Abstract
Background
Schistosomiasis japonica is a zoonotic parasitic disease. After nearly 70 years of control efforts in China, Schistosomiasis transmission has been reduced to a much lower level. The absence or near absence of infections in humans or livestock, based on traditional fecal and serological tests, has made the targets and priorities of future control efforts difficult to determine. However, detection of schistosome cercariae in waters using sentinel mice could be an alternative way of identifying remaining foci of infection, or even serve as a tool for evaluation of control efficacy. This method has been employed in China over last forty years. We therefore performed a meta-analysis of the relevant research to investigate if infections in sentinel mice mirror the ongoing trend of schistosomiasis transmission in China.
Methods
We conducted a meta-analysis of studies reporting infection rates of S. japonicum in sentinel mice in China before Sep 1, 2018 in accordance with the PRISMA guidelines. We retrieved all relative studies based on five databases (CNKI, WanFang, VIP, PubMed and Web of Science) and the reference lists of resulting articles. For each individual study, the infection rate in sentinel mice is presented together with its 95% confidence interval (CI). Point estimates of the overall infection rates and their 95% CIs were calculated. Subgroup analyses were performed according to study periods, seasons or regions.
Results
We identified 90 articles, including 290 studies covering eight endemic provinces. The overall rate in sentinel mice was 12.31% (95% CI: 10.14–14.65%) from 1980 to 2018. The value of 3.66% (95% CI: 2.62–4.85%) estimated in 2004 to 2018 was significantly lower than in 1980 to 2003 (22.96%, 95% CI: 19.25–26.89%). The estimate was significantly higher in the middle and lower reaches than in the upper reaches of the Yangtze River. The highest estimates were obtained in Hunan (30.11%, 95% CI: 25.64–34.77%) followed by Anhui (26.34%, 95% CI: 12.88–42.44%) and then Jiangxi (13.73%, 95% CI: 6.71–22.56%). Unlike the other provinces in the middle and lower reaches, no significant reduction was seen in Hubei after 2003. Even in Hubei two studies carried out after 2014 reported infections in sentinel mice, although no infected snails were reported across the province. Infections were most found in April (17.40%, 95% CI: 1.13–45.49%), July (24.98%, 95% CI: 15.64–35.62%) and October (17.08%, 95% CI 5.94–32.05%). High degrees of heterogeneity were observed.
Conclusion
This meta-analysis provides a comprehensive analysis of schistosome infection in sentinel mice across China. The estimates largely mirror the ongoing trends of transmission in terms of periods and regions. Infections were most likely to occur in April, July and October. In areas where no infected snails were reported infections in sentinel mice were still observed. Due to the presence of snails and infected wildlife, detection of schistosomes in waters using such a highly sensitive method as the deployment of sentinel mice, remains of importance in schistosomiasis monitoring. We would suggest the current criteria for transmission interruption or elimination of schistosomiasis in China be adjusted by integrating the results of sentinel mice based surveys.
Author summary
With the continued activities of the prevention and control programme in China, the prevalence and intensity of Schistosoma japonicum infection have been reduced to low levels. This makes it impossible to detect any infections in humans or livestock using the traditional approach of fecal and serological testing, so as to evaluate properly the risk map of infection. However, detection of existence of schistosome cercariae in waters could be an alternative way of detecting a potential focus of transmission. We therefore performed a meta-analysis of studies performed over the last 40 years to estimate the overall infection rates of S. japonicum in sentinel mice. The estimate across China in 2004 to 2018 was 3.66%, significantly lower than in 1980 to 2003. The highest estimates were observed in Hunan, followed by Anhui and Jiangxi. Two studies conducted in Hubei in 2015 and 2016 respectively, reported infected sentinel mice where no infected snails had been reported across the province since 2014. Transmission was found be most likely in April, July and October. The estimates largely mirror the ongoing trends of S. japonicum infections in terms of periods and regions. Due to the presence of snails and other infected wildlife, detection of schistosome cercariae in waters with a highly sensitive tool remains of great importance in schistosomiasis monitoring and evaluation. To the authors’ knowledge, this is the first time that the potential threat of S. japonicum in nature, to humans and livestock has been assessed in this manner. We would suggest that the current criteria for transmission interruption or elimination of S. japonicum in China be adjusted by integrating the results of sentinel mice.
Introduction
Schistosomiasis, caused by blood flukes of the genus Schistosoma (phylum Platyhelminthes; class Trematoda), is a zoonotic parasitic disease and is one of the 18 neglected tropical disease listed by the World Health Organization [1,2]. Currently, an estimated 240 million people are infected with the parasites, and about 800 million are at risk of infection in 78 tropical and subtropical countries [3,4]. The majority of human infections and morbidity are caused by three schistosome species: Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum [5]. Among three species, Schistosoma japonicum is considered to cause the most serious disease due to its female worms’ highest egg output and a possible longest lifespan of adult worms [6]. In China, Schistosomiasis is mainly caused by the infection of S. japonicum.
Schistosomiasis japonicum is a water-borne parasitic disease with amphibious Oncomelania hupensis snails serving as its intermediate host. In water infected snails release cercariae, which infect humans or animals when they have water activities nearby. After infection, schistosomula migrate to the liver where they develop and mate with an opposite-sex parasite. The paired worms then migrate into mesenteric veins where they reside and lay eggs. A fraction of the eggs are discharged out of the body with host’s stool, and then in water eggs hatch into free-swimming miracidia, which penetrate snails and then develop into cercariae [7]. Schistosomiasis japonicum was once a major public health problem in China with up to 11.6 million human cases in 1950s. After nearly 70 years of control efforts, the number of infected people has been gradually reduced to nearly 37.6 thousand in 2017 [8,9]. In 2014, the central government of China proposed a two-stage roadmap with aims to achieve transmission interruption by 2020 and then to achieve disease elimination by 2030 [10,11]. However, due to the complex lifecycle of S. japonicum and its easy colonization of new snail populations [12], the widely distributed snail habitats [9], and the existence of infected wildlife [13], schistosomiasis elimination in China remains a great challenge, and recently schistosomiasis has even been believed to be more serious than previous thought [14]. One important issue we have encountered in China is that much lower infection prevalences of S. japonicum, based on the traditional fecal and serological tests, in both humans and livestock have been frequently reported and documented [15], which may have led to no or unclear targets of further control efforts.
However, detection of existence of schistosome cercariae in waters could be an alternative way in determining potential foci of transmission, or even serve as an evaluation of current schistosomiasis situation. There are several approaches for detecting water infectivity, including use of sentinel mice or rabbits, sticking cercariae with specific membrane, detection of parasite DNA with PCR, and so on [16–18]. Among these, the sentinel mice method has been most commonly employed because of its high sensitivity and simple operability [19], and even recommended as one control measure [20]. The procedure is to put a group of 5 to 10 mice into a wire cage, which is tied with foam plastics at two ends and can float on water surface. This ensures mice to expose to water on limbs, tail and lower abdomen. The period of water exposure in practice generally lasts for a few hours per day and for two to three days. The exposed mice are then raised in the laboratory for 28 to 35 days and dissected for recovering worms or eggs [21].
This method has been employed in China over last forty years. Several research even reported an infection rate of up to 100% of S. japonicum in mice [22–30]. As infections in sentinel mice could also be an index of endemic situation in areas, we therefore performed a meta-analysis of research performed over the last 40 years to estimate the overall prevalence of S. japonicum in sentinel mice to see if it mirrors the ongoing trend of the parasite transmission in China. Subgroup analyses according to study periods, seasons and regions were also performed. To the authors’ knowledge, this is the first time to assess the potential and direct threat of S. japonicum infection in natural environments to humans and livestock. The purpose was to increase our knowledge on how to facilitate schistosomiasis control, particularly in China with low infection prevalence of the parasite in both humans and cattle [15].
Methods
Search strategy
A comprehensive literature search was carried out for publications published before September 1, 2018. Three Chinese and two English electronic bibliographic databases, namely China National Knowledge Infrastructure (CNKI), Wanfang, Chinese Scientific Journal Databases (VIP), PubMed and Web of Science (SCI), were searched to include all published studies that reported the infection rate of S. japonicum in sentinel mice within mainland china. We used search terms ‘Sentinel mice (or mouse)’, ‘Schistosomiasis’, and ‘China’ in the English databases and ‘shaoshu’, ‘xuexichong and/or xuexichongbing’ in the Chinese databases. No restrictions were imposed. To find additional studies, we also manually checked the relevant eligible literatures through cross-references of the identified articles in the reference lists. We did not contact authors of original studies for additional information. No attempt was made to identify unpublished studies. Full text articles were downloaded or obtained through library resources.
