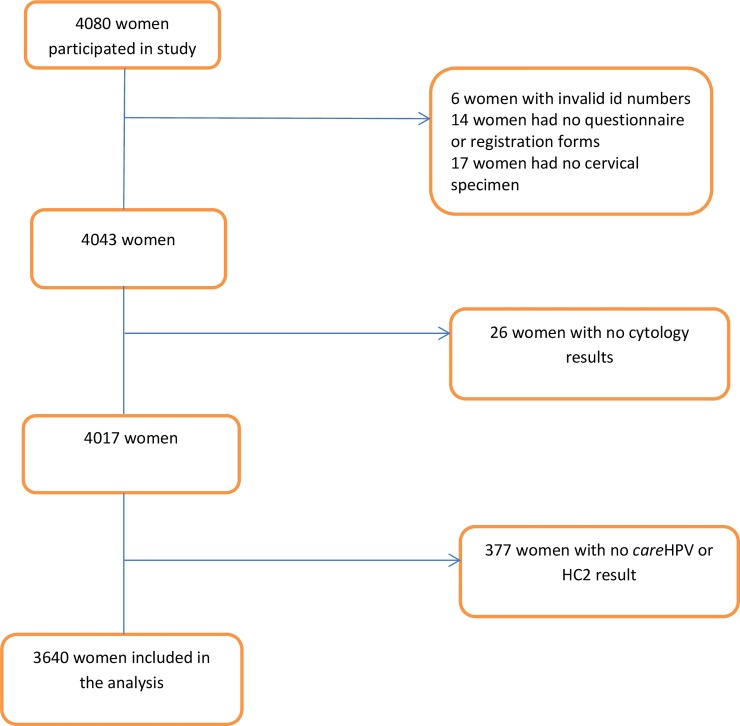
In all, 4080 women were enrolled in the CONCEPT project. A total of 37 women were excluded from the analysis due to incomplete questionnaire or registration forms (n = 14), lack of cervical specimens (n = 17) and invalid study number (n = 6). We also excluded 26 women where the cytology result was lacking, and 377 with no matched careHPV and HC2 results, leaving 3640 women for analysis (Fig 1).
Fig 1. Schematic flow chart of the study population.
Socio-demographic characteristics of the study population are displayed in Table 1. The mean age of the study women was 40 years (±4sd). Most women were married or cohabiting (71.1%), and the majority (64.7%) had attended primary school. Of the 3640 women included in this paper, 3209 (88.1%) had normal cytology, 299 (8.2%) had ASCUS/LSIL (of these 66% were ASCUS), and 135 (3.7%) had a cytological diagnosis of HSIL+.
Table 1. Socio-demographic and cytology characteristics of the study participants (N = 3640).
| Variable | Frequency (n) | Percentage (%) |
|---|---|---|
| Age | ||
| 25–29 | 459 | 12.6 |
| 30–34 | 522 | 14.3 |
| 35–44 | 1393 | 38.3 |
| 45–54 | 1058 | 29.1 |
| 54–64 | 206 | 5.7 |
| Missing | 2 | |
| Marital status | ||
| Married/cohabiting | 2572 | 71.1 |
| Single | 421 | 11.6 |
| Divorce/widow | 628 | 17.3 |
| missing | 19 | |
| Education | ||
| No formal education | 120 | 3.3 |
| Primary school | 2355 | 64.7 |
| Secondary school | 797 | 21.9 |
| College | 367 | 10.1 |
| missing | 1 | |
| Cytology | ||
| Normal | 3206 | 88.1 |
| LSIL | 102 | 2.8 |
| Atypical cells | 197 | 5.4 |
| HSIL+ | 135 | 3.7 |
Among all women, 23.6% (859/3640) tested positive for careHPV, 19.1% (696/3640) tested positive for HC2 and 6.3% (231/3640) tested positive for VIA. For all three methods, the prevalence of test positive women decreased with increasing age. The proportion of women positive to respectively careHPV, HC2 or VIA was higher among HIV positive women, with positivity rates of 36.4%, 33.8% and 10.6, respectively. The corresponding figures among HIV negative women were 20.0%, 15.9% and 5.4% respectively. Table 2.
