Abstract
Summary
Objectives.
Temporal lobe epilepsy (TLE) is known to affect large-scale gray and white matter networks, and these network changes likely contribute to the verbal memory impairments observed in many patients. In this study, we (1) investigate multimodal imaging patterns of brain alterations in TLE and (2) evaluate the sensitivity of different imaging measures to verbal memory impairment.
Methods.
Diffusion tensor imaging (DTI), volumetric MRI (vMRI), and resting-state functional MRI (rs-fMRI) were evaluated in 46 patients with TLE and 33 healthy controls to measure patterns of microstructural, structural, and functional alterations, respectively. These measurements were obtained within the white matter directly beneath neocortex (i.e., superficial white matter; SWM) for DTI and across neocortex for vMRI and rs-fMRI. The degree to which imaging alterations within left medial temporal lobe/posterior cingulate (LMT/PC) and left lateral temporal regions were associated with verbal memory performance was evaluated.
Results.
Patients with left TLE and right TLE both demonstrated pronounced microstructural alterations [i.e., decreased fractional anisotropy (FA) and increased mean diffusivity (MD)] spanning the entire frontal and temporo-limbic SWM that were highly lateralized to the ipsilateral hemisphere. Conversely, reductions in cortical thickness in vMRI and alterations in the magnitude of the rs-fMRI response were less pronounced and less lateralized than the microstructural changes. Both stepwise regression and mediation analyses further revealed that FA and MD within SWM in LMT/PC regions were the most robust predictors of verbal memory, and these associations were independent of left hippocampal volume.
Keywords: multimodal neuroimaging, superficial white matter, volumetric MRI, diffusion tensor imaging, cognitive ability
1. Introduction
Temporal lobe epilepsy (TLE) is the most common localization-related epilepsy in adults and is often resistant to anti-epileptic medication. TLE is associated with widespread temporal and extratemporal pathology, including changes to white matter microstructure1, reductions in cortical thickness2, and disruptions of functional connectivity3. However, few studies measure these changes simultaneously in the same cohort of patients, making it difficult to evaluate the co-localization of these changes and the potential importance of each measure to cognitive functioning. In a recent study, Liu et al. investigated the associations between white matter microstructure, cortical thickness, and resting-state functional MRI (rs-fMRI) in 61 patients with TLE4. Unlike previous studies that have focused on deep, long-range association tracts in the brain5, these researchers examined microstructural changes in white matter directly beneath the cortex (i.e., superficial white matter; SWM) given its key role in maintaining cortico-cortical connectivity6. They found that SWM abnormalities were independent from the adjacent cortical thinning, but were concomitant with functional activity reductions within anterior and posterior midline regions and lateral temporo-parietal cortices. These findings suggest that structural and functional activity changes are partially independent of one another and could differ in their sensitivity to TLE-related pathology.
Verbal memory impairment affects up to 64% of patients with TLE pre-operatively7. Studies have shown that both left medial and lateral temporal lobes are essential for verbal memory8,9, and that disruption to these regions contributes to verbal memory deficits in chronic TLE10. Although the role of the left hippocampus in verbal memory is well-appreciated11, other studies have emphasized that a network of structures beyond the hippocampus contributes to verbal memory impairment in patients with TLE. For example, several volumetric MRI (vMRI) studies have demonstrated the importance of perirhinal and entorhinal cortices to verbal memory12. In addition, reduced functional connectivity between the medial temporal lobe (including the hippocampus, parahippocampal gyrus and amygdala) and posterior cingulate has been implicated in verbal memory impairment13. Furthermore, studies examining white matter integrity have found an association between reduced verbal memory in TLE and microstructural compromise to the left uncinate fasciculus14, inferior longitudinal fasciculus, and parahippocampal cingulum15. Beyond the medial temporal regions, Saling (2009)16 has highlighted the importance of left lateral temporal regions, including the anterior and inferior temporal cortex, to associative/relational verbal learning and memory in TLE. Although these studies support the involvement of a broad network of medial and lateral temporal lobe regions in verbal memory, the degree to which microstructural, structural, and/or functional disruptions within these regions lead to verbal memory impairment remains unclear.