Study selection
All papers were imported to the literature management software Endnote X7 to eliminate duplicated records. Two authors (CQ and HZ) independently conducted an initial screening of identified titles and abstracts and then the full-text articles were downloaded for a second screening. Studies were considered eligible only if they: (i) were carried out within mainland China; (II) were neither experimental studies nor review articles; (iii) clearly reported the time performed, as least specific to year; (iv) provided geographical location, at least specific to provinces; (v) provided numbers of dissected and infected mice, or could calculate by formula; (vi) were available in full texts. Studies were excluded if they did not fulfill any of these criteria. We deemed data regarding S. japonicum infection rates in sentinel mice from the same place at the same time as a single study, and so sometimes an article can contain several studies. If the same study data were published in both English and Chinese sources, the articles with less detailed information would be excluded from this study.
Data extraction
The detailed characteristics of each study were extracted using a pre-designed data-collection excel form. Information was recorded as follows: surname of first author, year of publication, year of study, location of study, numbers of the infected and dissected mice.
Data analysis
The pooled infection rate and its 95% confidence intervals (CI) of S. japonicum in sentinel mice were calculated with the Freeman-Tukey double arcsine transformation [3,31]. Besides addressing the problem of variance instability, this approach also solves the problem of confidence limits falling outside the 0 to 1 range, as transformed infection rates are weighted slightly towards 50% and thus studies with infection rates of zero or one can be included in the analysis. Forest plots were used to visualize the results of each study and the heterogeneity among studies. Between studies heterogeneity was assessed using the Cochran’s Q (reported as p values), which is quantified by I2 values. The I2 index indicate the variation between studies attributed to heterogeneity rather than chance, with values of 25, 50 and 75% corresponding to low, moderate, and high degrees of heterogeneity, respectively [32]. When there was evidence of heterogeneity (I2 > 50%), infection rates were combined by using a random-effects model; otherwise, rates were combined by using a fixed-effects model [33].
We also conducted subgroup analyses to investigate potential sources of heterogeneity. Such analyses were performed on the following variables: 1) by study periods, i.e. 1980 to 2003 and 2004 to 2018 according to the schistosomiasis control strategy implemented [34]; 2) by river reaches, i.e the upper, middle, and lower reaches of the Yangtze River [35]. The upper reach includes Sichuan and Yunnan provinces, the middle includes Hunan, Hubei and Jiangxi provinces, and the lower includes Anhui, Jiangsu and Zhejiang provinces; 3) by provinces; 4) by seasons a study performed. The publication bias was visually examined by funnel plots and the statistical significance was assessed by the Egger’s regression asymmetry test [36]. A two-tailed p value < 0.05 was considered statistically significant. Extracted data were entered into Microsoft Office Excel 2016 and R3.5.1 was used in all statistical analyses. This study was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [37], and the PRISMA checklist (S1 Checklist) was used as the basis for inclusion of relevant information.
Results
Literature search
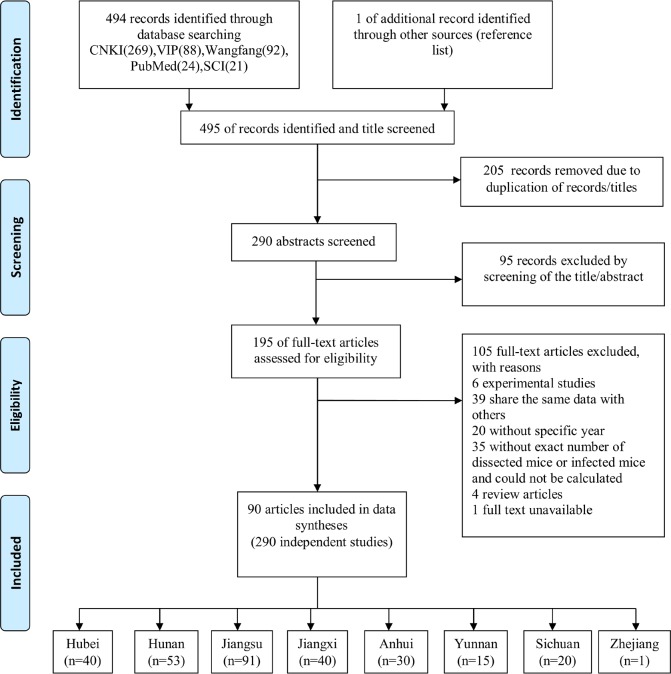
We identified 495 potentially relevant publications through five databases and the reference lists, of which 205 articles were excluded when taking duplication into consideration. After the initial screening of titles and abstracts, a further 95 articles were excluded. Employing the selection criteria, we obtained quantitative data for our meta-analysis after reading through full texts. The search strategy finally resulted in 90 articles (3 in English and 87 in Chinese)[22–30,38–118], reporting 290 studies. Fig 1 shows our systematic workflow for identifying, screening, and including studies in this study.
Fig 1. Flow chart of study selection.
The diagram shows the numbers of titles and studies reviewed in preparation of this meta-analysis of S. japonicum infection rates in sentinel mice associated with infectious waters. n represents the number of studies included in data syntheses.
Study characteristics
The years of the studies performed and published ranged from 1980 to 2018 and from 1985 to 2018, respectively. A total of 91 studies were reported from Jiangsu province, 53 from Hunan, 40 from Jiangxi, 40 from Hubei, 30 from Anhui, 20 from Sichuan, 15 from Yunnan, and one from Zhejiang. A total of 63998 sentinel mice were dissected and 7521 were identified with S. japonicum infection. A total of 153 studies were carried out during 1980 to 2003, and 137 during 2004 to 2018. The infection rates of S. japonicum in sentinel mice among the included studies varied between 0 and 100%. The detailed characteristics of each study are provided in Supporting Information file S1 Table, and its infection rate with 95% CI are also plot in Supporting Information file S1 Fig.
Pooling and heterogeneity analysis
A substantial heterogeneity was observed among studies (χ2 = 20412.5, p < 0.0001; I2 = 98.6%, 95% CI: 98.5–98.6%). When calculated using a random-effects model, the overall infection rate was 12.31% (95% CI: 10.14–14.65%).
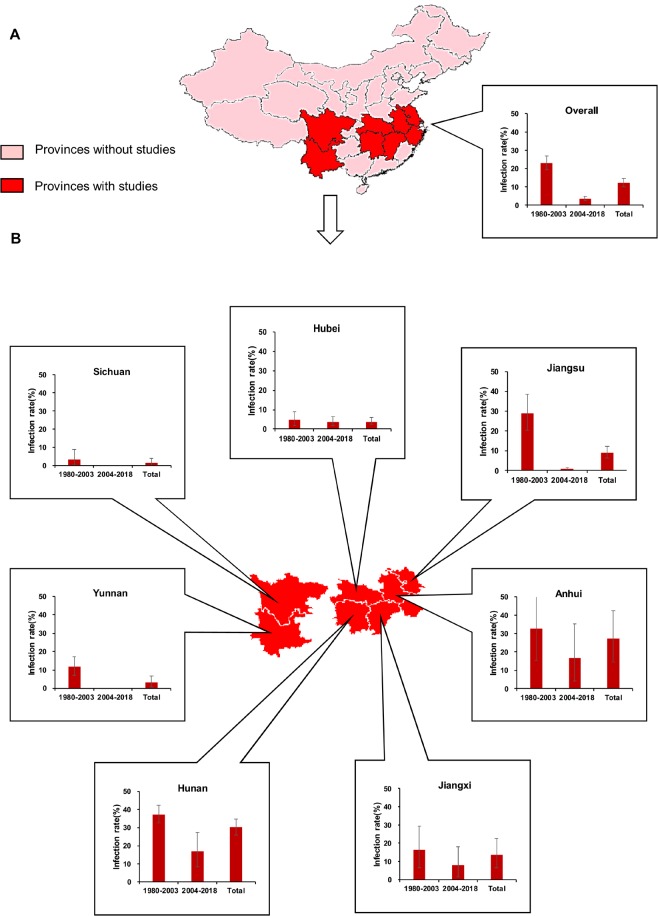
The estimates of infection rates for different subgroups and heterogeneities are presented in Table 1 and Fig 2. All pooled infection rates for each subgroup were calculated using a random-effects model because of the observed high heterogeneity among studies within subgroups. Based on study periods, the estimate had been significantly reduced since 2004 (1980–2003: 22.96% (95% CI: 19.25–26.89%), n = 153 vs 2004–2018: 3.66% (95% CI: 2.62–4.85%), n = 137). In terms of the River reaches, the estimate was highest in the middle reach (15.85%, 95% CI: 12.54–19.45%, n = 133), followed by that in the lower reach (12.80%, 95% CI: 9.55–16.42%, n = 122), and the lowest in the upper reach (2.12%, 95% CI: 0.63–4.32%, n = 35). At the level of provinces, the estimates ranged from 1.41% (95% CI: 0.05–4.09%, n = 20) in Sichuan to 30.11% (95% CI: 25.64–34.77%, n = 53) in Hunan. In terms of season, the estimate was highest in July (24.98%, 95% CI: 15.64–35.62%, n = 33) and lowest in September (5.36%, 95% CI: 2.25–9.57%, n = 27). The forest plots for each subgroup are provided in Supporting Information file S2–S4 Figs. In addition, we also made a further stratification of meta-analyses within provinces according to study periods. As seen in Fig 3, a rapid reduction in the pooled infection rate has been seen in all provinces since 2004 with the exception of Hubei province. Even in the latter, two studies reported infection rates of 1.75% in 2015 and 2.5% in 2016. See Supporting Information file S1 Table.