Table 2. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of careHPV, Hybrid Capture 2 (HC2), and visual inspection with aceto acid (VIA) in detection of cervical high-grade squamous intraepithelial neoplasia or cancer (HSIL+) among all women, and stratified by age, and HIV status.
| Total | HSIL+ | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|
| N (%) | n (%) | (95% CI) | (95% CI) | (%) | (%) | |
| Overall | ||||||
| (25–60) | ||||||
| CareHPV | 859 (23.6) | 120 (14.0) | 88.9 (82.4–93.6) | 78.9 (77.5–80.3) | 14.0 | 99.4 |
| HC2 | 696 (19.1) | 123 (17.8) | 91.1 (85.0–95.3) | 83.7 (82.4–84.9) | 17.7 | 99.6 |
| VIA | 231 (6.3) | 42 (18.2) | 31.1 (23.4–39.6) | 94.6 (93.8–95.3) | 18.2 | 97.3 |
| (30–60) | ||||||
| CareHPV | 729(22.9) | 115(15.8) | 88.5 (81.7–93.4) | 79.9 (78.4–81.3) | 15.8 | 99.4 |
| HC2 | 574(18.1) | 118(20.6) | 90.8 (84.4–95.1) | 85.0 (83.7–86.3) | 20.6 | 99.5 |
| VIA | 202(6.3) | 41 (20.3) | 31.5 (23.7–40.3) | 94.7 (93.9–95.5) | 20.3 | 97.0 |
| By age | ||||||
| 25–29 | ||||||
| CareHPV | 130 (28.1) | 5 (3.8) | 100 (47.8–100) | 73.0 (68.7–77.0) | 3.9 | 100 |
| HC2 | 122 (26.3) | 5 (4.1) | 100 (47.8–100) | 74.6 (70.3–78.5) | 4.2 | 100 |
| VIA | 29 (6.3) | 1 (3.4) | 20.0 (0.5–71.6) | 93.8 (91.2–95.8) | 3.5 | 99.1 |
| 30–34 | ||||||
| CareHPV | 132 (25.3) | 15 (11.4) | 93.8 (69.8–99.8) | 76.9 (73.0–80.5) | 11.4 | 99.7 |
| HC2 | 119 (22.8) | 15 (12.6) | 93.8 (69.8–99.8) | 79.4 (75.7–82.9) | 12.6 | 99.8 |
| VIA | 38 (7.3) | 7 (18.4) | 43.8 (19.8–70.1) | 93.9 (91.4–95.8) | 18.4 | 98.1 |
| 35–44 | ||||||
| CareHPV | 339 (24.3) | 50 (14.7) | 92.6 (82.1–97.9) | 79.2 (76.9–81.3) | 15.2 | 99.6 |
| HC2 | 267 (19.2) | 51 (19.1) | 94.4 (84.6–98.4) | 83.9 (81.8–85.8) | 19.1 | 99.7 |
| VIA | 99 (7.1) | 20 (37.0) | 37.0 (24.3–51.3) | 94.1 (92.7–95.3) | 20.2 | 97.4 |
| 45–54 | ||||||
| CareHPV | 216 (20.4) | 39 (18.8) | 79.6 (65.7–89.8) | 83.4 (80.9–85.6) | 18.8 | 98.8 |
| HC2 | 157 (14.8) | 43 (27.4) | 87.8 (75.2–95.4) | 88.7 (86.6–90.6) | 27.4 | 99.3 |
| VIA | 57 (5.3) | 14 (24.6) | 28.6 (16.6–43.3) | 95.7 (94.3–96.9) | 24.6 | 96.5 |
| 55–60 | ||||||
| CareHPV | 42 (20.4) | 9 (21.4) | 81.8 (47.2–97.7) | 83.1 (77.1–88.1) | 21.3 | 98.8 |
| HC2 | 31 (15.0) | 9 (29.0) | 81.8 (48.2–97.7) | 88.7 (83.4–92.8) | 21.3 | 98.9 |
| VIA | 8 (3.9) | 0 | 0.0 (0.0–28.5) | 95.5 (92.1–98.2) | 0.0 | 94.4 |
| By HIV status | ||||||
| HIV positive | ||||||
| CareHPV | 242 (37.2) | 61 (25.2) | 92.4 (83.2–97.5) | 69.1 (65.1–72.8) | 25.2 | 98.8 |
| HC2 | 220 (33.8) | 63 (28.6) | 95.5 (87.3–99.1) | 73.2 (69.4–76.7) | 28.6 | 99.3 |
| VIA | 69 (10.6) | 20 (29.0) | 30.3 (19.6–42.9) | 91.6 (89.1–93.7) | 29.0 | 92.1 |
| HIV negative | ||||||