The purpose of this study was twofold. First, we investigated the extent and magnitude of microstructural, structural, and functional alterations within the SWM and across the neocortex of patients with right TLE (RTLE) and left TLE (LTLE) using three different brain imaging modalities (i.e., diffusion tensor imaging; DTI, vMRI, and rs-fMRI). Second, we evaluated the relative sensitivity of each modality to verbal memory performance, focusing on the two broad regions most frequently implicated in verbal memory performance: the left medial temporal/posterior cingulate (LMT/PC) and left lateral temporal (LLT) regions17. As noted above, studies in TLE have mainly focused on the association between long-range white matter tracts and verbal learning and memory performance14,15. However, the role of the SWM in cognitive functioning is receiving increased attention, with recent studies demonstrating an association between SWM integrity and memory, processing speed, visuomotor-attention, and age-related cognitive decline18,19. Therefore, we adopted this measure to evaluate whether microstructural compromise in the LMT/PC and/or LLT regions is an independent and/or stronger predictor of verbal memory than the structural and functional measures within the adjacent cortex.
2. Methods
This study was approved by the Institutional Review Board at the University of California (UC), San Diego and the UC, San Francisco and all participants provided informed consent according to the Declaration of Helsinki.
2.1. Participants
All patients in this study were medically-refractory, under evaluation for surgical treatment at the UC San Diego or UC San Francisco Epilepsy Center, and diagnosed by board-certified neurologists with expertise in epileptology20. A total of 28 patients were recruited at UC San Diego, and a total of 18 patients were recruited at UC San Francisco. Patients were classified as either LTLE or RTLE based on seizure onsets recorded by video-EEG telemetry and supported by seizure semiology and neuroimaging results at each site. MRIs were visually inspected by a board-certified neuroradiologist for detection of mesial temporal sclerosis (MTS) and exclusion of contralateral temporal lobe structural abnormalities. Control participants were all recruited at UC San Diego and were included if they had no reported history of neurological or psychiatric conditions. Twenty-six patients with a diagnosis of LTLE, twenty patients with a diagnosis of RTLE, and thirty-three healthy controls were included in this study.
Demographic and clinical characteristics of patients with LTLE and RTLE and healthy controls are presented in Table 1. The mean ages and distributions of handedness and sex of the LTLE and RTLE groups were not statistically different from the control group. However, healthy controls attained more years of education than both patient groups.
Table 1.
Demographic and clinical information.
| LTLE | RTLE | Control | LTLE vs Control | RTLE vs Control | LTLE vs RTLE | |
|---|---|---|---|---|---|---|
| n | 26 | 20 | 33 | -- | -- | -- |
| Age | 34±13 | 35±13 | 38±15 | Z = 1.10, p = .271 | Z = 0.71, p = .480 | Z = 0.34, p = .731 |
| Years of education | 13.4±1.6 | 13.2±1.8 | 15.9±2.3 | Z = 4.05, p < .001* | Z = 3.64, p < .001* | Z = 0, p = 1 |
| Sex (M/F) | 11/15 | 10/10 | 12/21 | χ2 = 0.22, p = .642 | χ2 = 0.95, p = .329 | χ2 = 0.27, p = .604 |
| Handedness (L/R/A) | 4/22/0 | 2/16/2 | 1/32/0 | χ2 = 2.86, p = .091 | χ2 = 4.77, p = .092 | χ2 = 2.88, p = .237 |
| Presence of MTS | 18 | 12 | -- | -- | -- | χ2 = 0.43, p = .515 |
| Age of seizure onset | 17±15 | 21±15 | -- | -- | -- | Z = .10, p = .318 |
| Year of seizure duration | 17±16 | 14±12 | -- | -- | -- | Z = −.09, p = .929 |
| Number of medications | 2.2±1 | 2.5±1 | -- | -- | -- | Z = .85, p = .396 |
Note: Age, years of education, age of seizure onset, year of seizure duration, and number of medications were presented in mean ± SD; Handedness (L/R/A) = handedness (Left/Right/Ambidextrous); Presence of MTS = number of patients with mesial temporal sclerosis; Wilcoxon-Mann-Whitney tests were performed for the continuous variables, whereas chi-square tests were performed for the categorical variables.
p < .05
Due to excessive head motion (i.e. mean framewise displacement > 0.5 mm), rs-fMRI was removed for two patients with RTLE and one control. The mean head motion parameters (i.e., head-to-head, translation, and rotation) of the LTLE (t(56) = 0.2, p = .845, t(56) = 0.53, p = .595, and t(56) = −1.03, p = .307, respectively) and RTLE (t(48) = −1.45, p = .154, t(48) = −1.38, p = .174, and t(48) = −1.46, p = .15, respectively) groups were not statistically different from the control group. Due to the absence of B0 scans to correct for geometric distortions, diffusion data were removed for six patients with LTLE and two patients with RTLE.
