Abstract
Introduction
Response rates in health research are declining, and low response rates could result in biased outcomes when population characteristics of participants systematically differ from the non-respondents. Few studies have examined key factors of non-response beyond demographic characteristics, such as behavioral and psychological factors. The aim of the current study was to identify predictors of non-response and loss to follow-up in a longitudinal sexual health study.
Materials and methods
A longitudinal cohort study (iMPaCT) was conducted from November 2016 to July 2018 among heterosexual STI clinic visitors aged 18–24 years. At four different time points in one year, data was collected on sexual behavior, psychological determinants and chlamydia infections. The national STI surveillance database provided data on demographic, behavioral and sexual health-related characteristics for non-respondents. Predictors of non-response at baseline and of loss to follow-up were identified using multivariable logistic regression analyses.
Results
In total, 13,658 STI clinic visitors were eligible to participate, of which 1,063 (8%) participated. Male gender, low/medium education level, young age (≤ 20 years) and having a non-Dutch migration background were significant predictors of non-response at baseline. Furthermore, non-respondents at baseline were more likely to report STI-related symptoms, to have been notified by a partner, to have had condomless sex, and to have had ≤ 2 partners in the past six months, compared to participants. Psychological predictors of loss to follow-up differed between STI clinic regions, but low perceived importance of health at baseline was associated with loss to follow-up in all regions. The baseline chlamydia positivity rate was significantly higher in the non-respondents (17%) compared to the participants (14%), but was not a predictor of loss to follow-up.
Discussion
Targeted recruitment aimed at underrepresented groups in the population based on demographic, behavioral and psychological characteristics, might be necessary to decrease loss to follow-up, and to prevent non-response bias in health research.
Introduction
Response rates in health research have been declining over the past decades [1, 2]. This decline is concerning, especially because low response rates can lead to systematic differences in population characteristics between participants and non-respondents [3–5]. These differences in characteristics, hereafter referred to as non-response bias, could result in biased study outcomes [1, 2, 6, 7]. Strategies, such as data weighting techniques, can be used to reduce non-response bias, but the extent to which these strategies minimize bias is dependent on the availability of data on all potential factors that might be associated with non-response [8]. This highlights the importance of gaining insight into key factors of non-response.
Most studies exploring key factors of non-response examined differences in demographic characteristics between participants and non-respondents. These studies found that females, highly educated, or older individuals (> 24 years), are usually more likely to participate in health research than males, lower educated, or younger individuals (≤ 24 years) [3, 5, 8, 9]. Fewer studies also compared behavioral characteristics of participants and non-respondents. In sexual health-related research, these studies found that low-risk individuals, in terms of lower number of sexual partners or less often previously diagnosed with a sexually transmitted infection (STI), were less likely to participate than high-risk individuals [5, 10, 11]. One study explored psychological characteristics in sexual health research context and found that non-respondents had less reward dependent and more harm-avoidant personalities than participants [12]. These findings suggest that identifying key factors of non-response should not be limited to only demographic characteristics.
In addition to low response rates during recruitment, loss to follow-up in longitudinal cohort studies could also lead to non-response bias when characteristics and health outcomes of participants lost to follow-up are different from the participants who are retained in the study [13–15]. Previous research indicated that certain demographic characteristics related to non-response at recruitment, such as lower education or low socioeconomic status, were also associated with loss to follow-up in longitudinal cohort studies [14, 15]. Although some empirical studies identified general psychological drivers behind participation in longitudinal studies [16, 17], such as positive attitudes towards participating in surveys, few such studies were conducted in sexual health-related research. One study in sexual health context, found that patients with poor knowledge of STI and a higher level of perceived STI-related stigma were more likely to be lost to follow-up from STI care [18]. However, this study was conducted in a low-resource setting and only included STI diagnosed individuals.
The objectives of this study were to identify demographic, sexual health-related, and behavioral predictors of non-response during recruitment, and to identify demographic, sexual health-related, behavioral, and psychological predictors of loss to follow-up. Data from a longitudinal cohort study in the Netherlands called 'Mathematical models incorporating Psychological determinants: control of Chlamydia Transmission' (iMPaCT) offered a unique opportunity to analyze comprehensive information of both participants and non-participants during recruitment and loss to follow-up.
Materials and methods
Setting
A detailed description of the study design can be found in the iMPaCT study protocol [19]. In short, the iMPaCT study explored the link between sexual behavior, psychological determinants, and chlamydia infections over a period of one year, using online questionnaires and information routinely registered by STI clinics. All heterosexual males and females, and females who have sex with both males and females, aged 18 to 24 years, making an appointment at the STI clinics of the public health services in Amsterdam, Kennemerland, Hollands Noorden, and Twente in the Netherlands from November 2016 to June 2017 were eligible to participate in the iMPaCT study. Two different recruitment strategies were used. At the STI clinics in Amsterdam, Kennemerland, and Hollands-Noorden, individuals were invited to participate in the iMPaCT study after finishing the online intake assessment for an STI test. After agreeing to participate and signing the online informed consent form, they were automatically redirected to the online questionnaire assessing psychological and behavioral determinants (see S1 and S2 Files for original and English translation of the questionnaire, and the invitation). At the STI clinic in Twente, it was only possible to make an appointment by telephone, and individuals were informed about the iMPaCT study at the end of the intake assessment. If they agreed to participate, they received an e-mail with the link to the informed consent and the online questionnaire. Individuals who agreed to participate, provided informed consent and started the baseline questionnaire will hereafter be referred to as participants, and all other eligible STI clinic visitors will be referred to as non-respondents.
Participants were enrolled for one year, and online questionnaires were administered at four different time points: baseline, three-week follow-up, six-month follow-up, and one-year follow-up. Furthermore, participants were tested for chlamydia using nucleic acid amplification tests (NAAT) at enrolment at the STI clinic and through a self-sampling kit sent to a laboratory at six-month follow-up. Participants were invited for all follow-up data collection moments, if they completed the baseline questionnaire and provided a valid email address. Participants could be temporarily lost to follow-up (i.e., completed questionnaire at six-month and one-year follow-up, but did not respond to the three-week follow-up questionnaire) or permanently (i.e., completed questionnaire at three-week follow-up, but did not respond to subsequent questionnaires).