Table 1. Pooled infection rates of S. japonicum in sentinel mice across China and within subgroups.
| Factors related to infection rate | No. of papers included | No. of studies included | No. of infected mice | No. of total mice examined | Pooled Infection Rate (95%CI) |
Heterogenity | Egger's test | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q-χ2 | Q-P | I2(%) | t | p | |||||||
| Overall | 90 | 290 | 7521 | 63998 | 0.1231(0.1014–0.1465) | 20412.50 | <0.0001 | 98.6 | 3.97 | <0.0001 | |
| Periods | 1980–2003 | 49 | 153 | 6459 | 26667 | 0.2296(0.1925–0.2689) | 7836.80 | <0.0001 | 98.1 | -0.17 | 0.8682 |
| 2004–2018 | 56 | 137 | 1062 | 37331 | 0.0366(0.0262–0.0485) | 3719.61 | <0.0001 | 96.3 | 6.77 | <0.0001 | |
| Province | Hubei | 18 | 40 | 313 | 6089 | 0.0408(0.0230–0.0629) | 520.66 | <0.0001 | 92.5 | 1.45 | 0.1565 |
| Hunan | 18 | 53 | 4061 | 14870 | 0.3011(0.2564–0.3477) | 1642.94 | <0.0001 | 96.8 | 0.48 | 0.6361 | |
| Jiangsu | 28 | 91 | 1325 | 26194 | 0.0917(0.0647–0.1224) | 5053.29 | <0.0001 | 98.2 | 5.35 | <0.0001 | |
| Jiangxi | 14 | 40 | 635 | 4782 | 0.1373(0.0671–0.2256) | 2348.56 | <0.0001 | 98.3 | 2.71 | 0.0101 | |
| Anhui | 9 | 30 | 734 | 2772 | 0.2634(0.1288–0.4244) | 2332.46 | <0.0001 | 98.8 | 2.39 | 0.02381 | |
| Yunnan | 7 | 15 | 114 | 2237 | 0.0307(0.0064–0.0693) | 242.40 | <0.0001 | 94.2 | 0.91 | 0.3816 | |
| Sichuan | 6 | 20 | 339 | 6923 | 0.0141(0.0005–0.0409) | 891.27 | <0.0001 | 97.9 | -0.85 | 0.4074 | |
| Zhejiang | 1 | 1 | 0 | 131 | |||||||
| The reaches of the Yangtze River | Upper | 13 | 35 | 453 | 9160 | 0.0212(0.0063–0.0432) | 1133.13 | <0.0001 | 97 | -0.085 | 0.9329 |
| Middle | 46 | 133 | 5009 | 25741 | 0.1585(0.1254–0.1945) | 7116.84 | <0.0001 | 98.1 | -1.24 | 0.2161 | |
| Lower | 37 | 122 | 2059 | 29097 | 0.1280(0.0955–0.1642) | 8428.11 | <0.0001 | 98.6 | 6.79 | <0.0001 | |
| Season | Jan | 1 | 1 | 0 | 8 | ||||||
| Feb | 1 | 1 | 0 | 17 | |||||||
| Mar | 2 | 2 | 0 | 169 | |||||||
| Apr | 3 | 5 | 171 | 677 | 0.1740(0.0113–0.4549) | 247.35 | <0.0001 | 98.4 | -0.42 | 0.7051 | |
| May | 9 | 14 | 183 | 4153 | 0.0875(0.0309–0.1670) | 696.32 | <0.0001 | 98.1 | 3.33 | 0.0060 | |
| Jun | 15 | 19 | 466 | 5044 | 0.1827(0.0832–0.3089) | 1852.19 | <0.0001 | 99.0 | 3.24 | 0.0048 | |
| Jul | 21 | 33 | 718 | 6569 | 0.2498(0.1564–0.3562) | 2650.91 | <0.0001 | 98.8 | 5.11 | <0.0001 | |
| Aug | 14 | 20 | 155 | 4252 | 0.0561(0.0201–0.1066) | 595.90 | <0.0001 | 96.8 | 3.65 | 0.0018 | |
| Sep | 18 | 27 | 284 | 6445 | 0.0536(0.0225–0.0957) | 999.94 | <0.0001 | 97.4 | 3.09 | 0.0049 | |
| Oct | 6 | 7 | 87 | 535 | 0.1708(0.0594–0.3205) | 91.39 | <0.0001 | 93.4 | 1.15 | 0.3027 | |
| Nov | 2 | 2 | 4 | 42 | 0.0905(0.0157–0.2038) | 0.95 | 0.3292 | 0 | |||
| Dec | 1 | 1 | 0 | 9 | |||||||
Abbreviations: CI: Confidence interval; I2: Inverse variance index; Q-P: Cochran’s P-value.
Fig 2. Forest plot of infection rates of S. japonicum in sentinel mice.
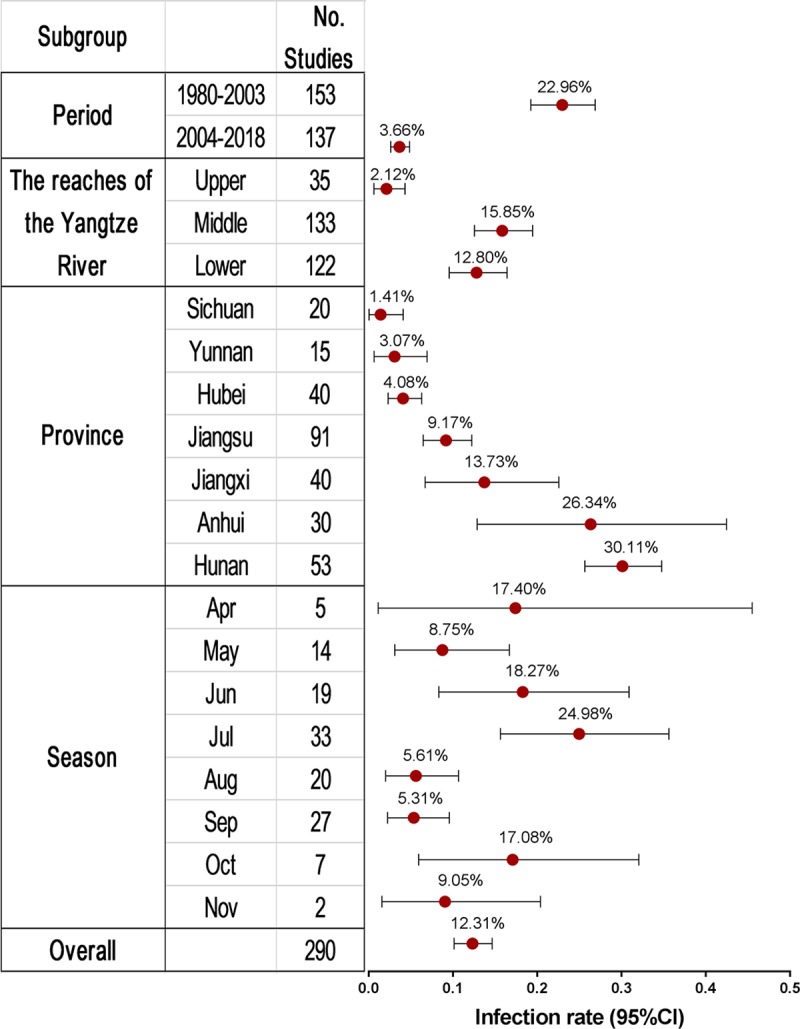
Red circles indicate infection rate estimated by random effects meta-analysis and whisker bars indicate the 95% CI. Results are shown for all included studies (bottom line, n = 290), and for subgroups according to periods, river reaches, provinces (excluding Zhejiang due to one study only) or seasons (for studies with the time of month specified).
