Abstract
The concepts of agency of one’s actions and ownership of one’s experience have proven useful in relating body-representations to bodily-consciousness. Here we apply these concepts to cognitive maps. Agency is defined as “the sense that I am the one who is generating the experience represented on a cognitive map”, while ownership is defined as “the sense that I am the one who is undergoing an experience, represented on a cognitive map”. The roles of agency and ownership are examined with respect to the transformation between egocentric and allocentric representations, the underlying neurocognitive and computational mechanisms, and within the neuropsychiatric domain including Alzheimer’s disease and other memory-related disorders, in which the senses of agency and ownership may be disrupted.
Keywords: Agency, ownership, memory, simulation, modeling, Alzheimer’s disease
Agency and ownership in the cognitive sciences
Two decades ago, in an influential paper, the philosopher Shaun Gallagher distinguished between two important concepts: self-agency (see Glossary) and self-ownership (or briefly, agency and ownership; [1,2]). Agency is defined as “the sense that I am the one who is causing or generating an action. For example, the sense that I am the one who is causing something to move, or that I am the one who is generating a certain thought in my stream of consciousness”, while ownership is “the sense that I am the one who is undergoing an experience. For example, the sense that my body is moving regardless of whether the movement is voluntary or involuntary” [1]. Together, these two highlight a substantial difference between a “minimal-self” (“oneself as an immediate subject of experience, unextended in time”), and a “narrative-self” (“self that is constituted with a past and a future”) [1]. The minimal-self refers to the world mainly through the body: agency is expressed while moving one’s body or body-parts, and ownership through the feeling of owning a body or body-part. The narrative-self (or experiencing-self) refers to the world through life-stories: in this manner, agency may be defined as the sense that ‘I am the one who tells the story’ and ownership as the sense that ‘I am the protagonist of the story’.
Following Gallagher’s work, the terms of agency and ownership have been used mostly in cognitive research examining the minimal-self, that is the bodily-self or body-consciousness [3–10]. In this domain, the sense of ownership has been defined as a “pre-reflective experience or sense that I am the subject of the movement, that I am the one moving …” and the sense of agency as “the pre-reflective experience or sense that I am the cause or author of the movement” [5,9]. Importantly, while body-processing was extensively investigated for more than a century, agency and ownership have added another explanatory level by relating the more mechanistic body-processing to a broader, self-referenced, bodily-consciousness [4,11]. In this paper we attempt to apply the concepts of agency and ownership also to the narrative-self through the construct of cognitive mapping.
Cognitive mapping
More than 70 years ago, Kenneth Craik speculated that “the organism carries a “small-scale model” of external reality and of its possible actions within its head, it is able to try out various alternatives, conclude which is the best of them, react to future situations before they arise, utilize the knowledge of past events in dealing with the present and the future” [12]. Tolman [13] further hypothesized that perceived stimuli are not connected by “simple one-to-one switches to the outgoing responses”, but rather, are represented on “a tentative, cognitive-like map of the environment. And it is this tentative map, indicating routes and paths and environmental relationships, which finally determines what responses, if any, the animal will finally release” (p.193). Decades later, mechanisms have been proposed to support mapping functions. O’Keefe and colleagues [14] studied freely moving rodents and revealed that specific hippocampal neurons fire whenever an animal arrives at a specific place in the environment (“place-cells”). Later on, another type of cells was discovered in the entorhinal cortex, firing periodically at multiple locations to form a six-fold rotationally symmetric grid-like pattern across space (“grid-cells”) [15]. Activity of such types of cells provides the so-called “cognitive map”, a map-like representation of the environment which is used for active navigation and planning. In addition, several other cell-types have been discovered [16]: head-direction-cells code for the animal’s direction, which is crucial for self-location based navigation; border-cells, coding for the borders within the environment (firing when one is close to a border), enable to tune navigation according to the specific arena’s size. Even an artificial network armed with the simplest navigational tools (linear and angular velocity) developed units manifesting in a similar manner to grid-, border-, and head-direction-cells [17,18]. Interestingly, cognitive-map-like organization and strategies are applied not only to spatial navigation but also to other modalities like time (coding for specific time-durations; [19]), social space (relation to one’s social network), localization of other animals in space, potential tasks and decisions to be made, predictions or even “concepts” of objects in the environment [16], suggesting further implication of this system in mentally modeling one’s surroundings. Such “mental models” [20] may thus extend beyond the spatial environment (see [21,22]).
Of special importance for the narrative-self are temporal cognitive maps, representing the relationships between temporally non-contiguous events [19,23]. In the same manner that spatial cognitive maps represent navigation between non-adjacent locations by combining multiple pieces of spatial information, temporal cognitive maps enable “navigation” in between non-contiguous events by combining temporal information regarding different events [23]. This process is similar to the way in which events are spatially represented with respect to the narrative-self as well as to other events on a “mental-time-line” [24–26]. Likewise, cognitive maps may be regarded as a general system [27–29] representing relations between items in the world as well as the location of the narrative-self, in the immediate or imagined spatial environment, along the timeline of past experiences and future simulations, or other planes such as social interactions with different individuals (unlike mere familiarity [30]).
The interplay between egocentric and allocentric representations
An important property of the cognitive map is the interplay between bodily-view (egocentric), and bird’s-eye view or allocentric representations (Box 1). In spatial cognition, navigation may be based on active movement of the body in the environment, while computing distances and angles of the body as one explores the environment; this strategy is known as “path-integration” or egocentric-navigation [31,32]. One’s self-location is then serving as the basis for one’s behavior in the environment. In contrast, an allocentric strategy encodes information to form a landmark-based cognitive map. In this strategy, relationships among landmarks, as well as the subject’s stationary location, assist in defining one’s location within the environment. Importantly, these two strategies are in continual dialogue: allocentric representations of one’s self-location are updated by egocentrically derived changes of one’s self-location, and vice-versa, one’s egocentric perception is changed according to the allocentric cognitive map, maintaining one’s ongoing relations with the environment [11,33,34].
Box 1: The ego- and allocentric views.
In their discussion of first-person-perspective, Vogeley and Finke [11] distinguished between the reference frame (which may be ego- or allo-centric) and the phenomenal level (which may be seen from first- or third-person perspective). The advancements both in cognitive mapping research and navigation technology invites a new typology.