| CareHPV | 617 (20.7) | 59 (9.6) | 85.5 (75.0–92.8) | 80.9 (79.4–82.3) | 9.7 | 99.6 |
| HC2 | 476 (15.9) | 60 (12.6) | 87.0 (76.7–93.9) | 85.8 (84.4–87.0) | 12.6 | 99.6 |
| VIA | 162 (5.4) | 22 13.6) | 31.9 (21.2–44.2) | 95.2 (94.4–96.0) | 13.6 | 98.3 |
The test performance of careHPV, HC2 and VIA in detecting HSIL+ lesions are shown in Table 2. Women in the age group 25–29 showed slightly higher sensitivity of both for CareHPV and HC2compared to age group 30–34, but a lower sensitivity of VIA. However, there was no significant differences in sensitivity in the overall analysis between the two groups 25–64 years and 30–64 years old women. Among all women, careHPV had a sensitivity of 88.9% (95% CI: 82.4–93.9) and a specificity of 78.9% (95% CI: 77.5–80.3). The HC2 test had a slightly higher sensitivity of 91.1% (95% CI: 85.0–95.3) and a slightly higher specificity of 83.7% (95% CI: 82.4–84.9). In contrast to these findings, VIA was found to have a low sensitivity of 31.1% (95% CI: 23.4–39.6) but a comparatively higher specificity of 94.6% (95% CI: 93.8–95.3). The sensitivity of both careHPV, HC2, and VIA tended to decrease with increasing age. For careHPV, and HC2, the specificity increased with older age, whereas for VIA the specificity was constantly high. Among HIV positive women, the sensitivity of careHPV (92.4%), and HC2 (95.5%) was higher than among HIV negative women (85.5%), and (87.0%), whereas the sensitivity did not vary according to HIV status for VIA. In contrast, specificity was lower among HIV positive women than among HIV negative women for all three tests.
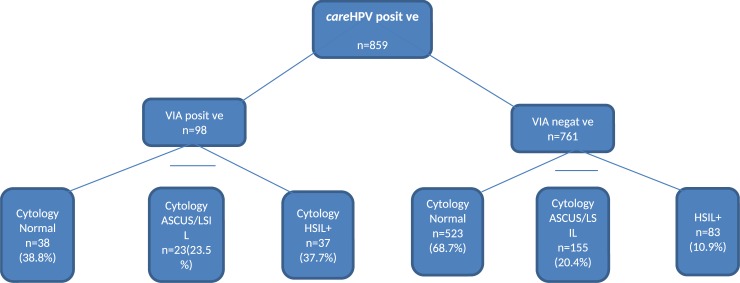
We also examined the performance of VIA as a triage test among the 859 careHPV positive women (Fig 2). In all, 98 women were both careHPV positive and VIA positive, among these 37 women (38.9%) had HSIL+, 38 (38.8%) had normal cytology, 23 (23.4%) had an ASCUS/LSIL diagnosis. Altogether 761 women were careHPV positive but VIA negative, and among these, 523 women (68.7%) had normal cytology and 155 (20.4%) had ASCUS/LSIL, whereas 83 women (10.9%) had HSIL+.
Fig 2. Overview of women’s cytology results distribution according to VIA results among careHPV positive.
Discussion
In more than 3600 women from a Sub-Saharan country (Tanzania), we evaluated test performance of careHPV, HC2 and VIA to detect cervical high-grade lesions with expert reviewed liquid-based cytology as the gold standard. Compared to HC2, careHPV had an only slightly lower sensitivity. In contrast, the test performance of VIA was poor. When using VIA as a triage test among careHPV positive women, the sensitivity decreased even further, whereas the specificity increased substantially.