Several passive recruitment strategies were used to increase response rates and retention in the iMPaCT study. First, the STI clinic visitors were informed of receiving a free of charge home-based test kit for chlamydia and gonorrhea after completing the questionnaire at six-month follow-up, and a monetary incentive after completing the questionnaire at one-year follow-up. Second, during follow-up the participants received two reminders per questionnaire by e-mail: one week and two weeks after the invitation for each follow-up questionnaire. The iMPaCT study was approved by the Medical Ethical Committee of the University Medical Centre Utrecht, the Netherlands (NL57481.094.16/METC18-363/D).
Data non-response at baseline
The national STI surveillance database provided consultation data of all eligible STI clinic visitors during the recruitment period (November 2016—June 2017) in the four participating STI clinics. Participants were distinguished from the non-respondents in the consultation data using a unique iMPaCT study identification number. For individuals who visited the STI clinic more than once in the recruitment period, only the first consultation was included in the analyses, or, in case they agreed to participate in the iMPaCT study, the consultation linked to the iMPaCT questionnaire was included.
Demographic information obtained from the national STI surveillance database included age, gender (female/male), education level defined as highest attained degree or education currently enrolled in (low/medium: no education, primary education only, lower general secondary education and vocational education, high: all other education levels), STI clinic region (Amsterdam/Non-Amsterdam: Hollands Noorden/Kennemerland/Twente), and migration background (ethnic Dutch/non-Dutch). Migration background was based on the birth country of the participant and both parents consistent to the definitions used by Statistics Netherlands [20] and was categorized into two groups: Dutch (participant and both parents are born in the Netherlands) and non-Dutch (first-generation and second generation migrants from all other countries).Sexual health-related information included the STI test results at baseline (positive at any anatomic location/negative), type of STI test at baseline (regular consultation/self-sampling test kit), as well as STI-test in the past year, prior chlamydia/gonorrhea/syphilis diagnosis in the past year, and STI-related symptoms (all yes/no). Finally, the STI surveillance databases contained information on sexual behavior, including number of partners in the past six months, received partner notification (yes/no), and condom use most recent sex act (yes/no).
Data loss to follow-up
For participants, additional data on behavioral and psychological data was available from the baseline online questionnaire. The development of the questionnaire has been described in detail in the iMPaCT study protocol [19]. Behavioral data included the number of sexual partners in the past six months, and age at sexual debut. Psychological data included perceived importance of (sexual) health or “health goals”, attitudes regarding prevention of chlamydia, intentions towards condom use and STI testing in the future, anticipated stigma, shame and anxiety with regard to chlamydia diagnosis, self-efficacy regarding condom use, expected social support after chlamydia diagnosis, subjective and social norms regarding condom use and STI testing, self-esteem, impulsiveness, risk perception, and knowledge regarding sexual health, prevention of chlamydia and consequences of chlamydia diagnosis.
The psychological characteristics were assessed on 5-point Likert scales, except for risk perception and knowledge. The 5-point Likert scales ranged from 1 (i.e., low level of the determinant) to 5 (i.e., high level of the determinant), and a mean score was calculated for all psychological scales. Risk perception was assessed on a scale from 0% to 100%. Risk perception for chlamydia own risk was defined as the mean of the participants’ estimate of their own risk in the coming year and in their lifetime, and risk perception for chlamydia peers’ risk was defined as the mean of the participants’ estimate of the risk of their peers in the coming year and in their lifetime. Knowledge of sexual health in terms of prevention of chlamydia and consequences of chlamydia diagnosis, was assessed by six true/false/I don’t know items, and was defined as the sum score of six items based on the number of correct answers (zero to six). Subjective and social norms, and social support were combined into one variable reflecting social environment. The score of each psychological determinant was divided in two categories at the median: low/median = lower than median, high = equal to or higher than median.
Statistical analyses
To identify predictors for non-response at baseline, univariable and multivariable logistic regression analyses were performed using data from the national STI surveillance database on participants and non-respondents. All demographic, sexual health-related, and behavioral baseline characteristics were included in the univariable and multivariable analyses. Chlamydia positivity rates at baseline were compared between participants and non-respondents using a chi-squared test.
Predictors for loss to follow-up were also identified with univariable and multivariable logistic regression analyses, using data collected at baseline. The response to each follow-up moment was analyzed separately, as participants could be temporarily lost to follow-up. First, baseline characteristics were compared between participants who completed the questionnaire at three-week follow-up and participants who did not respond to the questionnaire invitation at three-week follow-up. Second, baseline characteristics were compared between participants who completed the questionnaire at six-month follow-up and participants who did not respond to the questionnaire invitation at six-month follow-up. Last, baseline characteristics were compared between participants who completed the questionnaire at one-year follow-up and participants who did not respond to the questionnaire invitation at one-year follow-up. In the univariable analyses, the baseline chlamydia test results, and the behavioral and psychological variables from the baseline questionnaire, were included in addition to the demographic, sexual health-related and behavioral variables from the national STI surveillance database. As the number of potential variables for the multivariable model was relatively high in relation to the sample size, the baseline variables included in the multivariable analyses were pre-selected in the univariable analyses using a p-value criterion of 0.1. Variables were excluded from the multivariable model if the number of observations per outcome category was too small in relation to the number of predictors [21]. If a variable was associated with loss to follow-up at either the three-week, six-month and/or one-year follow-up in the univariable analyses, it was included in all multivariable models.