In an egocentric body-centered representation (Box Figure I, a) one sees (solid-lines) the world (blue) from her own point of view (red). It is from this subjective point of view that she also observes the interactions between other people or the relations between different locations, and imagines their relations to her own self (dashed lines). The green line represents a barrier preventing the subject from seeing a third point and its relation to other locations/people/events. Such a representation is illustrated by Google Street View (lower row).
On the contrary, in a pure allocentric map-view representation (Box Figure I, b) one (red) imagines an object-to-object or world-centered representation, independent of her subjective point ofview. In this representation the observer position (grey) is marked within the environment but processed as other objects (blue) are. In this representation all interrelations are imagined (dashed-lines). Such a representation is illustrated by a classical map (here from Google Maps, lower row).
In an “omniscient” point-of-view (Box Figure I, c), such as an online navigation system like Google Maps, one knows not only what she may see from her own position, but also what other people or locations see from their positions. This point-of-view may be regarded as a schematic ego-and allo-centric hybrid.
Finally, the world may be seen/imagined also from an elevated, bird’s-eye view perspective (Box Figure I, d). In this representation, the individual sees the world, as well as her own self, from an elevated point of view. This position enables her to see the interrelations not only between oneself and the world but also between the different locations/people/events (object-to-object, including the one beyond the green barrier), and is therefore considered here as allocentric. This point-of-view has a special importance with respect to the difference between cognitive mapping in rodents and primates [100]. Such a view is embedded in the 3D feature of Google Maps (here in a satellite view).
Note that while the above is illustrated in the domains of spatial navigation, a similar notion implies also to the representation of the temporal (events) and social (people) domains (Fig. 1). Like locations, they may be mutually related or only unidirectionally; or they may contain information known only to an omniscient agent (e.g. psychologist, confidant).
Figure I, Box 1:
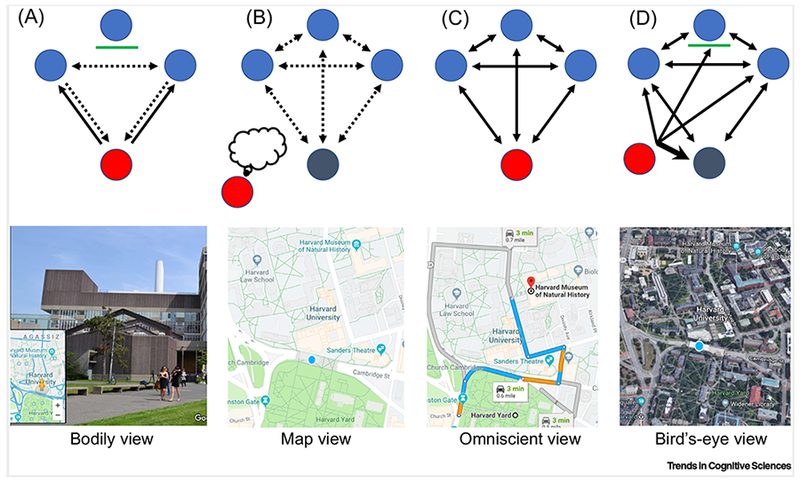
Illustrations and realizations of ego- and allo-centric views
The egocentric-allocentric interplay of perspective-taking is not restricted to spatial cognition. In temporal cognition, for instance, visuo-spatial imagery of a coherent scene from a single viewpoint is crucial for the simulation of an episode [35,36]. Memories can be retrieved from an egocentric perspective, as most events are initially experienced, as well as from an allocentric perspective, as if one is seeing oneself participating in the memory from outside. Moreover, the particular egocentric perspective adopted during memory retrieval may also shape the constructive nature of memories [37,38]. The relation between egocentric and allocentric perspectives is associated with the cognitive distance [39] from a remembered event: recent autobiographical memories are more frequently associated with egocentric perspective, whereas remote memories are associated with allocentric perspective [40,41]. Shifting visual perspective during memory retrieval may reduce the emotional intensity of memories [42–45]. The natural egocentric perspective in long-term memory retrieval can be manipulated and updated when people imagine the possible (spatial) movements they can make within the remembered scene [46]. Moreover, it was shown that shifting visual perspective during retrieval shapes remembering and memory constructions [46,47]. In social cognition the interplay between egocentric and allocentric perspectives lays the ground for “theory of mind” [48] in which mental states of others are modeled according to one’s egocentric reflections as if she is in another’s (allocentric) shoes, an ability learned early on in tales and stories [49], which may be crucial for social behavior.
Agency and ownership in cognitive mapping
We suggest that self-agency and self-ownership are critical components for cognitive mapping over the different domains (space, time and person) and for the egocentric-allocentric translation embedded in this process. In the same manner that agency and ownership help relating movements we initiate and sensations we perceive to our bodily (or minimal) self, we suggest that agency and ownership are crucial to relate our movement in the environment, which is continuously translated to a form of a cognitive map, to the narrative (or experiencing) self. Notably, agency and ownership are suggested not just as a metaphor or a conceptual framework but also as cognitive operators involved in the projection of information to and from the processing of the cognitive maps [1,9,18]. Gallagher’s [1] original definitions will therefore be applied such that agency is defined as “the sense that I am the one who is generating the experience represented on a cognitive map in my stream of consciousness”, while ownership is “the sense that I am the one who is undergoing an experience, represented on a cognitive map”. Following these definitions, agency is manifested here as a top-down process (self-to-map) which inserts self-generated “priors” into the process of cognitive mapping, including expectations, simulations and past memories regarding the environments and the self within it; ownership is a bottom-up process (map-to-self) in which one’s existing model about the way she experiences the environment is updated through information gathered from the environment, including path-integration and world-information (Fig. 2A; for a detailed model see Fig. 3).
Figure 2. From cognitive map to the narrative-self in the core network.