Screening is an essential component in the prevention of cervical cancer, however, in LICs conventional screening approaches are hampered by complex infrastructure and lack of expert staff. The World Health Organization therefore advocates a policy of 'screen and treat' for cervical screening in LICs. This involves VIA followed by cervical cryotherapy or LEEP if indicated. The performance of VIA as a primary screening tool for the detection of cervical pre-cancer and cancer has, however, shown rather inconsistent results. It is therefore increasingly being questioned whether VIA can stand alone as a scaled up screening procedure due to its high inter-operator variability and low sensitivity [22], and it has been suggested that HPV testing can be provided at point of care in LICs.
In the present study, the sensitivity of careHPV (88.9%) was slightly lower than that of HC2 (91%) in detecting HSIL+, and a similar picture was found for the specificity (80% for careHPV and 84% for HC2). This level of accuracy is similar to that from previous studies in Africa and other low resource settings [14,23,24] and is only slightly lower than in a recent study of more than 7,500 women from rural China [25]. The finding of the present study is promising, especially when keeping in mind that careHPV analyses were done at the point of care in a LIC by newly trained and inexperienced laboratory technicians, while HC2 analyses were performed in a sophisticated environment with experienced laboratory staff in a HIC.
In contrast, we found a poor sensitivity for VIA (~31%) to detect high-grade cervical precancer lesions; VIA failed to detect 97 out of 135 HSIL+ cases, and among women with a positive VIA test, only 18.2% had HSIL+ on cytology. These VIA results are similar to a recent study that we performed among more than 3000 Tanzanian women (sensitivity: 28%) [14] and in some other studies [24,26] but the sensitivity is lower than that from a recent meta-analysis reporting a pooled sensitivity estimate of 82% for detection of CIN2+ [27]. The low VIA sensitivity in our study may reflect the nature of the test itself, which is subjective and related to the competence of health provider. The health providers involved in this study were experienced and had refresher training just before implementing the study, however, the VIA test was still found to have a low sensitivity in our hands. The findings from the present study show that careHPV testing is efficient as a primary screening method for cervical cancer in Tanzania. However, the test cannot stand alone and HPV positive women should be offered triage. This information is important for Tanzanian policymakers and will help them in their efforts in better controlling cervical cancer.
The sensitivity varied with age for all three tests, and was lower in the oldest age group (55–64 years) (~82% for careHPV and HC2; 0% for VIA) than in the youngest (25–34 years) (~95% for careHPV and HC2; 38% for VIA). This is in line with some studies [14,28] but not all [29]. For VIA this could be due to the fact that as women age there is a tendency of the transformation zone to move inside the cervical channel, making it more difficult to visualize which may imply a decrease in sensitivity of the test. This underlines that one of the limitations of VIA is the low sensitivity, especially for older women [22,30]. The currently available results regarding the test performance of careHPV in relation to age are equivocal as some find an association with age and cut off value [31], whereas others do not [24], so more studies are needed to solve this. The sensitivity of both tests was higher among HIV positive women compared to HIV negative. Higher HPV positivity is noted among HIV positive women probably due to immune suppression that causes HPV to infect and grow easily. These findings are in line with other studies from LICs [14,32].
Although in our study careHPV was a little less sensitive than HC2, it performed substantially better than VIA, and would have treated the majority of HSIL+ cases. However, at the same time a large proportion of careHPV positive women had normal cytology, or ASCUS/LSIL lesions that in the majority of cases will regress. Consequently, there is a need for a triage test to minimize referral and overtreatment. When we applied VIA as a triage strategy, we substantially increased the positive predictive value and specificity, but at the cost of sensitivity, that dropped to an unacceptably low level (27%). Our results are in agreement with a recent, large study in a low-resource setting in China [24]. As performance of VIA is so variable in different settings, one solution does not seem to fit all, and in areas with poor VIA performance, alternative triage strategies such as different cut off value on the HPV test or use of other biomarkers should be considered. Finally, in contrast to VIA, careHPV testing allows for homebased testing which may help overcome many of the personal and system-level screening barriers, which are especially pronounced in low income countries.