All multivariable models (non-response and loss to follow-up) were constructed using a backward elimination procedure, based on the lowest Akaike information criterion (AIC) score. Interaction terms were added to the multivariable model, and if statistically significant, stratified analyses were shown. Missing values were included as a separate category if more than 5% of the observations were missing. Multicollinearity was evaluated using the Variance Inflation Factor (VIF) (VIF values above 5 indicating multicollinearity) [22], and variables that were highly correlated with other predictors were removed from the multivariable model. Goodness of fit of the model was examined using the Hosmer-Lemeshow (non-significant p-value indicating good fit) [23]. All statistical analyses were done using R version 3.4.0 [24].
Results
Study population and response
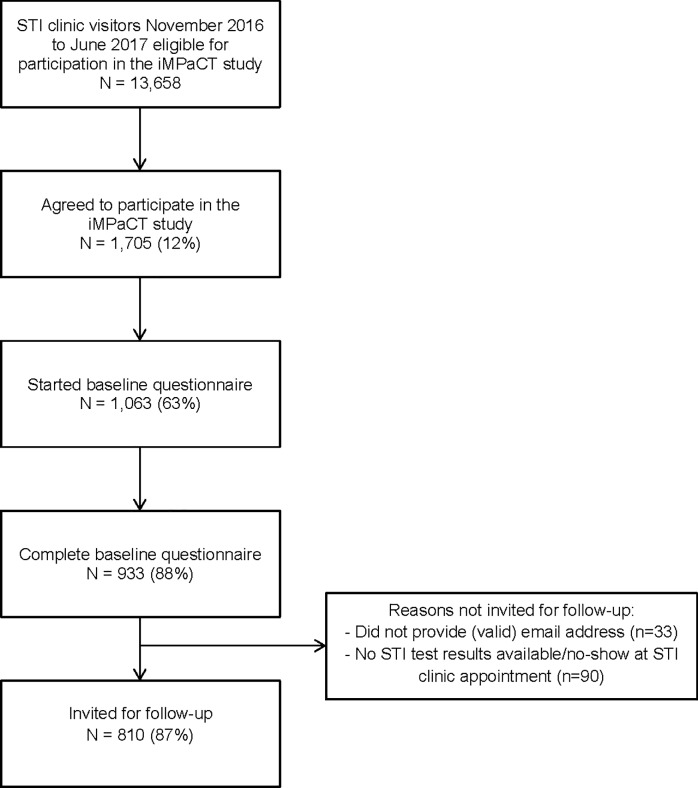
In total, 13,658 STI clinic visitors were eligible to participate in the iMPaCT study. The majority of those STI clinic visitors was ≥ 21 years (76%), female (72%), highly educated (71%), and ethnic Dutch (72%). Of those STI clinic visitors, 2,253 (16%) actively declined the online invitation, 1,705 (12%) agreed to participate, and 1,063 (8%) started the online questionnaire, and 933 (7%) completed the baseline questionnaire (Fig 1). The majority of the participants was ≥23 years old (52%), female (81%), highly educated (89%), and ethnic Dutch (81%). Furthermore, 79% of the participants were recruited at the STI clinic in Amsterdam (n = 838), 7% in Kennemerland (n = 81), 10% in Hollands Noorden (n = 105), and 4% in Twente (n = 39), resulting in response rates of 9%, 6%, 7%, and 3% respectively.
Fig 1. Baseline response rates in the iMPaCT study.
Abbreviations: STI = Sexually Transmitted Infection.
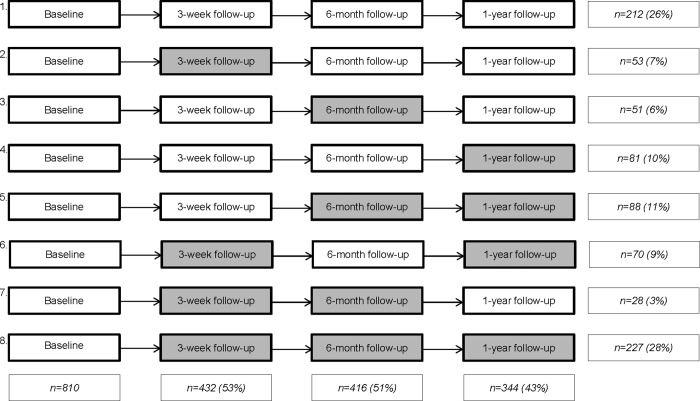
Of all participants who completed the baseline questionnaire), 810 participants could be invited to participate in the follow-up data collection moments. Of these 810 participants, 432 (53%) filled out the online questionnaire at three-week follow-up, 416 (51%) filled out the online questionnaire at six-month follow-up, and 344 (43%) filled out the last questionnaire at one-year follow-up (Fig 2). Furthermore, 26% of the participants completed all three follow-up questionnaires, 23% completed two follow-up questionnaires, 23% completed one follow-up questionnaire, and 28% did not respond to any of the follow-up questionnaires. All 416 participants who filled out the questionnaire at six-month follow-up received a home-based test kit, and 315 (76%) send the test kit to the laboratory for chlamydia and gonorrhea testing.
Fig 2. Response participants invited for follow-up, at three-week, six-month, and one-year follow-up.
Grey indicates (partial) lost to follow-up.
Predictors for non-response at baseline
All demographic, behavioral and sexual health-related variables were significant predictors of non-response in the univariable analysis (Table 1). In the multivariable analysis, type of STI test was highly correlated to other explanatory variables as type of STI test is dependent on a number of predictors in the model (i.e., triage criteria, such as age, and migration background), and was therefore excluded from the model. In the final multivariable model, there was no multicollinearity (VIF values around 1), and the Hosmer-Lemeshow test indicated good fit (p-value = 0.3). Male gender, low/medium education level, young age (≤ 20 years), and being non-Dutch, were significant predictors of non-response at baseline. Furthermore, non-respondents were more likely to report STI-related symptoms, being notified by a partner, ≤ 2 partners in the past six months, and having had condomless sex at the last sex act compared to the participants. The chlamydia positivity rate at baseline was significantly higher (p = 0.003) in the non-respondents (n = 2,153, 17%) compared to the participants (n = 143, 14%).