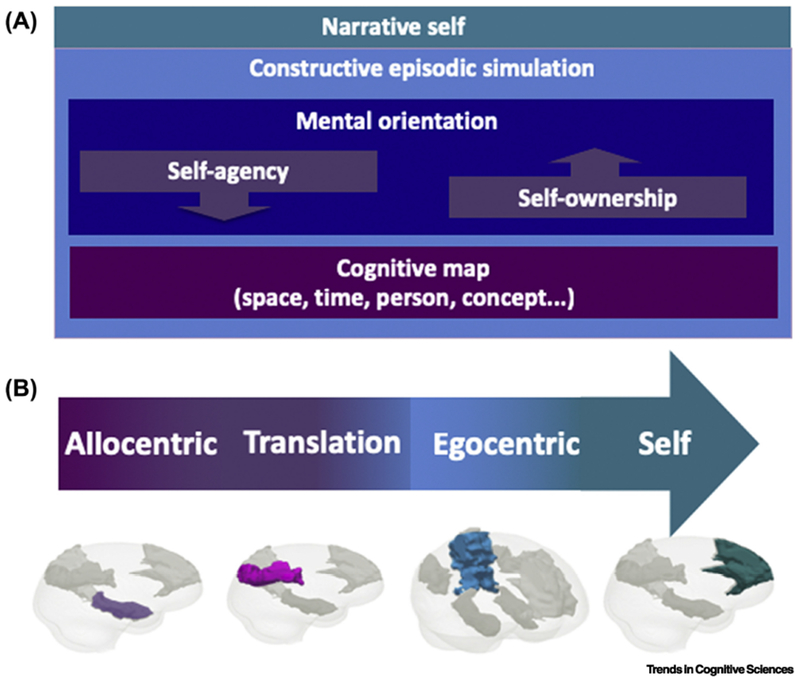
A. Cognitive operations from cognitive map to the narrative-self. Self-agency and self-ownership mediate in between the cognitive maps and the narrative-self. These operations are contained within mental orientation, which relates the internal representation of the self to the external world. Both are included in the operation of constructive episodic simulation. B. From allocentric representation to the self in the core network. The flow from allocentric representation through agency and ownership to egocentric representation and the self is shown, in parallel to the corresponding regions in the “core network” of brain activity for episodic simulation (medial temporal lobe, posterior cingulate/retrosplenial cortex, lateral parietal and temporal cortex, and medial prefrontal cortex, respectively).
Figure 3. Agency-ownership interaction model.
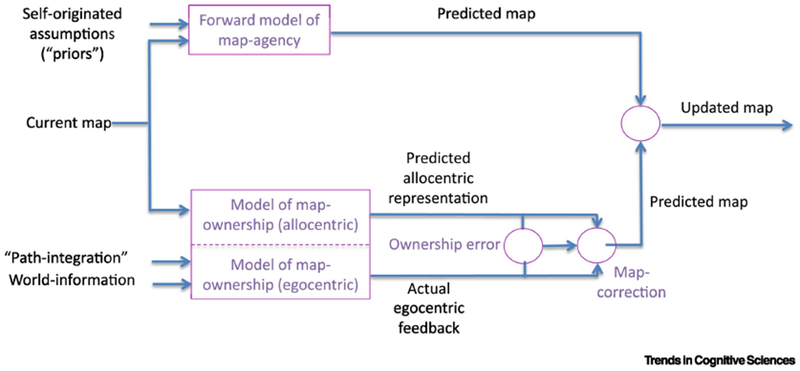
Modification of the model used by Wolpert et al. [60] is shown schematically, consisting of two processes. The agency-branch (up) uses self-originated (top-down) agency-derived priors and beliefs and the current generated cognitive map to predict the next version of the map using a forward model. The ownership-branch (bottom) uses one’s personal model of ownership and egocentric-allocentric translation (difference between the predicted allocentric map and the actual egocentric feedback perceived by path-integration and perceived world information) to correct the predicted map. The next generated map is dependent on these two processes (with different weighting). Circles represent operators along the model.
Agency and ownership in cognitive mapping may be better understood through Gallagher’s distinction between minimal-self and narrative-self. Minimal-self involves the immediate experience, unextended in time, and is therefore well expressed in bodily-consciousness, since the embodied-self is, by definition, here and now. The narrative-self, however, involves a continuum self that is more than a singular point, including an ongoing gradually changing qualitative duration (the Bergsonian durée [50]) from past to future and is also freely moving in space. Adapting a cognitive mapping model, rather than living in the here and now, the narrative-self “lives” on a cognitive map that contains one’s remembered past(s) and one’s imagined potential futures. Priors are inserted through agency into the newly generated map. World and egocentric feedback are influenced by an ownership-model that the individual creates as based on schemata used to process information [51–53] (Fig. 3). Agency in the temporal cognitive map [23] will be defined as ‘the sense that I am the one who is simulating this future or remembering this past scenario’, and therefore inserting memories and simulations to the experience. Ownership is ‘the sense that I am the one who underwent (or will undergo) this experience, combined by these events’. In space, the minimal-self is limited to the body and its peri-personal space (the immediate environment), whereas the narrative-self may reach more and more locations, beyond cities, countries and continents. In the social domain the narrative-self involves the experiencing subject as well as people around her [54]. Agency on the social cognitive map will be defined as ‘the sense that I am the one who generates and evaluate interactions with people around me (or social network)’. Ownership may be ‘the sense that I am the one who is involved in a social network’[55].
To summarize, self-agency and self-ownership may be appreciated through their role in broader cognitive operations in which the narrative-self shapes the cognitive maps and interacts with them, including episodic simulation [35], mental-orientation [56] (Fig. 2A) or “self-reference” [1,2,57,58]. The cognitive map depicts the relation between different “landmarks” within the environment – places, events or people – but how are these related to the narrative-self? We suggest that the agency process inserts into the map different beliefs, memories and concepts acquired by the narrative-self across time, and simulations of the map to-be-generated (Fig. 3, upper branch). One’s own model of ownership is shaped and operates according to one’s experience, which influences the information inserted (Fig. 3, lower branch). To reiterate, it is this information-flow in-between map-based representation of the environment and the previously learned information and beliefs about the environment that enables one to process the changing environment with respect to the self and its cognitive state and to imagine possible future states so as to plan further behavior [35,59]. Agency and ownership are the cognitive operators that provide the bi-directional link between the constructed map and the narrative-self.
Computational neuroscience: combining the agency-ownership and egocentric-allocentric operations in cognitive mapping
The rich information available on the different cell types underlying cognitive mapping in the medial temporal lobe (MTL) and their different characteristics, as well as on relational processes, have led to the development of detailed computational models for potential mechanisms underlying cognitive mapping [17,18,33,46]. However, many questions about the relations between the narrative-self and cognitive maps are still open: How is the dialogue between the two managed? How are errors either in concepts adopted by the narrative-self about the world or in the map-like representation corrected from one version of the map to another? How are the egocentric-allocentric translations made? Are predictions and plans represented a-priori on the maps? How are they updated over time? How does an updated map influence back the narrative-self? And how does the updated map in one domain, for instance space, influence a newly generated map of another domain (e.g. person)?