2.2. Materials and Procedures
Neuropsychological testing of verbal memory, DTI, vMRI, and rs-fMRI were performed on all participants. Participants were administered the California Verbal Learning Test-Second Edition (CVLT)21 and the Logical Memory (LM) subtest from the Wechsler Memory Scale-Third Edition22 to evaluate learning, immediate recall and delayed recall of verbal information. Age- and education-corrected scaled scores were calculated for the CVLT, and age-corrected scaled scores were calculated for LM.
All imaging was performed on a General Electric Discovery MR750 3T scanner with an 8-channel phased-array head coil, and acquired at either the Keck Center for Functional MRI at UC San Diego or the Surbeck Laboratory for Advanced Imaging at UC San Francisco. The imaging sequences were identical between sites and included a conventional three-plane localizer, GE calibration scan, a T1-weighted 3D customized FSPGR structural sequence scan [TR = 8.08 ms, TE = 3.16 ms, TI = 600 ms, flip angle = 8°, FOV = 256 mm, matrix = 256 × 192 (resampled to 256 × 256 with 1 mm isotropic resolution), a 30-directional diffusion weighted sequence scan with a b-value of 1000 s/mm2 with an additional b = 0 volume (TR = 8000 ms, TE = 82.9 ms, flip angle = 90°, FOV = 240 mm, matrix = 96 × 96, slice thickness = 2.5 mm, echo-spacing = 588 ms), and a rs-fMRI scan (TR = 3000 ms, TE = 30 ms, flip angle = 90°, FOV = 240 mm, matrix = 64 × 64, slice thickness = 3.5 mm). Both diffusion-weighted and rs-fMRI scans were corrected for geometric distortions using an integrated B0 scan acquired with opposite phase encoding polarities23.
2.3. Image Data Analysis
2.3.1. Image Preprocessing
All image quality inspection and processing were conducted at the UC San Diego Center for Multimodal Imaging and Genetics, using the same pre- and post-processing procedures.
T1-weighted images were corrected for non-linear warping caused by non-uniform fields created by the gradient coils23.
DTI data were preprocessed based on methods described in McDonald et al. (2014)15. In brief, the images were corrected for spatial and intensity distortions due to B0 magnetic field inhomogeneities, eddy currents distortion, gradient nonlinearity distortion, and head motion. The reverse gradient method was used to correct B0 distortion24. A method using least squares inverse and iterative conjugate gradient descent was used to correct for eddy currents25. Distortions due to gradient nonlinearity were corrected for each frame of the diffusion data according to Jovicich et al. (2006)23. Head motion was corrected by registering each frame to the corresponding volume synthesized from the parameters obtained through diffusion tensor fitting, accounting for variation in image contrast across diffusion orientations26.
The rs-fMRI data analysis was carried out using both Analysis of Functional NeuroImages (AFNI)27 and Matlab (MathWorks, Natick, MA). Individual fMRI data were preprocessed based on the methods described in Jernigan et al. (2016)28. In brief, the images were corrected for spatial distortions due to gradient nonlinearity23 and for B0 magnetic field inhomogeneities24. Each time series was shifted so that each slice was aligned to the first acquired slice using AFNI’s 3dTshift. Head motion was corrected by registering each time frame to the first using AFNI’s 3dvolreg. The mean frame-to-frame head motion estimates were calculated29 and used as covariates in the group analyses to account for motion effects30. The first four volumes were discarded to allow for equilibration of the T1-weighted signals. rs-fMRI time courses were then normalized to the mean of each voxel. This signal normalization was used to take the individual differences in the signal change into consideration. Linear regression was used to remove quadratic trends, signals correlated with estimated motion time courses and mean time courses of cerebral white matter and whole brain, as well as their first derivatives. To focus on low frequency oscillations, time courses were band-pass filtered between 0.01 and 0.08 Hz, and then sampled onto the cortical surface for each individual participant. The average variance of the resting-state blood oxygen-level dependent (rsBOLD) signal across the time series was used as the main rs-fMRI measure of interest. This measure is similar to the amplitude of low frequency fluctuations (ALFF)31 and can be interpreted as the average magnitude of the rsBOLD response within a voxel32.
2.3.2. Surface Reconstruction and Parcellation
Individual T1-weighted vMRIs were used for subcortical segmentation and cortical surface reconstruction and parcellation using FreeSurfer, 5.3.0 33 and the Desikan-Killiany atlas. The reconstructed surfaces were visually inspected for any topological defects and manually edited by a trained image analyst according to established software guidelines. Based on this parcellation, four imaging measures were derived. For DTI, fractional anisotropy (FA) and mean diffusivity (MD) were measured by sampling 1 mm below the white surface at each vertex placed with respect to the surface normal. Using vMRI, cortical thickness was measured point-by-point across the cortical surface. For rs-fMRI, rsBOLD was also measured across the cortical surface.