Fig 3. Distribution of eligible studies and pooled infection rate (95% CIs) of S. japonicum in sentinel mice.
(A) across China; (B) by province and period. These data are presented numerically in Table 1 and Supporting Information file S2 Table. Map was created using R 3.5.1 and data sources was from http://www.cnblogs.com/skyme/p/5182149.html.
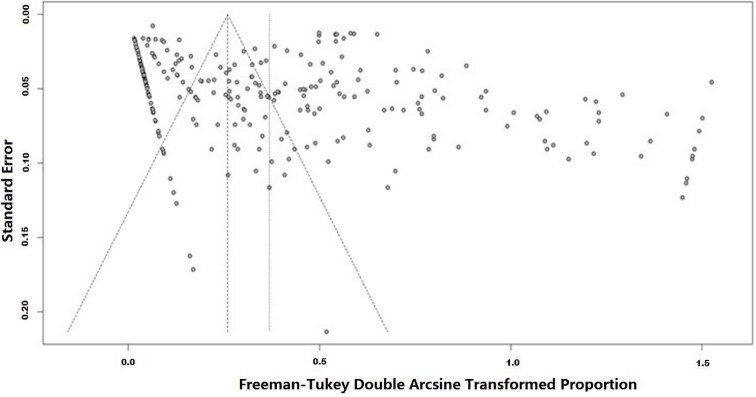
Publication bias
A potential publication bias was indicated by Egger linear regression test (bias coefficients b = 0.16, t = 3.97, p < 0.0001), which is showed in Fig 4. Subgroup analyses also suggested a publication bias for studies during the period of 2004 to 2018, within each of the three provinces (i.e. Jiangsu, Jiangxi, and Anhui) and in the lower reach of the Yangtze River (see Table 1).
Fig 4. Funnel plots of the arcsine transformed infection rate of S. japonicum in sentinel mice.
Discussion
As S. japonicum prevalence in humans and livestock has been reduced to a much lower level, it seems unlikely to detect any infections with fecal and serological testing [119,120] and thus unable to accurately assessing the current status of the parasite transmission. On the contrary, on-site water surveillance for S. japonicum cercariae using sentinel mice is able to determine potential foci of transmission in nature and has been employed in China over forty years for monitoring of schistosome infection [17]. Therefore, information on the overall prevalence of S. japonicum infections in sentinel mice across China, or by time or regions, could be of implications for further control work.
This meta-analysis retrieved 90 research involving 290 field studies. The overall infection rate of S. japonicum in sentinel mice from 1980 to 2018 was 12.31% (95% CI: 10.14–14.65%), which was comparable to the infection rate of 12.24% in wild rodents in 2011 [121]. There was high heterogeneity in infection rates among studies. Such variations may partially be attributed to factors including period or season of study, and/or different river reaches or provinces. Based on study periods, the estimate significantly decreased from 22.96% in 1980 to 2003 to 3.66% in 2004 to 2018. The rapid decrease was consistent with the observation in humans [8,122] and was mainly due to the newly developed integrated control strategy [123,124], which has been, with strong political, policy and financial support, successfully implemented across China since 2004 by the national schistosomiasis control programme [125–127]. The new strategy involves a series of interventions including replacing cattle with agricultural machines, supplying clean water, providing sanitation and egg-free latrines, together with annual routine control work.
In relation to the Yangtze River, the infection rate estimate of S. japonicum in sentinel mice was significantly higher in the middle (including Hunan, Hubei and Jiangxi provinces) and lower (including Anhui, Jiangsu and Zhejiang provinces) reaches than in the upper reaches (including Sichuan and Yunnan provinces). The highest estimate was observed in Hunan (30.11%), followed by Anhui (26.34%) and then Jiangxi (13.73%). The results were in agreement with the transmission levels defined according to infections in humans and livestock [128]. The middle and lower reaches of the River belong to a subtropical climate with sufficient precipitation, where many rivers are intertwined and the two largest lakes in China (i.e. Dongting Lake in Hunan and Poyang Lake in Jiangxi) form a shallow lake group that extensively exchanges water with the Yangtze River. The water level in the lakes and in the River changes according to the monsoon season, and therefore all marshes, beaches and islands in the middle and lower reaches of the River form scenarios of so-called ‘winter-land, summer-water’. This makes such areas more suitable to intermediate host snails and most of such endemic areas are classified into the swamp and lake regions. Currently a majority of snail habitats are distributed in such regions [122]. In addition, as over forty mammal species can serve as potential infection reservoirs for S. japonicum [129], existence of any infections in wildlife, for example infected rodents in hilly areas of Anhui [13,130], would have complicated local transmission of the parasite.
All provinces, with the exception of Hubei, have seen the rapid decrease in pooled infection rates since 2004. Besides thanks to the new control strategy, the Three Gorges Dam, which is located in the upper reaches of the Yangtze River and began to function in June 2003, might play a part in reducing S. japonicum infections in the middle and lower reaches. The dam has reduced the risk of flooding and the density of living snails in the downstream regions [131]. For example, by 2015 the mean density of living snails in the Dongting Lake area has been reduced to less than 5 snails/0.11 m2 [132]. The changes of the water level caused by the dam, coupled by the new control strategy, might be accelerating the progress towards transmission interruption in the middle and lower reaches of the Yangtze River [133]. However, we also noted that even in 2015 and 2016 in Hubei province, infections in sentinel mice was identified [42,43], although no infected snails had been found in all surveillance sites across the province since 2014 [122]. This, together with the insignificant change in the estimate in Hubei between two periods would await further investigations.
Provinces with larger numbers of sentinel mice used may well represent the infectivity of schistosomiasis affected areas, and are believed to provide more reliable findings. Among twelve endemic provinces in southern China, we obtained a total of 289 studies from seven provinces (i.e. Jiangsu, Jiangxi, Anhui, Hubei, Hunan, Sichuan and Yunnan). In each province more than 2000 sentinel mice were dissected for S. japonicum infections. We retrieved only one study in Zhejiang province, in which no infections was reported [115]. We could not obtain any data from the other four provinces. This may be partly due to their early success in schistosomiasis control as the four provinces have each reached the level of schistosomiasis interruption with both Guangdong and Shanghai in 1985, Fujian in 1987 and Guangxi in 1989 [126].
In terms of seasons surveys conducted, the estimate was 17.4% in April, then decreased in the following months. It again rose gradually and peaked in July (24.98%). Following that it decreased and again peaked in October (17.8%). This clearly showed that infections were most likely to occur in April, July or October. Snails usually go into the soil to hibernate during cold Winter. In April, rains increases and temperature rises, rain water floods high-risk areas for the first time of a year and over-winter infected snails, when contacting the water, release a large number of cercariae. The rising temperature from April to July may facilitate the development and mature of the parasite within a snail [134], and then in July the newly developed infected snails will increase the release of more schistosome larvae, thus making the areas of affected habitats most infectious. The peak of infections in Oct. could be due to the appearance of the newly borne and infected snails developed [135].
The sentinel mice method has high sensitivity in detecting infectivity of water bodies and then determining foci of transmission. However, the main disadvantage in practice is to find a fixed point (for example a tree) on a river bank, to which a mice cage is tied. This severely restricts the range of water surface to be detected, thus reducing its operability and efficiency. This method has recently been updated by Dr. Sun at Jiangsu Institute of Parasitic Diseases, who installed a propeller motor on the mice cage and enable it to move on water under remote control. This application expands the water area, and even make it possible to detect cercariae in more complicated environments where snail surveys can not be performed. Combined with the successful detection of schistosome DNA with the PCR assay in sera of mice at three-day post-infection [136], this would provide real-time risk information on potential foci of transmission and provide early warning.
Limitations
Though this study provided information on schistosomiasis transmission in China over the last 40 years and also directly assessed the threat of infections to humans and livestock, which would be useful in aiding future schistosomiasis control, it is not devoid of limitations. First, the estimations of infection rates using a random-effect model may not absolutely invalidate the heterogeneity between studies. Secondly, we don’t conduct the sensitivity analysis, as nearly 290 studies were included. Finally, we observed some evidence of publication bias in this work. Publication bias may exist when there is a preference to publish studies with significant findings. However, there is no certainty for a paper with high or low infection rates of S. japonicum in sentinel mice to get easily published; moreover, not all subgroup analyses showed publication bias. We thus think that publication bias is unlikely to have distorted our results.