Models of agency and ownership may help in answering such questions. In view of the analogous relations between the agency-ownership loop and the sensorimotor one, such a model may rely on the fundamental principles of sensorimotor models [1,9], since these models are a prototype dealing with top-down self-generated movements and perceived bottom-up feedback. Agency, similarly to motor control/command, is a top-down process (self-to-movement), and ownership, similarly to sensory response, is a bottom-up process. In a seminal paper, Wolpert, Ghahramani and Jordan [60] sketched the principles of such models. Of specific interest are forward models which are designed to predict the outcome of the interplay between top-down and bottom-up processes, as well as to tune and optimize their mutual effect [61,62]. Building on this work (but see [63]), we suggest a forward model that includes a feedback loop between agency and ownership processes, applied on cognitive maps (Fig. 3). In this model, an ownership branch (Fig. 3, lower branch) converges the allocentric representation of one’s self-location and world-representation on the map, as well as egocentric information (path-integration and world-information acquired during this process) through an ownership-model. This model reflects the way in which each individual processes such information. An agency branch (Fig. 3, upper branch) supplies self-originated assumptions (“priors”) to the information contained within the map. Notably, such a feedback model may capture the essence not only of the final maps but also of maps produced from each egocentric-allocentric translation. To reiterate, the proposed model is a combination of these agency and ownership processes, and together with the egocentric-allocentric translation leads the subject to draw a new cognitive map. This process recurs again and again to optimize the map. The agency-branch uses the current map and top-down self-originated information (priors, beliefs) to modify the next version of the agency-based map by simulating the map using a forward model. The ownership-branch uses a model of the subject’s actual “movement” in the represented environment (ownership-model) to predict the allocentric representation, which is also updated by the actual egocentric feedback [64]. The ownership-model may be viewed as a schema [51,52] that characterizes the way in which the individual cognitive system perceives and processes world-information, and thus inherently influences the production and update of the map representation. The ownership-error, that is the difference between the allocentric generated map and the actual egocentric (path-integration and world-information based) feedback [64], is used to correct the newly generated map. The relative contributions of the two processes may be modulated for each person to optimize the map-construction process. Future studies may unravel whether changes in one’s self-agency and self-ownership influence the generation, use and interpretation of cognitive maps. For instance, the question of whether altering one’s body-agency or body-ownership [65,66] would influence performance and strategies in navigation and orientation tasks could be investigated experimentally. Simulations and data applied to the model may also be tested through modification of certain parts of the model and prediction of the resulted outcome.
Neuroanatomy: agency and ownership in the core network
The mediation between the narrative-self and cognitive-maps through agency and ownership may be further clarified in view of their underlying neuroanatomical basis (Fig. 2B). The allocentric cognitive-maps are based on medial temporal lobe (MTL) sub-structures that are also home for the involved cell-populations [16]. The medial parietal region, including the retrosplenial cortex and the precuneus, is involved in the transformation, translation and integration of egocentric and allocentric frameworks [32,33]. Notable in this vein is the posteromedial – anterior-temporal (PMAT) theory. This theory postulates the posteromedial system (including the retrosplenial cortex and parahippocampal region) to be involved in processing online context information in the domains of space, time and person, referencing it to the perspective of one’s self, and storing that information into long-term memory (see evidence reviewed in [67]). On the lateral side, the temporoparietal junction (TPJ), mostly in the right hemisphere, was found crucial for a complete egocentric reference-frame [11,68,69]. Finally, the medial prefrontal cortex and the anterior cingulate cortex are hypothesized to be involved in a self-referential mental activity of the narrative-self [53,54,70,71]. Experimental studies in the bodily-consciousness domain have provided evidence relating the TPJ to agency processes and the medial prefrontal cortex to body-ownership [8,68,72], associations that may be related to the egocentric character of agency and to the self-referential character of ownership, respectively. Together, these regions comprise the “core network” of brain regions that supports both remembering past experiences and simulating future experiences [35,59,73,74]. This network includes the MTL, medial prefrontal cortex, posterior cingulate and retrosplenial cortices, and lateral parietal and temporal areas [75]. Notably, the network overlaps substantially with the default mode network (DMN) supporting self-referential processes [70,76,77].
The division of labor between cortical and MTL substructures of the core network was demonstrated in several recent studies [78–81]. One study [78] used intracranial brain recordings in humans (epileptic patients) with electrodes located both in cortical and MTL regions. Patients were asked to imagine themselves (or “travel” in time) in a specific time-point that may be the present-time (egocentric) or a potential past- or future-time (allocentric). They were then asked to make judgements about different events with respect to the imagined time-point. Results showed clear temporal difference between the lateral-cortical and MTL electrodes: cortical electrodes were active early on while “traveling” in time, and the MTL ones only later while specific events were referred to the narrative-self [78,82]. Application of agency and ownership may explain these distinctions: cognitive maps are based on cell populations in the MTL, while agency and ownership link these maps to the narrative-self, managed in cortical regions. The temporal distinction between the lateral temporal cortex and the MTL implies the involvement of agency processes in which the narrative-self (lateral cortex) may shape and affect cognitive mapping processes (MTL), in a top-down manner (self-to-map; Fig. 2). For instance, while one is entering a certain environment, there are several priors one generates regarding characters of this environment, that may shape its representation as a cognitive map [83]. Accordingly, the suggested framework predicts that while actively navigating a new environment and transforming egocentric and allocentric perspectives a TPJ activation will be found, based on previous literature linking TPJ with egocentric to allocentric transformation [11,69,70]. When an existing map is used and then transformed by the narrative-self (for instance, when expecting a certain environment but finally finding oneself in another type of environment), prefrontal or medial parietal regions will be active [53,67,83].
Neuropsychiatry of agency and ownership in cognitive mapping
Agency and ownership in cognitive mapping may also carry important clinical significance. In his original paper, Gallagher [1] used the concepts of agency and ownership to explain schizophrenic symptoms of delusions of control and thought insertion. Patients with schizophrenia, he claims, may suffer from loss of the sense of agency for their own thoughts, which, in turn, enables insertion of thoughts of reference by others into the minimal-self [85]. Indeed, several experimental works have shown distortion in these faculties in patients with schizophrenia [65,86,87].