Table 1. Univariable and multivariable logistic regression analysis of predictors for non-response in the iMPaCT study by comparing participants (n = 1,063) and non-respondents (n = 12,595) aged 18–24 years visiting an STI clinic in November 2016 to June 2017.
| Participants | Non-respondents | |||||
|---|---|---|---|---|---|---|
| N | % | N | % | OR (95% CI) | aOR (95%CI) | |
| Age | ||||||
| 18–20 years | 165 | 16 | 3057 | 24 | 1 | 1 |
| 21–22 years | 342 | 32 | 4567 | 36 | 0.72 (0.59–0.87) | 0.79 (0.65–0.96) |
| 23–24 years | 556 | 52 | 4971 | 40 | 0.48 (0.40–0.58) | 0.54 (0.45–0.65) |
| Gender | ||||||
| Female | 860 | 81 | 9018 | 72 | 1 | 1 |
| Male | 203 | 19 | 3577 | 28 | 1.68 (1.44–1.97) | 1.73 (1.47–2.05) |
| Education level | ||||||
| Low/medium | 120 | 11 | 3563 | 28 | 1 | 1 |
| High | 941 | 89 | 8692 | 69 | 0.31 (0.26–0.38) | 0.40 (0.32–0.48) |
| Migration background | ||||||
| Ethnic Dutch | 857 | 81 | 8955 | 71 | 1 | 1 |
| Non-Dutch | 206 | 19 | 3638 | 29 | 1.69 (1.45–1.98) | 1.27 (1.08–1.49) |
| Symptoms | ||||||
| No | 897 | 84 | 9649 | 77 | 1 | 1 |
| Yes | 166 | 16 | 2946 | 23 | 1.65 (1.39–1.96) | 1.35 (1.14–1. 62) |
| GO/CT/SYPH past year | ||||||
| No | 320 | 30 | 3092 | 24 | 1 | - |
| Yes | 129 | 12 | 1603 | 13 | 1.29 (1.04–1.60) | - |
| Not tested | 614 | 58 | 7900 | 63 | 1.33 (1.15–1.53) | - |
| Partner notification | ||||||
| No | 942 | 89 | 10684 | 85 | 1 | 1 |
| Yes | 121 | 11 | 1910 | 15 | 1.39 (1.15–1.70) | 1.23 (1.01–1.51) |
| Number of partners in past six months | ||||||
| 0–2 partners | 374 | 35 | 6005 | 48 | 1 | 1 |
| 3–4 partners | 396 | 37 | 3956 | 31 | 0.62 (0.54–0.72) | 0.66 (0.57–0.77) |
| ≥ 5 partners | 293 | 28 | 2634 | 21 | 0.56 (0.48–0.66) | 0.55 (0.47–0.65) |
| Condom use at last sexual contact | ||||||
| No | 828 | 78 | 10072 | 80 | 1 | 1 |
| Yes | 228 | 22 | 2386 | 19 | 0.86 (0.74–1.00) | 0.83 (0.71–0.98) |
Footnote: Categories do not all add up to 100%, as missing values are not shown. Statistical associations are shown in in italic when the p-value is equal to or smaller than 0.1, and in bold when the p-value is equal to or smaller than 0.05.
Abbreviations: CT = Chlamydia; OR = crude odds ratio, aOR = adjusted odds ratio; CI = Confidence Interval; IC = Informed Consent; STI = Sexually Transmitted Infection; SYPH = Syphilis.
Predictors for loss to follow-up
Interactions were found between STI clinic region (Amsterdam/non-Amsterdam) and the behavioral and psychological predictors. For these predictors, the analyses were stratified by STI region. Low/medium health goals at baseline was a significant predictor of non-response at six-month, and/or one-year follow-up in both Amsterdam (Table 2) and non-Amsterdam region (Table 3). Predictors of loss to follow-up in Amsterdam were low/medium social norms and support (at three-week follow-up), and having had ≥ 5 partners in the past six months (at six-month follow-up). For non-Amsterdam, high impulsiveness (at six-month follow-up), and high intentions (at one-year follow-up) were predictors of loss to follow-up. High risk perception at baseline in Amsterdam, and low/medium risk perception at baseline in non-Amsterdam was a significant predictor of loss to follow-up at six-month and one-year.
Table 2. Multivariable logistic regression analyses of predictors of non-response at the three follow-up data collection moments for participants who visited the STI clinic in Amsterdam.
| Baseline | 3-week follow-up non-response | 6-month follow-up non-response | 1-year follow-up non-response | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | aOR | N | % | aOR | N | % | aOR | |
| (95%CI) | (95%CI) | (95%CI) | |||||||||
| Total | 647 | 292 | 45 | 303 | 47 | 365 | 56 | ||||
| Number of partners in past six months | |||||||||||
| 0–2 partners | 222 | 34 | 94 | 32 | - | 93 | 31 | 1 | 117 | 32 | - |
| 3–4 partners | 261 | 40 | 123 | 42 | - | 114 | 38 | 0.99 (0.68–1.43) | 143 | 39 | - |
| ≥ 5 partners | 164 | 25 | 75 | 26 | - | 96 | 32 | 1.73 (1.14–2.64) | 105 | 29 | - |
| Condom use at last sex act | |||||||||||
| No | 504 | 78 | 235 | 81 | - | 239 | 79 | - | 300 | 82 | 1 |
| Yes | 143 | 22 | 57 | 20 | - | 64 | 21 | - | 65 | 18 | 0.70 (0.47–1.03) |
| Age at sexual debut | |||||||||||
| < 16 years | 205 | 32 | 102 | 35 | - | 100 | 33 | - | 123 | 34 | - |
| ≥ 16 years | 442 | 68 | 190 | 65 | - | 203 | 67 | - | 242 | 66 | - |
| Health goals | |||||||||||
| Low/med (score < 4.00) | 314 | 49 | 159 | 55 | 1 | 168 | 55 | 1 | 200 | 55 | 1 |
| High (score ≥ 4.00) | 333 | 52 | 133 | 46 | 0.77 (0.55–1.07) | 135 | 45 | 0.65 (0.47–0.90) | 165 | 45 | 0.65 (0.47–0.90) |
| Attitudes* | |||||||||||
| Low/med (score < 4.25) | 265 | 41 | 136 | 47 | 1 | 140 | 46 | - | 167 | 46 | - |
| High (score ≥ 4.25) | 382 | 59 | 156 | 53 | 0.76 (0.54–1.06) | 163 | 54 | - | 198 | 54 | - |
| Social norms and support | |||||||||||
| Low/med (score < 3.20) | 229 | 35 | 122 | 42 | 1 | 119 | 39 | - | 140 | 38 | - |
| High (score ≥ 3.20) | 418 | 65 | 170 | 58 | 0.65 (0.47–0.91) | 184 | 61 | - | 225 | 62 | - |
| Risk perception for CT (own risk) | |||||||||||
| Low/med (score < 27.50) | 311 | 48 | 134 | 46 | - | 126 | 42 | 1 | 151 | 41 | 1 |
| High (score ≥ 27.50) | 336 | 52 | 158 | 54 | - | 177 | 58 | 1.51 (1.09–2.08) | 214 | 59 | 1.70 (1.23–2.33) |
* Attitudes regarding prevention of chlamydia
Footnote: Categories do not all add up to 100%, as missing values are not shown. Statistical associations are shown in in italic when the p-value is equal to or smaller than 0.1, and in bold when the p-value is equal to or smaller than 0.05. Only variables that were pre-selected in the univariable analyses are shown here.