Conclusions
This meta-analysis provides a comprehensive analysis of S. japonicum infection in sentinel mice across China. The estimates largely mirror the ongoing trends of S. japonicum infections in terms of periods and regions. Infections were most likely to occur in April, July and October. However, in areas where no infected snails were reported infections of S. japonicum in sentinel mice were still observed. Due to the wide distribution of snails and the existence of any infected wildlife, detection of schistosome in waters using such a highly sensitive method remains of importance in the monitoring and objective evaluation of the disease. We would suggest that the current criteria for transmission interruption or elimination of S. japonicum in China [137] be adjusted by integrating the results of sentinel mice method. The recently updated method will facilitate its wide application and make the index easily obtained.
Supporting information
(DOC)
(DOCX)
(DOCX)
(TIFF)
(DOC)
(DOC)
(DOC)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The work was supported by the Chinese National Research and Development Plan (YSL, grant number 2016YFC1200500) and by the Jiangsu Provincial Project of Invigorating Health Care through Science, Technology and Education (DBL).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Colley DG, Bustinduy AL, Secor E, King CH (2014) Human schistosomiasis. Lancet 383: 2253–2264. 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jannin J, Solano P, Quick I, Debre P (2017) The francophone network on neglected tropical diseases. PLoS Negl Trop Dis 11: e0005738 10.1371/journal.pntd.0005738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T (2013) Meta-analysis of prevalence. Journal of Epidemiology and Community Health 67: 974–978. 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- 4.Assare RK, Knopp S, N'Guessan NA, Yapi A, Tian-Bi YNT, et al. (2014) Sustaining control of schistosomiasis mansoni in moderate endemicity areas in western Cote d'Ivoire: a SCORE study protocol. Bmc Public Health 14: 1290 10.1186/1471-2458-14-1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross AGP, Cripps AW (2013) Enteropathogens and chronic illness in returning travelers. N Engl J Med 369: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 6.Chen MG (2014) Assessment of morbidity due to Schistosoma japonicum infection in China. Infectious Diseases of Poverty 3: 6 10.1186/2049-9957-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu B, Gong P, Biging G, Liang S, Seto E, et al. (2004) Snail density prediction for schistosomiasis control using ikonos and ASTER images. Photogrammetric Engineering and Remote Sensing 70: 1285–1294. [Google Scholar]
- 8.Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, et al. (2005) The public health significance and control of schistosomiasis in China—then and now. Acta Tropica 96: 97–105. 10.1016/j.actatropica.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 9.Zhang LJ, Xu ZM, Dai SM, Dang H, Lu S, et al. (2018) Endemic status of schistosomiasis in People’s Republic of China in 2017 Chin J Schisto Control 30: 481–488. [DOI] [PubMed] [Google Scholar]
- 10.Lei ZL, Zhou XN (2015) Eradication of schistosomiasis: a new target and a new task for the National Schistosomiasis Control Porgramme in the People's Republic of China. Chin J Schisto Control 27: 1–4. [PubMed] [Google Scholar]
- 11.Zhou XN (2016) Implementation of precision control to achieve the goal of schistosomiasis elimination in China. Chin J Schisto Control 28: 1–4. [PubMed] [Google Scholar]
- 12.Attwood SW, Ibaraki M, Saitoh Y, Nihei N, Janies DA (2015) Comparative phylogenetic studies on Schistosoma japonicum and its snail intermediate host Oncomelania hupensis: origins, dispersal and coevolution. PLoS Negl Trop Dis 9: e0003935 10.1371/journal.pntd.0003935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudge JW, Webster JP, Lu DB, Wang TP, Fang GR, et al. (2013) Identifying host species driving transmission of schistosomiasis japonica, a multihost parasite system, in China. Proc Natl Acad Sci U S A 110: 11457–11462. 10.1073/pnas.1221509110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colley DG, Andros TS, Campbell CH (2017) Schistosomiasis is more prevalent than previously thought: what does it mean for public health goals, policies, strategies, guidelines and intervention programs? Infectious Diseases of Poverty 6: 63 10.1186/s40249-017-0275-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang LJ, Xu ZM, Qian YJ, Dang H, L S, et al. (2017) Endemic status of schistosomiasis in People’s Republic of China in 2016. Chin J Schisto Control 29: 669–677. [DOI] [PubMed] [Google Scholar]
- 16.MOH (1982) Schistosomiasis control manual. Shanhai: Shanghai Scientific and Technical Publishers. [Google Scholar]
- 17.Wu F, Huang YX (2010) Application of determination of infested water in schistosomiasis forecast and early warning. Chin J Schisto Control 22: 500–503. [Google Scholar]
- 18.Hamburger J, He N, Abbasi I, Ramzy RM, Jourdane J, et al. (2001) Polymerase chain reaction assay based on a highly repeated sequence of Schistosoma haematobium: a potential tool for monitoring schistosome-infested water. Am J Trop Med Hyg 65: 907–911. [DOI] [PubMed] [Google Scholar]
- 19.Liang YS, Sun LP, Dai JR, Hong QB, Huang YX, et al. (2009) Surveillance and forecast system of schistosomiasis in Jiangsu province I Establishment of indicators and approaches on monitoring and forecasting water infectivity. Chin J Schisto Control 21: 363–367. [Google Scholar]
- 20.Guo-Jing Y, Lu L, Hong-Ru Z, Griffiths SM, Marcel T, et al. (2014) China's sustained drive to eliminate neglected tropical diseases. Lancet Infectious Diseases 14: 881–892. 10.1016/S1473-3099(14)70727-3 [DOI] [PubMed] [Google Scholar]
- 21.Wan XH, Tu ZX, Chen YW (2013) Research progress on examination and proction of Schistosoma Japonicum Cercariae. JIANGXI SCIENCE 31: 754–758. [Google Scholar]
- 22.Xie MS (2002) A survey on water infectivity with schistosome during flooding season in the eastern Dongting Lake and the Yangtze River. Practical Preventive Medicine 9: 588–599. [Google Scholar]
- 23.Zhang YP, Zhu NP (1993) Experimental study on water-preserving ecological snail control in schistosomiasis-susceptible zone of Dongting Lake. Chinese Journal of Ecology 12: 23–25. [Google Scholar]
- 24.Jiang QW, Yuan HC, Chen M, Wei JG, Yang QJ, et al. (1991) Longitudinal evaluation of the effects of two different control strategies for controlling schistosomiasis. Chin J Schisto Control 3: 260–263. [Google Scholar]
- 25.Jiang Q, Zhang S, Yuan H, Liu Z, Zhao G, et al. (1996) The effect of a combined approach to schistosomiasis control on the transmission of Schistosoma japonicum in Xingzi of Poyang Lake area, China. Southeast Asian J Trop Med Public Health 27: 535–541. [PubMed] [Google Scholar]
- 26.Wang XH (2007) Epidemiological survey of schistosomiasis in Shili Lake basin, Xingzi County. Chinese Journal of Veterinary Parasitology 15: 29–30. [Google Scholar]
- 27.Xie CY, Xu GY (2000) Report on detecting schistosome-infested water in Baguazhou ferry terminal from 1998 to 1999. Journal of Practical Parasitic Diseases 8: 89. [Google Scholar]
- 28.Xu GY, Yang HM, Chen GM, Pang H, Wu WQ, et al. (1999) Report on the measurement of schistosome-infested water during the flood in 1998 in Nanjing. Journal of Practical Parasitic Diseases 7: 129. [Google Scholar]
- 29.Qiu L, Xu GY, Hu HB (1998) Comparative observation of the detection of schistosome-infested water using sentinel mice and rabbits. Journal of Practical Parasitic Diseases 6: 88. [Google Scholar]