The suggested agency-ownership model may help in the classification of several memory-related neuropsychiatric disorders [88] in the domain of space, time and person. Most prominently, and as based on recent findings [89–92], we claim that Alzheimer’s disease (AD) is a disorder in which the orientation of the narrative-self to the world is disturbed, encompassing agency and ownership processes. AD is clinically ill-defined, and mere memory or cognitive complaints and tests are insufficient for diagnosis and monitoring of disease progression [90,93]. It was further hypothesized that the egocentric-allocentric transformation, crucial for the creation of cognitive maps, and thus spatial and episodic memories, plays a major role in the disease [90,94,95]. Ecologically-valid, self-related approaches to test orientation and navigation helped to identify early disruption in young adults at genetic risk for AD and at an early stage on the Alzheimer’s continuum [89,91]. Neuroimaging studies showed that brain regions affected by AD highly overlap with the core network, default mode network and orientation system [89,90,96]. Based on this evidence, it is suggested that agency and ownership in cognitive mapping, and specifically the translations between egocentric to allocentric reference-frames and back, are impaired in very early stages of AD, and might therefore be of particular importance for detecting very early signs of the disease. Moreover, we propose that AD involves a failure of the agency-ownership interplay early on in the course of the disease that declines monotonically as the disease progresses. This is compensated by cognitive mapping activity, which fails later on with disease progression, leading to amnestic symptoms as known in AD. Experimental support for these hypotheses is needed [97].
Other, less prominent disorders may be intimately related to agency, ownership and cognitive maps, separately (Table 1). Agency-related disorders involve conditions in which the relations of the narrative-self to the world are disturbed, as well as conditions in which the narrative-self falsely applies priors and beliefs to its surroundings. Such disorders mostly involve parietal lesions. In ownership-related disorders there is a difficulty in correctly representing the world and appropriately processing world information, usually due to frontal lesions. In map-related disorders, the mapping process itself is disturbed, due to lesions in the medial temporal lobe. The utility of this distinction may be appreciated through the case of topographic-disorientation. Topographic-disorientation, a disorder of spatial orientation and topographical memory, has been described by Meyer [98], and further detailed by several other prominent authors (see in [99]) in the beginning of the 20th century. 100 years later, Aguirre and D’Eposito [99] further divided topographic-disorientation into several disorders including egocentric-disorientation, heading-disorientation and anterograde-disorientation. The first involves patients who are “unable to represent the location of objects with respect to self”, that is an agency-related disorder; the second involves patients who are “unable to represent direction of orientation with respect to external environment”, considered here as an ownership disorder; the third involves patients who are “unable to create new representations of environmental information”, a map-based disturbance (for the temporal and social domains, see Table 1).
Table 1.
Memory-related disorders classified according to agency, ownership and cognitive mapping disturbances
| Category | Domain | Disorder | Phenomenology | Anatomy |
|---|---|---|---|---|
| Agency | Space | Egocentric disorientation | Inability to represent the location of objects with respect to self | P |
| Time | Age disorientation | Insistence on being younger than one’s real age, even with correct knowledge of the current year and year of birth | Insula, P | |
| Person | Depersonalization | Feeling detached from one’s mental processes or body, as if one is an outside observer | TPJ, P | |
| Ownership | Space | Heading disorientation | Inability to represent direction of orientation with respect to the external environment | F, RSC |
| Reduplicative paramnesia | Delusion that a place simultaneously exists in two or more locations or is transferred to a different location | F | ||
| Time | Reduplicative paramnesia for time | Delusion that an event happens simultaneously in two or more locations or is transferred to a different time | F | |
| Person | Capgras / Fregoli | Familiar persons are believed to be imposters / Belief that a stranger is a familiar person | F | |
| Map | Space | Anterograde disorientation | Inability to create new representations of environmental information | MTL |
| Time | Limbic encephalitis | Deficits in past remembering and future imagination | MTL | |
| Déjà vu | Feeling that events have already happened | MTL | ||
| Timeline disorganization | Inability to obtain the correct sequence of life events | MTL | ||
| Transient global amnesia | loss of memory for recent events and an impaired ability to retain new information | MTL |
P – Parietal, TPJ – Temporoparietal junction, F – Frontal, MTL – Medial temporal lobe, RSC – Retrosplenial cortex; For references see [88].
Concluding Remarks
In conclusion, we suggest that agency and ownership of one’s experience as represented on cognitive maps in the spatial, temporal, social or other, more conceptual, domains are crucial in linking cognitive maps to the narrative-self and translating self-generated priors and egocentric perceptions to allocentric representations and back. It is this link that enables relating the more mechanistic map-like representation to the self who undergoes the ongoing experience in both top-down (self-to-map) and bottom-up (map-to-self) manners. This conceptualization invites further investigation of agency and ownership in cognitive mapping by means of theoretical hypotheses, psychological experiments, computational models, neuroimaging analyses, and neuropsychiatric explorations (see Outstanding Questions).
Outstanding Questions.
How do changes in one’s self-agency and self-ownership influence the generation, use, and interpretation of cognitive maps?
What are the relations between agency and ownership in cognitive mapping and other related processes such as bodily agency and ownership, sensorimotor processes, or action perception?
How well may the forward and feedback model of agency and ownership in cognitive mapping predict experimental findings?
How does the ownership model operate and how does it treat egocentric and allocentric processing?
How is this model expressed neuroanatomically?
How are disorders of agency and ownership in cognitive mapping represented in the different parts of the model?
How is the interplay between agency and ownership expressed in the clinical phenomenology, neuropsychological measures, and functional neuroanatomy of patients with AD?
Figure 1. Egocentric and allocentric perspectives in space, time and person.
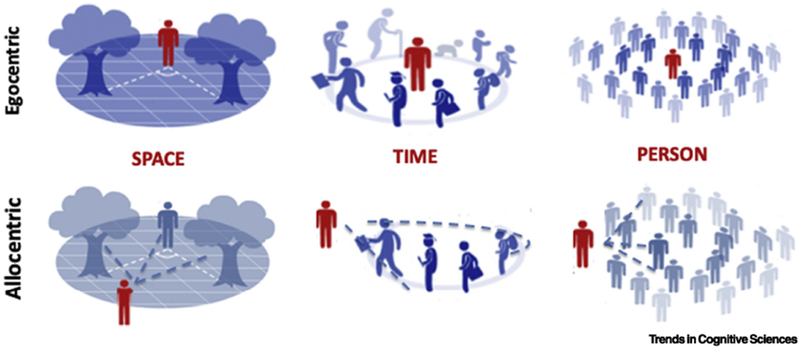
Illustration of egocentric (upper row) and allocentric (lower row) representations of the spatial, temporal and social world. Note that in both representations, the narrative-self is embedded within the environment, but how the represented objects are related to one another differs (see also Box 1; courtesy: Gregory Peters-Founshtein).