This study has several strengths. First, it has a large sample size making it possible to conduct stratified analyses according to age and HIV status. In addition, the study was performed in both an urban and a rural area, which enhances the representativity of the study population. We used presence of HSIL+ as threshold, since lower grade lesions are common abnormal cytological findings which usually regress spontaneously. Hence if women with lower grade lesions are considered as test positive it would lead to a high number of false positive findings and risk of overtreatment and treatment complications. In low-income countries, such an approach will have great negative impact on the scarce resources available. We did not have access to histological diagnosis and had to rely on a cytological diagnosis of HSIL+ as gold standard. This could be considered a limitation, as it may be argued that our findings are not fully comparable with studies where the diagnosis has been histologically verified. Consequently, our results should be interpreted with caution.
VIA is an inexpensive and simple test, but it is subjective and highly dependent of the skills and experience of the user, and therefore the accuracy is not optimal and inter-observer variability is high. Still, the implementation of VIA in many LICs may have paved the way for a screening infrastructure. However, the poor performance of the VIA test in some settings may indicate a need for rethinking its use in the future. As VIA mostly has a good specificity, it has been suggested that screen positive women subsequently could be triaged by VIA. However, if such an approach is to be successful, it requires that the health staff undergo thorough and continued training in how to interpret VIA results. With the increasing use of the Internet and smart-phones also in low-resource areas, a future solution for improved cervical cancer screening could be to consider computer assisted visual evaluation such as digital cervicography where pictures of the VIA test are shared with different experts for 2nd opinion to optimize quality. This program has been implemented elsewhere and has shown promising results for rapid scale up of cervical cancer screening [33,34]. Another option is the use of telepathology where cytologies/biopsies are obtained among HPV positive women, processed into slides and stained. The pictures are subsequently shared via the Internet [35].
In conclusion, our results show that the careHPV and HC2 tests had high sensitivity whereas VIA had much lower sensitivity in detecting cytologically diagnosed high-grade lesions or cancer (HSIL+). Based on our findings, implementation of an HPV-based test such as careHPV as a primary screening test should be considered in Tanzania. An additional advantage of introducing careHPV testing is the possibility of obtaining self-collected specimens, which makes it possible to reach more women with the screening program and focus resources only on those who test HPV positive. However, to improve the specificity and positive predictive value of HPV-based screening tests, it is necessary to have a secondary test in screen positive women. Moreover, it is important that the triage test does not significantly lower the sensitivity of the primary test. Finally, in areas with high prevalence of HIV positive women, it is important that the performance of the tests is as good in HIV positive women as in HIV negative women.
Supporting information
(PDF)
(PDF)
Acknowledgments
We thank the nursing staff at ORCI, KCMC and Mawenzi hospital cervical cancer screening clinics for their assistance and devotion in performing the interviews and obtaining cervical samples from the women. We also thank all PhD students in the CONCEPT project for the coordination and smooth running of the project.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Authors VR and JM received award from Danish International Development Agency (DANIDA) (project no 14-TAN-P02). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.International Agency for Research on Cancer (IARC). Latest world cancer statistics, Global cancer burden raises to 14.1 million new cases in 2012. 2013 Available at www.iarc.fr