Abbreviations: aOR = adjusted odds ratio, CI = Confidence Interval; Low/med = Low/medium, CT = Chlamydia; STI = Sexually Transmitted Infection.
Table 3. Multivariable logistic regression analyses of predictors of non-response at the three follow-up data collection moments for participants who visited the STI clinics in Kennemerland, Hollands Noorden, and Twente (non-Amsterdam).
| Baseline | 3-week follow-up non-response | 6-month follow-up non-response | 1-year follow-up non-response | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | aOR | N | % | aOR | N | % | aOR | |
| (95%CI) | (95%CI) | (95%CI) | |||||||||
| Total | 163 | 86 | 53 | 91 | 56 | 101 | 62 | ||||
| Health goals | |||||||||||
| Low/med (score < 4.00) | 83 | 51 | 49 | 57 | 1 | 49 | 54 | - | 57 | 56 | 1 |
| High (score ≥ 4.00) | 80 | 49 | 37 | 43 | 0.58 (0.31–1.08) | 42 | 46 | - | 44 | 44 | 0.40 (0.18–0.75) |
| Intentions | |||||||||||
| Low/med (score < 2.67) | 84 | 52 | 44 | 51 | - | 45 | 50 | - | 46 | 46 | 1 |
| High (score ≥ 2.67) | 79 | 48 | 42 | 49 | - | 46 | 51 | - | 55 | 55 | 2.16 (1.08–4.44) |
| Impulsiveness | |||||||||||
| Low/med (score < 2.63) | 78 | 48 | 39 | 45 | - | 35 | 39 | 1 | 45 | 45 | - |
| High (score ≥ 2.63) | 85 | 52 | 47 | 55 | - | 56 | 62 | 2.97 (1.52–5.99) | 56 | 55 | - |
| Knowledge* | |||||||||||
| Low/med (score < 6.00) | 91 | 56 | 48 | 56 | - | 56 | 62 | 1 | 60 | 59 | - |
| High (score ≥ 6.00) | 72 | 44 | 38 | 44 | - | 35 | 39 | 0.58 (0.30–1.12) | 41 | 41 | - |
| Risk perception for CT (own risk) | |||||||||||
| Low/med (score < 27.50) | 84 | 52 | 44 | 51 | - | 52 | 57 | 1 | 58 | 57 | 1 |
| High (score ≥ 27.50) | 79 | 48 | 42 | 49 | - | 39 | 43 | 0.45 (0.23–0.88) | 43 | 43 | 0.47 (0.23–0.94) |
* Knowledge regarding sexual health, prevention of chlamydia and consequences of chlamydia diagnosis
Footnote: Categories do not all add up to 100%, as missing values are not shown. Statistical associations are shown in in italic when the p-value is equal to or smaller than 0.1, and in bold when the p-value is equal to or smaller than 0.05. Only variables that were pre-selected in the univariable analyses are shown here.
Abbreviations: aOR = adjusted odds ratio, CI = Confidence Interval; Low/med = Low/medium, CT = Chlamydia; STI = Sexually Transmitted Infection.
Stratified multivariable analyses by STI clinic region using demographic and sexual health-related predictors was not possible due to the small number of observations in each cell (S1 and S2 Tables), and these predictors were analyzed separately without stratification. Male gender, low/medium education level, and younger age (≤ 20 years) were associated with non-response at either three-week, six-month and/or one-year follow-up (S3 Table). Chlamydia infection at baseline was not a predictor of loss to follow-up.
Discussion
Our results showed that male, younger age (≤ 20 years), and low/medium educated individuals were more likely to be non-respondents at baseline and more likely to be lost to follow-up after baseline participation. Furthermore, behavioral and psychological variables appeared to play a role in non-response at long-term follow-up. Behavioral and psychological predictors of loss to follow-up were different between STI clinic regions, except for low perceived importance of health at baseline, which was predictive of loss to follow-up at six-month and one-year follow-up in all STI clinic regions. The chlamydia positivity rate was significantly higher among non-respondents than among the participants, but chlamydia infection itself at baseline was not a predictor of loss to follow-up.