Highlights.
The concepts of agency and ownership were described two decades ago to explain the crucial relationship between the narrative – or experiencing – self and the represented experience. These concepts were applied mostly to bodily-consciousness.
The construct of cognitive mapping allows for these concepts to be applied to the relations between the narrative-self and the experience as represented on a cognitive map.
Computational models can be used to explain the integration of self-generated information (e.g. priors, beliefs, memories, simulations), the interplay between egocentric and allocentric views, and personal schemata into the cognitive maps.
The concepts of agency and ownership may help to understand psychological and clinical phenomena as well as their underlying neurocognitive and computational mechanisms.
Acknowledgments
The authors are grateful to Drs. Morris Moscovitch, Mor Nitzan, Michael Peer, Greg Peters-Founshtein, Nimrod Shaham, Hugo Spiers and Peggy St. Jacques for helpful discussions and comments on parts of the manuscript. The authors’ research contributing to this review is funded by the Israel Science Foundations (Grants No. 2598/16 and 1306/18 to SA), The Orion Foundation, and the National Institute of Aging (Grant R01 NIA008441) and National Institute of Mental Health (Grant R01 MH060941) to DLS.
Glossary
- Cognitive-map
A schematic-like mental representation of the relationships between entities in the world including places, events, people or even concepts.
- “Core” network
A network of brain regions, including medial temporal lobe, medial prefrontal cortex, posterior cingulate and retrosplenial cortices, and lateral parietal and temporal areas, that is engaged during both episodic simulation and memory.
- Ecologically-valid
Psychological approaches or tests in which the settings, materials and methods reflect the real-world environment. Ecological-validity is especially important in personalized environments, as is the case in autobiographical memory or social cognition.
- Environmental relationships
The way in which different entities in one’s mental environment (e.g. places in the spatial environment, events in the temporal one and people in the social one) refer to one another (and further on to the experiencing self). Relations may include cognitive distances (Euclidean or non-Euclidean), angles, phases scales or frequencies, among others.
- Episodic simulation
The mental construction of a specific hypothetical autobiographical event.
- Forward model
Forward model uses an existing representation to simulate the next representation, based on error between internal, predicted outcomes and actual outcomes.
- Mental model
A small scale representation of the external world, the relationships between its various parts as well as the individual who represents them. A mental model enables an individual to use past experiences to predict future ones.
- Mental-orientation
The relations between the self and the internal representation she forms about the corresponding reference system (that is, the external world), in space (places), time (events) and person (people).
- Schemata
Organized existing models that modify inputs perceived from the external world in relation to previous experiences, thus mediating between concepts one holds regarding the world and its actual perception.
- Self-agency (in cognitive mapping)
The sense that ‘I am the one who is generating an experience represented on a cognitive map’. The agency process may insert different priors or beliefs one has with respect to the represented environment.
- Self-ownership (in cognitive mapping)
The sense that ‘I am the one who is undergoing an experience, represented on a cognitive map’. This implies an “ownership-model” that processes newly acquired world-and self-related information in an individual manner.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gallagher S (2000) Philosophical conceptions of the self: implications for cognitive science. Trends Cogn Sci 4, 14–21 [DOI] [PubMed] [Google Scholar]
- 2.Gallagher S (2005) How the Body Shapes the Mind, Oxford University Press. [Google Scholar]
- 3.Ehrsson HH et al. (2004) That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science 305, 875–877 [DOI] [PubMed] [Google Scholar]
- 4.Blanke O (2012) Multisensory brain mechanisms of bodily self-consciousness. Nat. Rev. Neurosci 13, 556–571 [DOI] [PubMed] [Google Scholar]
- 5.Tsakiris M et al. (2007) On agency and body-ownership: Phenomenological and neurocognitive reflections. Conscious. Cogn 16, 645–660 [DOI] [PubMed] [Google Scholar]
- 6.Darby RR et al. (2018) Lesion network localization of free will. Proc. Natl. Acad. Sci 115, 10792–10797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsakiris M et al. (2007) Neural signatures of body ownership: a sensory network for bodily self-consciousness. Cereb Cortex 17, 2235–2244 [DOI] [PubMed] [Google Scholar]
- 8.Tsakiris M et al. (2010) Having a body versus moving your body: Neural signatures of agency and body-ownership. Neuropsychologia 48, 2740–2749 [DOI] [PubMed] [Google Scholar]
- 9.Schwabe L and Blanke O (2007) Cognitive neuroscience of ownership and agency. Conscious. Cogn 16, 661–666 [DOI] [PubMed] [Google Scholar]
- 10.Gallagher S (2012) Multiple aspects in the sense of agency. New Ideas Psychol. 30, 15–31 [Google Scholar]
- 11.Vogeley K and Fink GR (2003) Neural correlates of the first-person-perspective. Trends Cogn Sci 7, 38–42 [DOI] [PubMed] [Google Scholar]
- 12.Craik K (1943) The nature of explanation, Cambridge University Press. [Google Scholar]
- 13.Tolman EC (1948) Cognitive maps in rats and men. Psychol. Rev 55, 189–208 [DOI] [PubMed] [Google Scholar]
- 14.O’Keefe J and Nadel L (1978) The Hippocampus as a Cognitive Map, Oxford University Press. [Google Scholar]
- 15.Moser EI et al. (2008) Place cells, grid cells, and the brain’s spatial representation system. Annu. Rev. Neurosci 31, 69–89 [DOI] [PubMed] [Google Scholar]
- 16.Behrens TEJ et al. (2018) What is a cognitive map? Organising knowledge for flexible behaviour. Neuron 100, 490–509 [DOI] [PubMed] [Google Scholar]
- 17.Banino A et al. (2018) Vector-based navigation using grid-like representations in artificial agents. Nature 557, 429–433 [DOI] [PubMed] [Google Scholar]
- 18.Kanitscheider I and Fiete I (2017) Training recurrent networks to generate hypotheses about how the brain solves hard navigation problems. Conf. Neural Inf. Process. Syst at <http://arxiv.org/abs/1609.09059> [Google Scholar]