- 2.World Health Organization. Human papillomavirus and cervical cancer.2015; Available at www.who.int
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo N et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer, 2015; 136(5), pp. E359–E386. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 4.Surveillance, Epidemiology and End Results Program (SEER). Cancer statistics. 2018, available at seer.cancer.gov
- 5.Luchters S, Broeck D, Chersich M, Nel A, Delva W, Mandaliya K et al. Association of HIV infection with distribution and viral load of HPV types in Kenya: a survey with 820 female sex workers. BMC Infectious Diseases 2010, 10:18 10.1186/1471-2334-10-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agaba P, Thacher T, Ekwempu C, Idoko J. Cervical dysplasia in Nigerian women infected with HIV. International Journal of Gynecology and Obstetrics 2009, 107:99–102 10.1016/j.ijgo.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 7.Surveillance, Epidemiology and End Results Program (SEER). Cancer Statistics Review (CRS) 1975–2015. 2018, Available seer.cancer.gov
- 8.Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S and Bray F. Worldwide trends in cervical cancer incidence: Impact of screening against changes in disease risk factors. European Journal of Cancer 49 2013; 3262–3273 10.1016/j.ejca.2013.04.024 [DOI] [PubMed] [Google Scholar]
- 9.Somdyala N, Parkin D, Sithole N and Bradshaw D. Trends in cancer incidence in rural Eastern Cape Province; South Africa, 1998–2012. International Journal of Cancer. 2015; 136(5), pp. E470–E474. 10.1002/ijc.29224 [DOI] [PubMed] [Google Scholar]
- 10.Wabinga HR, Parkin DM, Wabwire-Mangen F and Bradshaw D. Trends in cancer incidence in Kyadondo County. British Journal of Cancer. 2000; 82(9), pp. 1585–1592. 10.1054/bjoc.1999.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louie K, de Sanjose S and Mayaud P. Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: a comprehensive review. Tropical Medicine and International Health. 2009; 14(10) pp 1287–1302 10.1111/j.1365-3156.2009.02372.x [DOI] [PubMed] [Google Scholar]
- 12.Sauvaget C, Fayette J, Muwonge R, Wesley R and Sankaranarayanan R. Accuracy of visual inspection with acetic acid for cervical cancer screening. International Journal of Gynecology and Obstetrics. 2011; 113, 14–24 10.1016/j.ijgo.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 13.Qiao L, Li B, Long M, Wang X, Wang S and Zhang G. Accuracy of visual inspection with acetic acid and with Lugol’s iodine for cervical cancer screening: Meta-analysis. Journal of Obstetrics and Gynaecology Research. 2015; 41(9), pp. 1313–1325. 10.1111/jog.12732 [DOI] [PubMed] [Google Scholar]
- 14.Dartell M, Rasch V, Iftner T, Kahesa C, Mwaiselage J, Junge J et al. Performance of visual inspection with acetic acid and human papillomavirus testing for detection of high-grade cervical lesions in HIV positive and HIV negative Tanzanian women. International Journal of Cancer. 2014; 135(4), pp. 896–904. 10.1002/ijc.28712 [DOI] [PubMed] [Google Scholar]
- 15.Costa S, venturoli S, Origoni M, Preti M, Mariani L, Cristoforoni P et al. Performance of HPV DNA testing in the follow–up after treatment of high grade cervical lesions, adenocarcinoma in situ (AIS) and micro invasive carcinoma. Ecancer. 2015; 9: 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cronje HS. Screening for cervical cancer in developing countries. International jounal of gynecology and obstetrics. 2014; 84 (2): 101–108. [DOI] [PubMed] [Google Scholar]
- 17.Tanzania national bureau of statistics. Population and housing census. 2012; available at www.nbs.go.tz
- 18.Ministry of health, community development, gender, elderly and children of Tanzania. Tanzania service delivery guideline for prevention and control. Tanzania. 2011.