- 30.Xu GY, Yang HM, Yang PC, Hu HB, Qiu L, et al. (1996) Report on the determination of schistosome-infested water using sentinel mice in Nanjing city from 1993 to 1995. Journal of practical parasitic diseases 4: 66–68. [Google Scholar]
- 31.Freeman MF, Tukey JW (1950) Transformations related to the angular and the square root. Annals of Mathematical Statistics 21: 607–611. [Google Scholar]
- 32.Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 21: 1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 33.Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Bmj 327: 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao Z, Huang Y, Wang T (2017) Schistosomiasis japonica control in domestic animals: progress and experiences in China. Front Microbiol 8: 464 10.3389/fmicb.2017.00464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu SJ (1998) Comparative study on economic development and the Upper, Middle and Lower Reaches of the Yangtze River basin Wuhan: Central China Normal University Press. [Google Scholar]
- 36.Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Xiong YL, Zhang JJ, Li Y, Zuo YT, et al. (2018) Assessment of schistosomiasis transmission risk after flood damage in Wuhan city. Chin J Schisto Control: 1–5. [DOI] [PubMed] [Google Scholar]
- 39.Shen XH, Fu ZY, Dai JR, Sun LP, W L, et al. (2017) Role of goats in transmission of schistosomiasis japonica VI Interrupting transmission of schistosomiasis based on elimination of Schistosoma japonicum-infected goats. China Tropical Medicine 17: 464–469. [Google Scholar]
- 40.Shen MF, Feng XG, Z Y, Wu MS, Xiong MT, et al. (2017) Surveillance with sentinel mice in key water areas of schistosomiasis endemic regions in Yunnan province, 2015. Chin J Schisto Control 29: 209–211,215. [DOI] [PubMed] [Google Scholar]
- 41.Liu ZC, Li JB, Luo ZH, Ren GH, Liu HX, et al. (2017) Effect of integration optimal measures for schistosomiasis prevention based on livestock infection source control in lake and marshland regions. Journal of Tropical Diseases and Parasitology 15: 68–71+77. [Google Scholar]
- 42.Li G, Chen YY, Tu Z, Shan XW, Cai SX (2017) Surveillance results and risk analysis of Schistosoma japonicum-infected sentinel mice in key water region in Hubei province in 2016. Chin J Schisto Control 29: 412–415. [DOI] [PubMed] [Google Scholar]
- 43.Li G, Chen YY, Shan XW, Li B, Wan L, et al. (2017) Surveillance for Schistosomiasis infected sentinel mice in key water areas of Hubei, 2015. Disease Surveillance 32: 405–408. [Google Scholar]
- 44.Li G (2017) Analysis of monitoring results of schistosomiasis based on sentinel mouse technique in key water regions of Wuhan city, Hubei province (2014–2016). J of Pub Health and Prev Med 28: 49–51. [Google Scholar]
- 45.Chen L, Wan NN, Wan JJ, Xu J, Li RZ, et al. (2017) Surveillance report of schistosomiasis in sentinel mice in key waters in Sichuan province from 2010 to 2016. Parasitoses and Infectious Diseases 15: 186–189. [Google Scholar]
- 46.Zuo YP, Zhu DJ, Du GL, Tang K, Ma YC, et al. (2016) Surveillance and risk assessment system of schistosomiasis in Jiangsu province Ⅲ Risk of schistosomiasis transmission in the area along the Yangtze River in Yangzhou City. Chin J Schisto Control 28: 353–357. [DOI] [PubMed] [Google Scholar]
- 47.Xiang RD, Yu B, Shan XW, Deng F, Xu XW, et al. (2016) Efficacy of the sentinel mouse method in monitoring Schistosoma japonicum infection in key water areas of Hanchuan city. Chin J Parasitol Parasit Dis 34: 46–51. [PubMed] [Google Scholar]
- 48.Wang DJ (2016) Schistosomiasis control effect of agricultural integrated development in Tieban marshland of the Yangtze River. Chin J Schisto Control 28: 338–339. [DOI] [PubMed] [Google Scholar]
- 49.Wan LX, You J, Zhou Q, You QH (2015) Analysis and emergency response of sentinel surveillance and early warning of schistosomiasis infection in key waters of Pukou district, Nanjing city in 2009. Medical frontier 5: 374–374. [Google Scholar]
- 50.Sun LP (2015) Integration and demonstration of key techniques in surveillance and forecast of schistosomiasis in Jiangsu province I Layout and effect of the demonstration sites for schistosomiasis surveillance and forecast. Chin J Schisto Control 27: 221–228. [PubMed] [Google Scholar]
- 51.Shen MF (2015) Monitoring of sentinel mice and risk assessment of schistosomiasis transmission in key regions of Yunnan province in 2014. Chin J Schisto Control 27: 174–176. [PubMed] [Google Scholar]
- 52.Wu ZS, Wang CX, Xie MK, Zhou QF, Zhon B (2014) Epidemic situation and prevention strategy of schistosomiasis in Ya'an city after Lushan earthquake on April 20, 2013. Chin J Schisto Control 26: 79–80. [PubMed] [Google Scholar]
- 53.Wanng CF, Wang CX, Mou LR, Zhon B, Liu Y, et al. (2014) Strategy and effect of schistosomiasis emergency control after earthquake in Lushan county. Chin J Schisto Control 26: 557–558+572. [PubMed] [Google Scholar]
- 54.Liu ZC, Ren GH, He HB, Yi P, Yu JH, et al. (2014) Implementation effect of integrated schistosomiasis control strategy with emphasis on livestock infection source in lake and marshy areas. China Tropical Medicine 14: 151–155. [Google Scholar]
- 55.Li G (2014) Surveillance of schistosoma japonicum-infected sentinel mice in key water regions of Hubei province (2012–2013). J of Pub Health and Prev Med 25: 27–30. [Google Scholar]
- 56.Zhen H, Li SZ, Cao CL, Zhang LJ, Sun LP, et al. (2013) Surveilance and response for schistosomiasis japonica based on sentinel mice examination for cercariae-infested water in risk regions, 2012. Chin J Parasitol Parasit Dis 31: 428–432. [PubMed] [Google Scholar]
- 57.Yang K, Sun L-P, Liang Y-S, Wu F, Li W, et al. (2013) Schistosoma japonicum risk in Jiangsu province, People's Republic of China: identification of a spatio-temporal risk pattern along the Yangtze River. Geospatial Health 8: 133–142. 10.4081/gh.2013.61 [DOI] [PubMed] [Google Scholar]
- 58.Ren DH (2013) Report on the surveillance of schistosomiasis in Xinmin Village, Gaoyou city in 2012. Jiangsu Health Care 15: 18–19. [Google Scholar]
- 59.Zhen H, Sun LP, Zhu R, Tu ZW, Li YY, et al. (2012) Surveillance and forecast of Schistosoma japonicum⁃infected sentinel mice in key water regions of China in 2010. Chin J Schisto Control 24: 5–9. [PubMed] [Google Scholar]
- 60.Zen XJ, Chen HG, Hong XL, Hu ZH, Jiang WS, et al. (2012) Evaluation on medium⁃term effect of schistosomiasis comprehensive control strategy based on infectious source control in Poyang Lake area. Chin J Schisto Control 24: 382–386. [PubMed] [Google Scholar]
- 61.Wang H (2012) Surveillance and forecast of schistosomiasis based on sentinel mouse technique in key water regions of Wuhan city in 2011. Chin J Schisto Control 24: 415–419. [PubMed] [Google Scholar]
- 62.Tu ZW (2012) Surveillance and forecast for schistosome infectivity of the Yangtze River and the Hanbeihe River during flooding in Hubei province. Chin J Schisto Control 24: 193–195. [PubMed] [Google Scholar]
- 63.Jiang RJ, Shen XH, Li YF, Chen XP (2012) Comprehensive control of Xitan in Gaoqiao town, Dantu district, Zhenjiang city to control the spread of schistosomiasis. Journal of Tropical Diseases and Parasitology 10: 161–163. [Google Scholar]
- 64.Huang CQ (2012) Systematic surveillance of water body in schistosomiasis susceptible areas in Nanchang county by sentinel mice. Chin J Schisto Control 24: 688–690. [PubMed] [Google Scholar]
- 65.Xie CY, Yang PC, Wei DH, Yin WG (2011) Observation on the off-site infection of floating Schistosoma japonicum cercarira. Modern Preventive Medicine 38: 730–731. [Google Scholar]
- 66.Wan QZ, Zhang SQ, Wang M, Wang FF, Wang ZL, et al. (2011) Temporal distribution of schistosome infectivity in water along the Yangtze River and its branch in flood season. Journal of Tropical Diseases and Parasitology 9: 78–80,103. [Google Scholar]