- 19.Eichenbaum H (2017) On the integration of space, time, and memory. Neuron 95, 1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson-Laird PN (1980) Mental models in cognitive science. Cogn. Sci 115, 71–115 [Google Scholar]
- 21.Warren WH et al. (2017) Wormholes in virtual space: From cognitive maps to cognitive graphs. Cognition 166, 152–163 [DOI] [PubMed] [Google Scholar]
- 22.Meilinger T et al. (2018) Humans construct survey estimates on the fly from a compartmentalised representation of the navigated environment In Spatial Cognition XI. Spatial Cognition 2018. Lecture Notes in Computer Science, vol 11034 (Creem-Regehr S et al. , eds), pp. 15–26, Springer [Google Scholar]
- 23.Schmajuk NA and Buhusi CV (1997) Spatial and temporal cognitive mapping: A neural network approach. Trends Cogn. Sci 1, 109–114 [DOI] [PubMed] [Google Scholar]
- 24.Howard MW et al. (2014) A Unified mathematical framework for coding time, space, and sequences in the hippocampal region. J. Neurosci 34, 4692–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arzy S et al. (2009) The mental time line: An analogue of the mental number line in the mapping of life events. Conscious. Cogn 18, 781–785 [DOI] [PubMed] [Google Scholar]
- 26.Symons CS and Johnson BT (1997) The self-reference effect in memory: A meta-analysis. Psychol. Bull 121, 371–394 [DOI] [PubMed] [Google Scholar]
- 27.Bellmund JLS et al. (2018) Navigating cognition: Spatial codes for human thinking. Science 362, eaat6766. [DOI] [PubMed] [Google Scholar]
- 28.Balkenius C and Gärdenfors P (2016) Spaces in the brain: From neurons to meanings. Front. Psychol 7, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiller D et al. (2015) Memory and space: Towards an understanding of the cognitive map. J. Neurosci 35, 13904–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumaran D and Maguire EA (2005) The Human Hippocampus: Cognitive maps or relational memory? J. Neurosci 25, 7254–7259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buzsáki G and Moser EI (2013) Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci 16, 130–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epstein RA et al. (2017) The cognitive map in humans: Spatial navigation and beyond. Nat. Neurosci 20, 1504–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bicanski A and Burgess N (2018) A neural-level model of spatial memory and imagery. Elife 7, 1–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallach A et al. (2018) A time-stamp mechanism may provide temporal information necessary for egocentric to allocentric spatial transformations. Elife DOI: 10.7554/eLife.36769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schacter D et al. (2012) The future of memory: Remembering, imagining, and the brain. Neuron 76, 677–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.St Jacques PL et al. (2017) Shifting visual perspective during retrieval shapes autobiographical memories. Neuroimage 148, 103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.St Jacques PL et al. (2018) Remembering and imagining alternative versions of the personal past. Neuropsychologia 110, 170–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDermott KB et al. (2016) Visual perspective in remembering and episodic future thought. Q. J. Exp. Psychol. (Hove). 69, 243–53 [DOI] [PubMed] [Google Scholar]
- 39.Liberman N and Trope Y (2008) The Psychology of transcending the eere and now. Science 322, 1201–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nigro G and Neisser U (1983) Point of view in personal memories. Cogn. Psychol 15, 467–482 [Google Scholar]
- 41.Rice HJ and Rubin DC (2009) I can see it both ways: First- and third-person visual perspectives at retrieval. Conscious. Cogn 18, 877–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson JA and Swanson KL (1993) Field and observer modes of remembering. Memory 1, 169–84 [DOI] [PubMed] [Google Scholar]
- 43.Gallagher S and Cole J (2011) Dissociation in self-narrative. Conscious. Cogn 20, 149–155 [DOI] [PubMed] [Google Scholar]
- 44.Andringa E (1996) Effects of “narrative distance” on readers’ emotional involvement and response. Poetics 23, 431–452 [Google Scholar]
- 45.Lothe J (2000) Narrative in Fiction and Film, Oxford University Press. [Google Scholar]
- 46.Byrne P et al. (2007) Remembering the past and imagining the future: A neural model of spatial memory and imagery. Psychol. Rev 114, 340–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolbers T et al. (2008) Spatial updating: how the brain keeps track of changing object locations during observer motion. Nat. Neurosci 11, 1223–30 [DOI] [PubMed] [Google Scholar]
- 48.Frith C and Frith U (2005) Theory of mind. Curr Biol 15, R644–6 [DOI] [PubMed] [Google Scholar]
- 49.Hutto DD (2007) The narrative practice hypothesis: Origins and applications of folk psychology. Narrat. Underst. Pers. R. Inst. Philos. Suppl 60 DOI: 10.1017/CBO9780511627903.004 [DOI] [Google Scholar]
- 50.Bergson H (2001) Time and Free Will: An Essay on the Immediate Data of Consciousness, Dover Publication. [Google Scholar]
- 51.Rumelhart DE (1980) Schemata: the building blocks of cognition In Theoretical Issues in Reading Comprehension (Spiro R, Bruce B and Brewer W eds), Lawrence Erlbaum, 33–58. [Google Scholar]
- 52.Brewer WF and Treyens JC (1981) Role of schemata in memory for places. Cogn. Psychol 13, 207–230 [Google Scholar]
- 53.Preston AR and Eichenbaum H (2013) Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol 23, R764–R773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumaran D et al. (2016) Computations underlying social hierarchy learning: Distinct neural mechanisms for updating and representing self-relevant information. Neuron 92, 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parkinson C et al. (2017) Spontaneous neural encoding of social network position. Nat. Hum. Behav 1, 72 [Google Scholar]
- 56.Peer M et al. (2015) Brain system for mental orientation in space, time, and person. Proc. Natl. Acad. Sci. U. S. A 112, 11072–11077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wittgenstein L (1958) The Blue and Brown Books, Blackwell. [Google Scholar]
- 58.Shoemaker SS (1968) Self-Reference and Self-Awareness. J. Philos 65, 555 [Google Scholar]
- 59.Schacter DL et al. (2017) Episodic future thinking: Mechanisms and functions. Curr. Opin. Behav. Sci 17, 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolpert DM et al. (1995) An internal model for sensorimotor integration. Science 269, 1880–2 [DOI] [PubMed] [Google Scholar]
- 61.Jordan MI and Rumelhart DE (1992) Forward models: Supervised learning with a distal teacher. Cogn. Sci 16, 307–354 [Google Scholar]