The main strength of this study is the comprehensive data consisting of behavioral characteristics, sexual health outcomes and demographic characteristics on both participants and non-respondents. Moreover, to our knowledge, this is the first study identifying psychological predictors for loss to follow-up among both chlamydia diagnosed and undiagnosed heterosexual STI clinic visitors. Furthermore, the extensive non-response analysis provided insights into potential bias and generalizability of the study population. This study was, however, not without some limitations. First, reasons for non-response at baseline or loss to follow-up were not recorded. Nevertheless, reasons for non-response in health research, such as lack of time, being forgetful, or privacy concerns [10, 25–27], are well documented, and were not the purpose of this study. Second, the actual number of eligible STI clinic visitors who were invited for participation during the recruitment period was not known. As recruitment was only done through the online registration form at the STI clinics in Amsterdam, Kennemerland and Hollands Noorden, individuals who booked an appointment via telephone were not invited for participation (4%-17% of all eligible STI clinic visitors (M.S. van Rooijen, personal communication, October 17, 2018). At the STI clinic in Twente, no online registration exists, and recruitment was only done when people booked an appointment via telephone. However, not all eligible participants were invited due to lack of time or forgetting or omitting to inform eligible STI clinic visitors, but we were not able to distinguish invitees from non-invitees, meaning that actual response rates are higher.
We found that male gender, lower education, and a lower number of sexual partners were predictors of non-response, which was consistent with the literature [3, 5, 11, 28–30]. Male gender, and lower education level were, as well as being predictors of non-response at baseline, also predictors of loss to follow-up, which is also in line with findings from previous studies [17, 31]. In contrast to other STI studies, we found that individuals who reported STI-related symptoms were less likely to participate at baseline. This might be explained by the differences in the study design. Participants in two other Dutch STI studies [5, 30], received a free chlamydia test kit if they agreed to participate, which might have provided extra motivation for individuals with STI-related symptoms to participate. In our study, participation was not required to receive the first STI test, as individuals who were invited to participate had already made an appointment for an STI test at the clinic.
We found that chlamydia positivity rates were significantly higher in non-respondents compared to participants at baseline, while individuals reporting a higher number of partners in the past six months, (classically categorized as high-risk [32, 33]), participated more than individuals reporting lower number of partners. A possible explanation for this contradiction might be that non-respondents, although having fewer partners, more often reported condomless sex at last sexual contact, STI-related symptoms, and being notified by their partner, which are also known risk factors for chlamydia infection [34]. Condomless sex at last sexual contact should, however, be interpreted with caution, as reporting condomless sex in a monogamous relationship does not necessarily reflect higher chlamydia risk [35].
Low perceived importance of health at baseline was a predictor of long-term loss to follow-up (at six-month and one-year follow-up) in all STI clinic regions. This finding might be related to earlier findings that showed that long-term health goals might influence certain behaviors, such as participation behavior (i.e., motivation to participate in health research) [36, 37]. In non-Amsterdam, high intentions towards condom use and STI testing at baseline and high impulsiveness were predictors of loss to follow-up, and in Amsterdam, less positive attitudes regarding prevention of chlamydia and lower social norms and support were associated with loss to follow-up. These results partly reflect the theory of planned behavior, that links attitudes and social norms to intended behavior [38], and with previous studies that found that individuals are not always able to carry out intended behavior [39–41]. Low perceived risk of chlamydia at baseline was associated with loss to follow-up at six-month and one-year follow-up in non-Amsterdam, which is in line with the health belief model (i.e., perceived seriousness/susceptibility associated with likelihood of engaging in behavior [42]) and has previously been described as the main reason for non-response [10]. However, in Amsterdam, high risk perception was a predictor of loss to follow-up. A possible explanation for this contradictory finding is that we examined the association between risk perception at baseline and loss to follow-up one year later, and not risk perception after one year. It might be that risk perception decreased after baseline in Amsterdam, because individuals believed they were engaging in less risky sexual behavior than before or they believed they overestimated their risk at baseline [43]. Lower risk perception at the follow-up moments might have negatively influenced the motivation to participate in the follow-up moments.
The findings of this study indicate that to prevent non-response bias, recruitment strategies should put more effort into recruiting underrepresented behavioral and demographic groups in sexual health-related research. For example, targeted recruitment or cultural adaptations (e.g., flyers and/or personalized invitations adapted to migrants' culture and language) [44], or adapting the recruitment method to increase interest and motivation among males (e.g., raising awareness or a greater sense of responsibility in terms of health in males) [29], might be effective in improving response rates in these underrepresented groups. Furthermore, the psychological predictors of loss to follow-up identified in this study could also be used as potential targets for recruitment strategies to increase retention. Recruitment strategies focusing on increasing perceived importance of health (i.e., health goals) by stimulating (health) goal pursuit [36, 37], or improving perceived risk [45] using risk communication targeting different elements of risk perception simultaneously (e.g., perceived severity and self-efficacy) [46], might increase response rates and retention in sexual health-related research. Moreover, implementation intentions (i.e., formulating a specific plan or implementation intention) might be an effective strategy to improve participation behavior, as it targets a variety of factors related to (intended) behavior [39], including the different psychological predictors of loss to follow-up identified in this study. Future research should be undertaken to investigate the impact of targeted recruitment strategies on response and retention in underrepresented demographic, behavioral, and psychological groups.