- 67.Sun LP, Liang YS, Wu HH, Tian ZX, Dai JR, et al. (2011) A Google Earth-based surveillance system for schistosomiasis japonica implemented in the lower reaches of the Yangtze River, China. Parasites & Vectors 4: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He LC (2011) Survey of causes of infected Oncomelania snails and infectious sources of schistosomiasis in marshland and lake region of Jingzhou city. Chin J Schisto Control 23: 381–385. [PubMed] [Google Scholar]
- 69.Yu Q (2010) Prevalence study on source of infection of schistosomiasis in marshland with infected snails in lower reaches of the Yangtze River. Chin J Schisto Control 22: 351–354. [Google Scholar]
- 70.Xie CY (2010) Effect of schistosomiasis control in Bianmin river of Nanjing city. Chin J Schisto Control 22: 47–50. [Google Scholar]
- 71.Wang SR, Xiang RD, Zhang ZH, Zhang JM, Xu XW, et al. (2010) Evaluation on the outcome of "herd-prohibiting and tractor-ploughing" for schistosomiasis control on river banks. J of Pub Health and Prev Med 21: 55–59. [Google Scholar]
- 72.Liu ZC, He HB, Wang ZX, Ding L, Guo FY, et al. (2010) Effect of marshland isolation and grazing prohibition on schistosomiasis control in Dongting Lake region. Chin J Schisto Control 22: 459–463. [Google Scholar]
- 73.Jia TW (2010) Surveillance of schistosomiasis in 36 pilot villages implementing comprehensive control strategy with focus on infectious source in China, 2008. Chin J Schisto Control 22: 333–338. [Google Scholar]
- 74.Yang PC, Gao Y, Xie CY, Chen XJ, Qiu L, et al. (2009) Monitoring of schistosoma cercariae in the Yangtze River after large-scale snail control in Nanjing. Parasitoses and Infectious Diseases 7: 152–153. [Google Scholar]
- 75.Yang GF (2009) Killing effect of slow release niclosamide ball on cercariae of Schistosoma japonicum in the field. China Tropical Medicine 9: 2087–2088. [Google Scholar]
- 76.Chen HG, Zeng XJ, Xiong JJ, Jiang WS, Hong XL, et al. (2009) Study on comprehensive schistosomiasis control strategy with emphasison infectious source control in Poyang Lake areas. Chin J Schisto Control 21: 243–249. [Google Scholar]
- 77.Yin WG, Gao Y, Xie CY, Qiu L, Yang PC (2008) Epidemic water monitoring before and after dredging project of the Bianmin river system in Nanjing. Parasitoses and Infectious Diseases 6: 80–81. [Google Scholar]
- 78.Li RM, Fu HS (2007) Evaluation of endemic situation and control strategy of schistosomiasis in Gaochun county. Chin J Schisto Control 19: 468–470. [Google Scholar]
- 79.Cao QY (2007) Field investigation on fluctuation of infected snails and infectivity of water body at marshlands with infected Oncomelania snail habitats along the Yangtze River in Jiangzhou. Anhui Agri Sci Bull 13: 52–53. [Google Scholar]
- 80.Yang PC, G Y, Xie CY, Qiu L, Yin WG, et al. (2006) Observation on the effect of schistosomiasis control after the slope protection of the bianmin river. parasitoses and Infectious Diseases 4: 74–75. [Google Scholar]
- 81.Xie CY, Gao Y, Qiu L, Yin WG, Zhou W, et al. (2005) Observation on the susceptibility zone of schistosomiasis by the sentinel mice. Chin J Parasitol Parasit Dis 23: 315–316. [PubMed] [Google Scholar]
- 82.Yu H, Wu GC, Wang XH, Fang JH, Liu Y, et al. (2004) Schistosomiasis control in the lake area. Chinese Journal of Veterinary Medicine 40: 18–20. [Google Scholar]
- 83.Li RM (2004) Recurrence of schistosomiasis and Oncomelania snails in Gaoxuan Marshland of Gaochun country in 2003. Chin J Schisto Control 16: 209. [Google Scholar]
- 84.Dai JR (2004) Field investigation on fluctuation of infected snails and infectivity of water body at marshlands with infected Oncomelania snail habitats along the Yangtze River in Jiangsu. Chin J Schisto Control 16: 185–188. [Google Scholar]
- 85.Xie MS (2001) Studies on schistosome water body infectivity during risk water level and falling stages in Dongting Lake region. Chin J Schisto Control 13: 289–291. [Google Scholar]
- 86.Yang HM, Xu GY, Qiu L, Hu HB, Xie CY, et al. (2000) Five-year prevention and control work and schistosomiasis epidemic trends in Nanjing Xiaba Pilot Project. Journal of Practical Parasitic Diseases 8: 34–35. [Google Scholar]
- 87.Yang AG, Mao GQ, Xie ZM, Shi Q, Su XW (2000) Establishment and statistical analysis of animal schistosomiasis epidemic detection network in mountainous areas in Changqiu. Chinese Journal of Veterinary Parasitology 8: 36–38. [Google Scholar]
- 88.Zhou Y, Xia DH, Zeng GX, Yan MW, Gan CX, et al. (1999) Preliminary observation on the detection of Schistosoma japonicum cercariae in marshland water bodies by C-6 membran. Chinese Journal of Parasitology and Parasitic Diseases 17: 66. [PubMed] [Google Scholar]
- 89.Zhao LG, Yi HZ, Li YX, Li XJ, Li CD (1998) Epidemiological survey of schistosomiasis in Qionghai Lake, Xichang. Journal of Practical Parasitic Diseases 6: 80. [Google Scholar]
- 90.Zhang Q, Qing SG, Hu SG, Zhu CX, Ding GH, et al. (1998) Four-year longitudinal observation on the epidemiology of animal schistosomiasis in Matangyuan of Yueyang County. Chinese Journal of Veterinary Parasitology 6: 51–53. [Google Scholar]
- 91.Chen Y, Yang RQ, Wu ZW, Peng XS (1998) Chemotherapy reduces the infection rate of human and animal schistosomiasis and the observation on infection effect of susceptible zone. Chinese journal of zoonosis 14: 93–94. [Google Scholar]
- 92.Wang YH, Ke ZH, Chen CH, Dong XH, Yi YZ, et al. (1997) Control of Oncomelania hupensis by destroying land and cultivating forest in river beach areas. Chin J Schisto Control 9: 232–233. [Google Scholar]
- 93.Shen HS, Lei JQ, Shen HZ, Zhen YH, Quan H, et al. (1997) Longitudinal study of schistosomiasis epidemiology in Xiongkou town, Qianjiang city, Hubei provine. Chinese Journal of Veterinary Parasitology 5: 40–42. [Google Scholar]
- 94.Liu YH, Cun YQ, Sun HY, Yang Z, Qiu ZL (1997) Observation on the effect of on-site detection of schistosomiasis water by C-6 membrane adhesion method. Chin J Schisto Control 9: 311. [Google Scholar]
- 95.Hong QB, Gao ZH, Cao Q, Wu ZX, Xu GK, et al. (1997) Longitudinal observation on epidemic factors and control effects of marshland schistosomiasis in Datong village. Chin J Schisto Control 9: 206–209. [Google Scholar]
- 96.Zhang SQ, Mei JD, Wang TP, Li QY, Su GB, et al. (1996) Study on epidemic factors and control strategy of acute schistosomiasis in marshland areas. Chin J Schisto Control 9: 31–35. [Google Scholar]
- 97.Xu FS, Qian XH, Liang S, Zhao WX, Gu XG, et al. (1996) Observation on the effect of schistosomiasis control in Chuanxing district, Xichan city from 1987 to 1995. Journal of practical parasitic diseases 4: 129–130. [Google Scholar]
- 98.Wu ZW (1996) Observation on the effect of controlling schistosomiasis in planting trees in the marshland of junshan farm. Practical preventive medicine 3: 149–151. [Google Scholar]
- 99.Pu PA, Chen WZ, Zhang KY, Tian SL, Qiu XH, et al. (1996) Field experiment on preventing schistosomiasis infection by skin application in Zixiang. J Prev Med Chin PLA 01: 62. [Google Scholar]
- 100.Liu LM, Su ZW, Fu Y (1996) Two methods for measuring water infectivity in schistosomiasis-affected areas. Hubei journal of preventive medicine 7: 43–44. [Google Scholar]
- 101.Liao ZJ (1996) Observation on the effect of chemotherapy-based measures on schistosomiasis control in the new marshland of Yangtze River. Chin J Schisto Control 8: 219–220. [Google Scholar]
- 102.Li XQ (1996) Investigation of Schistosoma japonicum infection in wild mice and sentinel mice in suburban marshland. Chin J Schisto Control 8: 242. [Google Scholar]