- 62.Miall RC and Wolpert DM (1996) Forward models for physiological motor control. Neural Networks 9, 1265–1279 [DOI] [PubMed] [Google Scholar]
- 63.Synofzik M et al. (2008) Beyond the comparator model: A multifactorial two-step account of agency. Conscious. Cogn 17, 219–239 [DOI] [PubMed] [Google Scholar]
- 64.Chen G et al. (2019) Differential influences of environment and self-motion on place and grid cell firing. Nat. Commun 10, 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farrer C et al. (2003) Modulating the experience of agency: a positron emission tomography study. Neuroimage 18, 324–333 [DOI] [PubMed] [Google Scholar]
- 66.Lenggenhager B et al. (2007) Video ergo sum: manipulating bodily self-consciousness. Science 317, 1096–1099 [DOI] [PubMed] [Google Scholar]
- 67.Ranganath C and Ritchey M (2012) Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci 13, 713–726 [DOI] [PubMed] [Google Scholar]
- 68.Arzy S et al. (2006) Neural basis of embodiment: distinct contributions of temporoparietal junction and extrastriate body area. J Neurosci 26, 8074–8081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruby P and Decety J (2001) Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nat Neurosci 4, 546–550 [DOI] [PubMed] [Google Scholar]
- 70.Gusnard DA et al. (2001) Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A 98, 4259–4264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D’Argembeau A et al. (2007) Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J. Cogn. Neurosci 19, 935–944 [DOI] [PubMed] [Google Scholar]
- 72.Yomogida Y et al. (2010) The neural basis of agency: an fMRI study. Neuroimage 50, 198–207 [DOI] [PubMed] [Google Scholar]
- 73.Benoit RG and Schacter DL (2015) Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia 75, 450–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arzy S et al. (2009) Subjective mental time: the functional architecture of projecting the self to past and future. Eur. J. Neurosci 30, 2009–2017 [DOI] [PubMed] [Google Scholar]
- 75.Addis DR et al. (2007) Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45, 1363–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buckner RL et al. (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci 1124, 1–38 [DOI] [PubMed] [Google Scholar]
- 77.Raichle ME (2015) The brain’s default mode network. Annu. Rev. Neurosci 38, 433–47 [DOI] [PubMed] [Google Scholar]
- 78.Schurr R et al. (2018) Temporal dissociation of neocortical and hippocampal contributions to mental time travel using intracranial recordings in humans. Front. Comput. Neurosci 12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baldassano C et al. (2017) Discovering event structure in continuous narrative perception and memory. Neuron 95, 709–721. e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moser EI et al. (2014) Grid cells and cortical representation. Nat. Rev. Neurosci 15, 466–481 [DOI] [PubMed] [Google Scholar]
- 81.Brown TI et al. (2018) Differential medial temporal lobe and parietal cortical contributions to real-world autobiographical episodic and autobiographical semantic memory. Sci. Rep 8, 6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsao A et al. (2018) Integrating time from experience in the lateral entorhinal cortex. Nature 561, 57–62 [DOI] [PubMed] [Google Scholar]
- 83.Javadi A-H et al. (2017) Hippocampal and prefrontal processing of network topology to simulate the future. Nat. Commun 8, 14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruby P and Decety J (2001) Effect of subjective perspective taking during simulation of action: A PET investigation of agency. Nat. Neurosci 4, 546–550 [DOI] [PubMed] [Google Scholar]
- 85.Gallagher S (2000) Self-reference and schizophrenia: A cognitive model of immunity to error through misidentification In Exploring the Self: Philosophical and Psychopathological Perspectives on Self-experience (Zahavi D, ed), pp. 203–239, John Benjamins [Google Scholar]
- 86.Farrer C and Frith CD (2002) Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage 15, 596–603 [DOI] [PubMed] [Google Scholar]
- 87.Blakemore S et al. (2002) Abnormalities in the awareness of action. Trends Cogn Sci 6, 237–242 [DOI] [PubMed] [Google Scholar]
- 88.Peer M et al. (2014) Orientation and disorientation: Lessons from patients with epilepsy. Epilepsy Behav. 41C, 149–157 [DOI] [PubMed] [Google Scholar]
- 89.Peters-Founshtein G et al. (2018) Mental-orientation: A new approach to assessing patients across the Alzheimer’s disease spectrum. Neuropsychology DOI: 10.1037/neu0000463 [DOI] [PubMed] [Google Scholar]
- 90.Coughlan G et al. (2018) Spatial navigation deficits — overlooked cognitive marker for preclinical Alzheimer disease? Nat. Rev. Neurol 14, 1–11 [DOI] [PubMed] [Google Scholar]
- 91.Kunz L et al. (2015) Reduced grid-cell-like representations in adults at genetic risk for Alzheimer’s disease. Science (80-. ). 350, 430–433 [DOI] [PubMed] [Google Scholar]
- 92.Fu H et al. (2017) Tau pathology induces excitatory neuron loss, grid cell dysfunction, and spatial memory deficits reminiscent of early Alzheimer’s disease. Neuron 93, 533–541. e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jack CR et al. (2018) NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 14, 535–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruggiero G et al. (2018) Allocentric to egocentric spatial switching: Impairment in aMCI and Alzheimer’s disease patients? Curr. Alzheimer Res 15, 229–236 [DOI] [PubMed] [Google Scholar]
- 95.Serino S et al. (2014) The role of egocentric and allocentric abilities in Alzheimer’s disease: A systematic review. Ageing Res. Rev 16, 32–44 [DOI] [PubMed] [Google Scholar]
- 96.Buckner RL et al. (2005) Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci 25, 7709–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scahill RI et al. (2002) Mapping the evolution of regional atrophy in Alzheimer’s disease: unbiased analysis of fluid-registered serial MRI. Proc. Natl. Acad. Sci. U. S. A 99, 4703–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meyer O (1900) Ein- und doppelseitige homonyme Hemianopsie mit Orientierungsstörungen. Eur. Neurol 8, 440–456 [Google Scholar]
- 99.Aguirre GK and D’Esposito M (1999) Topographical disorientation: a synthesis and taxonomy. Brain 122, 1613–1628 [DOI] [PubMed] [Google Scholar]
- 100.Meister MLR and Buffalo EA (2018) Neurons in primate entorhinal cortex represent gaze position in multiple spatial reference frames. J. Neurosci 38, 2430–41 [DOI] [PMC free article] [PubMed] [Google Scholar]