In conclusion, differences in demographic, behavioral, and psychological characteristics need to be taken into consideration in recruitment strategies. Tailoring recruitment strategies to demographic characteristics, behavioral, and psychological characteristics, might be needed to increase response rates and retention, and to prevent non-response bias in health research.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(PDF)
(PDF)
Acknowledgments
The authors would like to thank Joran Slager, who assisted with the data collection and contributed to the data analyses. Furthermore, the authors thank the staff at the STI clinics of Amsterdam, Kennemerland, Hollands Noorden, Twente, especially Karin Westra, Anne de Vries, Karlijn Kampman, and Titia Heijman, who were involved in the recruitment and data collection of participants at baseline. The authors are also grateful to Marlous Ratten and Klazien Visser from Soapoli-online, who coordinated the laboratory testing of the home-based test kits at six-month follow-up, and to the staff at the STI department at the National Institute for Public Health and the Environment, especially Birgit van Benthem.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the Strategic Programme (SPR) of the National Institute for Public Health and the Environment (RIVM) (project number S/113004/01/IP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Galea S, Tracy M. Participation rates in epidemiologic studies. Annals of epidemiology. 2007;17(9):643–53. 10.1016/j.annepidem.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 2.Mindell JS, Giampaoli S, Goesswald A, Kamtsiuris P, Mann C, Männistö S, et al. Sample selection, recruitment and participation rates in health examination surveys in Europe–experience from seven national surveys. BMC medical research methodology. 2015;15(1):78 10.1186/s12874-015-0072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung KL, Peter M, Smit C, de Vries H, Pieterse ME. The impact of non-response bias due to sampling in public health studies: A comparison of voluntary versus mandatory recruitment in a Dutch national survey on adolescent health. BMC public health. 2017;17(1):276 10.1186/s12889-017-4189-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallander L, Tikkanen RH, Mannheimer LN, Östergren P, Plantin L. The problem of non-response in population surveys on the topic of HIV and sexuality: a comparative study. The European Journal of Public Health. 2014;25(1):172–7. 10.1093/eurpub/cku154 [DOI] [PubMed] [Google Scholar]
- 5.Op de Coul ELM, Götz HM, van Bergen JEAM, Fennema JSA, Hoebe CJPA, Koekenbier RH, et al. Who participates in the Dutch Chlamydia screening? A study on demographic and behavioral correlates of participation and positivity. Sexually transmitted diseases. 2012;39(2):97–103. 10.1097/OLQ.0b013e3182383097 [DOI] [PubMed] [Google Scholar]
- 6.Kypri K, Samaranayaka A, Connor J, Langley JD, Maclennan B. Non-response bias in a web-based health behaviour survey of New Zealand tertiary students. Prev Med 2011;53(4–5):274–7. 10.1016/j.ypmed.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 7.Meiklejohn J, Connor J, Kypri K. The effect of low survey response rates on estimates of alcohol consumption in a general population survey. PLoS One. 2012;7(4):e35527 10.1371/journal.pone.0035527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corry NH, Williams CS, Battaglia M, McMaster HS, Stander VA. Assessing and adjusting for non-response in the Millennium Cohort Family Study. BMC medical research methodology. 2017;17(1):16 10.1186/s12874-017-0294-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maclennan B, Kypri K, Langley J, Room R. Non-response bias in a community survey of drinking, alcohol-related experiences and public opinion on alcohol policy. Drug and alcohol dependence. 2012;126(1–2):189–94. 10.1016/j.drugalcdep.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 10.Greenland KE, Op de Coul ELM, van Bergen JEAM, Brouwers EEHG, Fennema JSA, Götz HM, et al. Acceptability of the internet-based Chlamydia screening implementation in the Netherlands and insights into nonresponse. Sex Transm Dis. 2011;38(6):467–74. 10.1097/OLQ.0b013e318204546e [DOI] [PubMed] [Google Scholar]
- 11.Carey MP, Senn TE, Vanable PA, Coury-Doniger P, Urban MA. Do STD clinic patients who consent to sexual health research differ from those who decline? Findings from a randomized controlled trial with implications for the generalization of research results. Sexually transmitted diseases. 2008;35(1):73 10.1097/OLQ.0b013e318148b4ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunne MP, Martin NG, Bailey JM, Heath AC, Bucholz KK, Madden PA, et al. Participation bias in a sexuality survey: psychological and behavioural characteristics of responders and non-responders. International Journal of Epidemiology. 1997;26(4):844–54. 10.1093/ije/26.4.844 [DOI] [PubMed] [Google Scholar]
- 13.Bauman A, Phongsavan P, Cowle A, Banks E, Jorm L, Rogers K, et al. Maximising follow-up participation rates in a large scale 45 and Up Study in Australia. Emerging themes in epidemiology. 2016;13(1):6 10.1186/s12982-016-0046-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booker CL, Harding S, Benzeval M. A systematic review of the effect of retention methods in population-based cohort studies. BMC public health. 2011;11(1):249 10.1186/1471-2458-11-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powers J, Tavener M, Graves A, Loxton D. Loss to follow-up was used to estimate bias in a longitudinal study: a new approach. Journal of clinical epidemiology. 2015;68(8):870–6. 10.1016/j.jclinepi.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 16.Rogelberg SC, Spitzmüeller C, Little I, Reeve CL. Understanding response behavior to an online special topics organizational satisfaction survey. Personnel Psychology. 2006;59(4):903–23. 10.1111/j.1744-6570.2006.00058.x [DOI] [Google Scholar]
- 17.Gustavson K, von Soest T, Karevold E, Røysamb E. Attrition and generalizability in longitudinal studies: findings from a 15-year population-based study and a Monte Carlo simulation study. BMC public health. 2012;12(1):918 10.1186/1471-2458-12-918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsadik M, Berhane Y, Worku A, Terefe W. The magnitude of, and factors associated with, loss to follow-up among patients treated for sexually transmitted infections: a multilevel analysis. BMJ open. 2017;7(7):e016864 10.1136/bmjopen-2017-016864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Wees DA, Heijne JCM, Heijman T, Kampman CJG, Westra K, de Vries A, et al. Study protocol of the iMPaCT study: a longitudinal cohort study assessing psychological determinants, sexual behaviour and chlamydia (re)infections in heterosexual STI clinic visitors. BMC Infectious diseases. 2018;18(1):559 10.1186/s12879-018-3498-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statistics Netherlands (CBS). Person with a Dutch background, Person with a western migration background, Person with a non-western migration background 2018. [updated 07-03-2018; cited 2018 6 August]. Available from: https://www.cbs.nl/en-gb/our-services/methods/definitions?tab=m#id=migration-background. [Google Scholar]