- 103.Zhan ZW, Li XQ, Zhang XS, Zhou YL, Xu MX (1995) Control measures and effect evaluation of the snail marshland test area in Wuhan section of the Yangtze River. Chinese Journal of Parasitic Disease Control 8: 154–155. [Google Scholar]
- 104.Yang HM, Xu GY, Hu HB, Yang PC, Huang LG, et al. (1995) Epidemiological dynamics and trends of schistosomiasis in Nanjing. Chin J Schisto Control 7: 357–358. [Google Scholar]
- 105.Wan Z, Zhao ZX, Cheng LC, Xiang GX, Yu F, et al. (1995) Dynamic analysis of schistosomiasis in Guanghui village, Tongling county. Control and research on parasitic diseases 24: 181–182+192. [Google Scholar]
- 106.Nie GX (1994) Determination of Infectivity of Lake water after snail control in susceptible areas in Huzhou. Chin J Schisto Control 6: 103. [Google Scholar]
- 107.Yang WS (1992) Preliminary analysis on the determination of schistosomiasis water in Zhonghe village of Weishan county. Chin J Schisto Control 4: 187. [Google Scholar]
- 108.Pan NG, Wan YH (1992) Effects of chemotherapy on schistosomiasis control in Nanyang. Hunan medical 9: 35–36. [Google Scholar]
- 109.Jiang QW, Yuan HC, Chen M, Wei JG, Yang QJ, et al. (1992) Longitudinal study on blocking the transmission of schistosomiasis in Jijiba, Guichi, Anhui. Chin J Schisto Control 4: 85–87. [Google Scholar]
- 110.Zhu DP (1991) Snail-killing effect of spraying bromoacetamide in the hilly area of Pengze county. Chin J Schisto Control 3: 287–289. [Google Scholar]
- 111.Wu JZ, Xie FX, Zhang XQ, Xu RF, Duan YR, et al. (1991) Observation on the effect of niclosamide on snail control and cockroach killing in the mountainous area of Yunnan province. Journal of parasitology and parasitology 9: 75–76. [Google Scholar]
- 112.Tang XY (1991) Investigation and analysis on the prevalence of schistosomiasis in the Qilihu basin. Chin J Schisto Control 3: 303–305. [Google Scholar]
- 113.Li TS, Wang TP, Qin XY, Mei JD, Cui XM, et al. (1990) Study on the prevalence of growth and decline after schistosomiasis control in marshland areas. Chin J Schisto Control 2: 24–28+55. [Google Scholar]
- 114.Xu YF, Hu YM, Xiong ZF, Hu ZG, Xu CD (1989) Experience in eliminating schistosomiasis in Ertang township. Jiangxi Medical Journal 24: 236–237. [Google Scholar]
- 115.Tao HQ, Shen BR, Mao YZ, Jiang YM, Wang FS, et al. (1989) Experimental observation on blocking the transmission of schistosomiasis in Zhuangbu village in the short term. Chin J Schisto Control 1: 5–8. [Google Scholar]
- 116.Zhang R, Tan HQ, Hua XJ, Wu Q, Zhao J, et al. (1988) Chemotherapy combined with positive snail zone local snail control of schistosomiasis in lakes and marshes. Chinese journal of parasitology and parasitology 6: 10–13. [Google Scholar]
- 117.Xu ST, Wu WM, Dai YH, Wang DB, Wei T, et al. (1988) Epidemiology and control strategies of schistosomiasis in cattle in Muping Lake area, Hunan province. Chinese Journal of Veterinary Medicine: 7–13. [Google Scholar]
- 118.Hu LS, Xie ZW, Qiu YX, Hu QL, Zhang SJ, et al. (1985) Report on the countermeasures against schistosomiasis in Poyang Lake area. Jiangxi Medical Journal: 1–5. [Google Scholar]
- 119.Cao CL, Guo JG (2018) Challenge and strategy of prevention and control of important parasitic diseases under the Belt and Road Initiative. Chin J Schisto Control 30: 111–116. [DOI] [PubMed] [Google Scholar]
- 120.Wang W, Dai JR, Liang YS (2014) Apropos: factors impacting on progress towards elimination of transmission of schistosomiasis japonica in China. Parasites & Vectors 7: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu XP, Wang TP, Wang QZ, Yi XM, Zhou L, et al. (2013) Infaction status of sources of schistosomiasis japonica in marshland and hilly regions. Journal of Pathogen Biology 8: 445–447+410. [Google Scholar]
- 122.Zhang LJ, Xu ZM, Qian YJ, Dang H, Lu S, et al. (2016) Endemic status of schistosomiasis in People's Republic of China in 2015. Chin J Schisto Control 28: 611–617. [DOI] [PubMed] [Google Scholar]
- 123.Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, et al. (2009) A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med 360: 121–128. 10.1056/NEJMoa0800135 [DOI] [PubMed] [Google Scholar]
- 124.Wang LD, Guo JG, Wu XH, Chen HG, Wang TP, et al. (2009) China's new strategy to block Schistosoma japonicum transmission: experiences and impact beyond schistosomiasis. Trop Med Int Health 14: 1475–1483. 10.1111/j.1365-3156.2009.02403.x [DOI] [PubMed] [Google Scholar]
- 125.Zhou Y-B, Liang S, Jiang Q-W (2012) Factors impacting on progress towards elimination of transmission of schistosomiasis japonica in China. Parasites & Vectors 5: 275–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Song LG, Wu XY, Sacko M, Wu ZD (2016) History of schistosomiasis epidemiology, current status, and challenges in China: on the road to schistosomiasis elimination. Parasitology Research 115: 4071–4081. 10.1007/s00436-016-5253-5 [DOI] [PubMed] [Google Scholar]
- 127.Fan KW (2012) Central-provincial relations for anti-schistosomiasis policy in china. Iran J Public Health 41: 1–11. [PMC free article] [PubMed] [Google Scholar]
- 128.Ross AG, Sleigh AC, Li Y,., Davis GM, Williams GM, et al. (2001) Schistosomiasis in the People's Republic of China: prospects and challenges for the 21st century. Clinical Microbiology Reviews 14: 270 10.1128/CMR.14.2.270-295.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.He YX, Salafsky B,., Ramaswamy K,. (2001) Host—parasite relationships of Schistosoma japonicum in mammalian hosts. Trends in Parasitology 17: 320–324. [DOI] [PubMed] [Google Scholar]
- 130.Lu DB, Wang TJ, Donnelly CA, Fang GR, Webster JP (2010) Contrasting reservoirs for Schistosoma japonicum between marshland and hilly regions in Anhui, China—a two-year longitudinal parasitological survey. Parasitology 137: 99–110. 10.1017/S003118200999103X [DOI] [PubMed] [Google Scholar]
- 131.Wu JY, Zhou YB, Chen Y, Liang S, Li LH, et al. (2015) Three Gorges Dam: impact of water level changes on the density of schistosome-transmitting snail Oncomelania hupensis in Dongting Lake area, China. Plos Neglected Tropical Diseases 9: e0003882 10.1371/journal.pntd.0003882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li F, Ma S, Li Y, Tan H, Hou X, et al. (2017) Impact of the Three Gorges project on ecological environment changes and snail distribution in Dongting Lake area. PLoS Negl Trop Dis 11: e0005661 10.1371/journal.pntd.0005661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou YB, Liang S, Chen Y, Jiang QW (2016) The Three Gorges Dam: Does it accelerate or delay the progress towards eliminating transmission of schistosomiasis in China? Infectious Diseases of Poverty 5: 63 10.1186/s40249-016-0156-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang GJ, Utzinger J, Sun LP, Hong QB, Vounatsou P, et al. (2007) Effect of temperature on the development of Schistosoma japonicum within Oncomelania hupensis, and hibernation of O. hupensis. Parasitology Research 100: 695–700. 10.1007/s00436-006-0315-8 [DOI] [PubMed] [Google Scholar]
- 135.He JC, Xia CG, Dan XM, Wan EM, Yao ZQ, et al. (2004) Study on the Schistosoma japonicum infection pattern of puman and cattle in different seasons in the areas along the Changjiang River. Journal of Tropical Medicine 4: 364–367. [Google Scholar]
- 136.Xu J, Duan ZL, Guan ZX, Wang YY, Lin C, et al. (2017) Early detection of circulating DNA of Schistosoma japonicum in sentinel mice models. Experimental Parasitology 176: 82–88. 10.1016/j.exppara.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 137.Xu J, Li SZ, Chen JX, Wen LY, Zhou XN (2017) Playing the guiding roles of national criteria and precisely eliminating schistosomiasis in P. R. China. Chin J Schisto Control 29: 1–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(TIFF)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.