- 19.National guideline for the management of HIV and AIDS. 5th edition National AIDS control program; 2015 [Google Scholar]
- 20.Thomsen L, Frederiksen K, Munk C, Junge J, Iftner T and Kjaer S. Long-term risk of cervical intraepithelial neoplasia grade 3 or worse according to high-risk human papillomavirus genotype and semi-quantitative viral load among 33,288 women with normal cervical cytology. International Journal of Cancer. 2015;137, 193–203 10.1002/ijc.29374 [DOI] [PubMed] [Google Scholar]
- 21.Nayar R. and Wilbur D. C. The Pap test and Bethesda 2014. Cancer Cytopathology. 2015; 123(5), pp. 271–281. 10.1002/cncy.21521 [DOI] [PubMed] [Google Scholar]
- 22.Silkensen SL, Schiffman M, Sahasrabuddh V and Flanigana JS. Is It Time to Move Beyond Visual Inspection With Acetic Acidfor Cervical Cancer Screening? Global Health: Science and Practice. 2018;6 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeronimo J, Bansil P, Lim J, Peck R, Paul P, Amador JJ et al. A Multicountry Evaluation of careHPV Testing, Visual Inspection With Acetic Acid, and Papanicolaou Testing for the Detection of Cervical Cancer. International Journal of Gynecological Cancer. 2014; 24 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Hu S, Zhao S, Zhang W, Pan Q, Zhang X et al. Accuracy of triage strategies for human papillomavirus DNA- positive women in low-resource settings: A cross-sectional study in China. Chin J Cancer Res 2017;29(6):496–509 10.21147/j.issn.1000-9604.2017.06.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao F, Jeronimo J, Qiao Y, Schweizer J, Chen W, Valdez W et al. An Evaluation of Novel, Lower-Cost Molecular Screening Tests for Human Papillomavirus in Rural China. American Association for cancer research. 2013 [DOI] [PubMed] [Google Scholar]
- 26.Umulisa1CM, Franceschi S, Baussano I, Tenet V, Uwimbabazi M, Rugwizangoga B et al. Evaluation of human-papillomavirus testing and visual inspection for cervical cancer screening in Rwanda. BMC Women's Health.2018; 18:59 10.1186/s12905-018-0549-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly H, Mayaud P, Segondy M, Pai NP, Peeling RW. A systematic review and meta-analysis of studies evaluating the performance of point-of-care tests for human papillomavirus screening. Sex Transm Infect 2017;(93):S36–S45. [DOI] [PubMed] [Google Scholar]
- 28.Holt HK, Zhang L, Zhao F, Hu S, Zhao X, Zhang X et al. Evaluation of multiple primary and combination screening strategies in postmenopausal women for detection of cervical cancer in China. International Journal of Cancer. 2017;140, 544–554 10.1002/ijc.30468 [DOI] [PubMed] [Google Scholar]
- 29.Poomtavorn Y and Suwannarurk K. Accuracy of Visual Inspection with Acetic acid in Detecting High-Grade cervical Intraepithelial Neoplasia in Pre- and Post-Menopausal Thai Women with Minor Cervical Cytological Abnormalities. Asian Pacific Journal of Cancer Prevention. 2015;16 (6), 2327–2331. 10.7314/apjcp.2015.16.6.2327 [DOI] [PubMed] [Google Scholar]
- 30.Cremer M, Conlisk E, Maza M, Bullard K, Peralta E, Siedhoff M et al. Adequacy of visual inspection with acetic acid in women of advancing age. International Journal of Gynecology and Obstetrics;2011(113): 68–71 [DOI] [PubMed] [Google Scholar]
- 31.Kang L, Jeronimo J, Qiao Y, Zhao F, Chen W, Valdez M, et al. Optimal Positive Cutoff Points for careHPV Testing of Clinician- and Self-Collected Specimens in Primary Cervical Cancer Screening: an Analysis from Rural China. Journal of Clinical Microbiology. 2014; 52 (6), 1954–1961. 10.1128/JCM.03432-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKenzie ND, Kobetz EN, Ganjei-Azar P, Rosa-Cunha I, Potter JE, Morishita A et al. HPV in HIV-infected women: implications for primary prevention. Front. Oncol; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bateman AC, Parham GP, Sahasrabuddhe VV, Mwanahamuntu MH, Kapambwe S, Katundu K et al. Clinical Performance of Digital Cervicography and Cytology for Cervical Cancer Screening in HIV-infected Women in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2014; 67(2): 212–215 10.1097/QAI.0000000000000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Firnhaber C, Mao L, Levin S, Faesen M, Lewis DA, Goeieman BJ et al. Evaluation of a Cervicography-Based Program to Ensure Quality of Visual Inspection of the Cervix in HIV-Infected Women in Johannesburg, South Africa. Journal of Lower Genital Tract Disease, 2015;(19): 7–11 10.1097/LGT.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams S, Henricks W, Becich M, Toscano M and Carter AB. Telepathology for Patient Care: What Am I Getting Myself Into? Adv Anat Pathol, 2010;17:130–149 10.1097/PAP.0b013e3181cfb788 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.