- 21.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. American journal of epidemiology. 2007;165(6):710–8. 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
- 22.Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36(1):27–46. 10.1111/j.1600-0587.2012.07348.x [DOI] [Google Scholar]
- 23.Hosmer DW, Lemesbow S, Methods. Goodness of fit tests for the multiple logistic regression model. Communications in statistics-Theory. 1980;9(10):1043–69. [Google Scholar]
- 24.R Development Core Team. R: A language and environment for statistical computing. 3.4.2 ed. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 25.Korkeila K, Suominen S, Ahvenainen J, Ojanlatva A, Rautava P, Helenius H, et al. Non-response and related factors in a nation-wide health survey. European journal of epidemiology. 2001;17(11):991–9. [DOI] [PubMed] [Google Scholar]
- 26.van Loon AJ, Tijhuis M, Picavet HS, Surtees PG, Ormel J. Survey non-response in the Netherlands: effects on prevalence estimates and associations. Ann Epidemiol 2003;13(2):105–10. 10.1016/S1047-2797(02)00257-0 [DOI] [PubMed] [Google Scholar]
- 27.Rosser BRS, Capistrant B. Online Versus Telephone Methods to Recruit and Interview Older Gay and Bisexual Men Treated for Prostate Cancer: Findings from the Restore Study. JMIR Cancer. 2016;2(2):e9 10.2196/cancer.5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jørgensen MJ, Maindal HT, Christensen KS, Olesen F, Andersen B. Sexual behaviour among young Danes aged 15–29 years: a cross-sectional study of core indicators. Sex Transm Infect. 2015;91(3):171–7. 10.1136/sextrans-2014-051814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ek S. Gender differences in health information behaviour: a Finnish population-based survey. Health promotion international. 2013;30(3):736–45. 10.1093/heapro/dat063 [DOI] [PubMed] [Google Scholar]
- 30.Van Bergen JEAM, Götz HM, Richardus JH, Hoebe CJPA, Broer J, Coenen AJT. Prevalence of urogenital Chlamydia trachomatis increases significantly with level of urbanisation and suggests targeted screening approaches: results from the first national population based study in the Netherlands. Sexually transmitted infections. 2005;81(1):17–23. 10.1136/sti.2004.010173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wærsted M, Børnick TS, Twisk JWR, Veiersted KB. Simple descriptive missing data indicators in longitudinal studies with attrition, intermittent missing data and a high number of follow-ups. BMC Research Notes. 2018;11(1):123 10.1186/s13104-018-3228-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzog SA, Heijne JC, Scott P, Althaus CL, Low N. Direct and Indirect Effects of Screening for Chlamydia trachomatis on the Prevention of Pelvic Inflammatory Disease: A Mathematical Modeling Study. Epidemiology. 2013;24(6):854–62. 10.1097/EDE.0b013e31829e110e . [DOI] [PubMed] [Google Scholar]
- 33.Johnson AM, Mercer CH, Erens B, Copas AJ, McManus S, Wellings K, et al. Sexual behaviour in Britain: partnerships, practices, and HIV risk behaviours. The Lancet. 2001;358(9296):1835–42. 10.1016/S0140-6736(01)06883-0 [DOI] [PubMed] [Google Scholar]
- 34.Visser M, van Aar F, Op de Coul ELM, Slurink IAL, van Wees DA, Hoenderboom BM, et al. Sexually transmitted infections in the Netherlands in 2017. Bilthoven: Centre for Infectious Disease Control, National Institute for Public Health and the Environment (RIVM), 2018. [Google Scholar]
- 35.Levine EC, Herbenick D, Martinez O, Fu TC, Dodge B. Open Relationships, Nonconsensual Nonmonogamy, and Monogamy Among US Adults: Findings from the 2012 National Survey of Sexual Health and Behavior. Archives of sexual behavior. 2018:1–12. 10.1007/s10508-018-1178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Custers R, Aarts H. The unconscious will: How the pursuit of goals operates outside of conscious awareness. Science. 2010;329(5987):47–50. 10.1126/science.1188595 [DOI] [PubMed] [Google Scholar]
- 37.den Daas C, Häfner M, de Wit J. The impact of long-term health goals on sexual risk decisions in impulsive and reflective cognitive states. Arch Sex Behav. 2014;43(4):659–67. 10.1007/s10508-013-0183-0 [DOI] [PubMed] [Google Scholar]
- 38.Ajzen I. The theory of planned behavior. Organizational behavior and human decision processes. 1991;50(2):179–211. 10.1016/0749-5978(91)90020-T [DOI] [Google Scholar]
- 39.Ajzen I, Czasch C, Flood MG. From Intentions to Behavior: Implementation Intention, Commitment, and Conscientiousness Journal of Applied Social Psychology. 2009;39(6):1356–72. 10.1111/j.1559-1816.2009.00485.x|. [DOI] [Google Scholar]
- 40.Sheeran P. Intention—behavior relations: a conceptual and empirical review. European review of social psychology. 2002;12(1):1–36. 10.1080/14792772143000003 [DOI] [Google Scholar]
- 41.Moshier SJ, Ewen M, Otto MW. Impulsivity as a moderator of the intention–behavior relationship for illicit drug use in patients undergoing treatment. Addictive behaviors. 2013;38(3):1651–5. 10.1016/j.addbeh.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janz NK, Becker MH. The health belief model: A decade later. Health education quarterly. 1984;11(1):1–47. 10.1177/109019818401100101 [DOI] [PubMed] [Google Scholar]
- 43.van Wees DA, den Daas C, Kretzschmar MEE, Heijne JCM. Double trouble: modelling the impact of low risk perception and high-risk sexual behaviour on chlamydia transmission. J R Soc Interface. 2018;15(141):20170847 10.1098/rsif.2017.0847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. 10.1146/annurev.publhealth.27.021405.102113 [DOI] [PubMed] [Google Scholar]
- 45.Caldwell PHY, Hamilton S, Tan A, Craig JC. Strategies for increasing recruitment to randomised controlled trials: systematic review. PLoS medicine. 2010;7(11):e1000368 10.1371/journal.pmed.1000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheeran P, Harris PR, Epton T. Does heightening risk appraisals change people’s intentions and behavior? A meta-analysis of experimental studies. Psychological bulletin. 2014;140(2):511 10.1037/a0033065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.