All surface-based measures were smoothed on the average surface (i.e., FA, MD, cortical thickness, and rsBOLD) using a 20-mm full width at half maximum (FWHM) Gaussian kernel. This kernel only smooths across the surface and not across the gray matter and white matter boundaries, and therefore, does not result in tissue mixing. This smoothed data was used to perform surface-based analysis in an atlas space to improve the signal-to-noise ratio. The 20-mm FWHM smoothing kernel was selected based on previous studies of cortical thickness34 and was motivated by our focus on increasing sensitivity in our surface-based analyses (i.e., large kernels produce large, more contiguous signals). Vertex-wise surface maps of FA, MD, cortical thickness, and rsBOLD were created for each individual and then averaged into a spherical representation to align sulcal and gyral features allowing for accurate matching of FA, MD, and cortical thickness measurement locations at the individual level, while minimizing metric distortion35.
FA, MD, cortical thickness, and rsBOLD estimates were also measured within gyral-based region of interests (ROIs)36 that were based on average estimates at individuals’ native space obtained from the unsmoothed data at the vertex level within a given ROI. All ROI averages were computed on unsmoothed data in native space to ensure that the values included in the average are localized within the ROI and are not influenced by neighboring regions. Hippocampal and amygdala volumes were derived using Freesurfer’s subcortical segmentation pipeline and corrected for total intracranial volume. A total of eleven left hemisphere brain regions (i.e., posterior cingulate, isthmus cingulate, fusiform, entorhinal temporal pole, parahippocampal, hippocampus, amygdala, superior temporal, middle temporal and inferior temporal) were selected in this study based on a meta-analysis of 74 fMRI studies in verbal memory17. To reduce the number of statistical comparison (i.e., control the Type I error rate), eight brain regions were used to create one composite LMT/PC ROI and three brain regions were selected to create one composite LLT ROI (see Figure 1).
Figure 1.
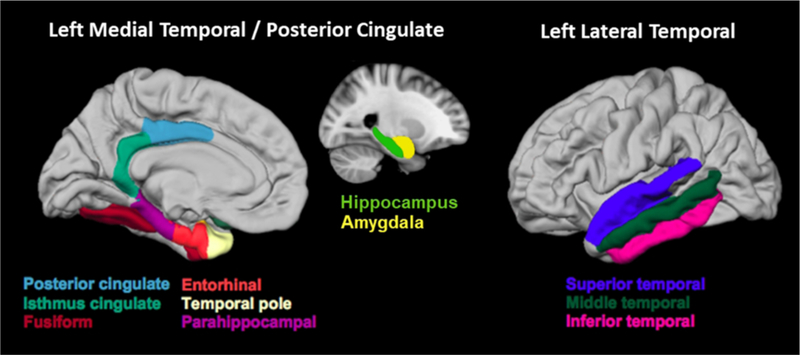
Desikan ROIs in the left medial temporal/posterior cingulate (LMT/PC) and in the left lateral temporal (LLT) regions. The LMT/PC region consists of entorhinal, parahippocampal, temporal pole, fusiform, amygdala, hippocampus, posterior cingulate, and isthmus cingulate. The LLT region consists of inferior, middle, and superior temporal gyri.
2.4. Statistical Analyses
Group differences in neuropsychological measures between each patient group and controls were tested with Wilcoxon-Mann-Whitney tests. For surface-based comparisons, we calculated vertex-wise t-tests between each TLE group and control group using the group average maps and corrected for multiple comparisons using a false discovery rate (FDR) correction.
Principal component analysis was performed within LMT/PC and LLT regions for each of the four imaging measures, to summarize each into a single measure to control the Type I error rate. The first principal component was used to represent each imaging modality in each of the two regions, yielding a total of eight principal components (i.e., two principal components in LMT/PC and LLT regions for each of the four imaging measures). Because the CVLT (i.e., list learning) and LM (prose recall) assess different aspects of verbal memory and we also wished to consider learning, short-delay free recall, and long-delay recall separately, we did not create a principle component for verbal memory16. Detailed results of the principal component analyses are provided in Supplementary Tables 1 and 2.
Forward stepwise regression was then used to determine the contribution of each imaging measure to the verbal memory performance, and the model with the minimum corrected Akaike Information Criterion (AIC) was selected. Backward stepwise regression was also performed to verify the robustness of the results. All participants were included in the original model in order to increase power and extend the range of imaging and verbal memory values37. Secondary analyses were performed to determine (1) whether demographic variables (i.e., age, years of education, handedness, and sex) contributed additional variance to verbal memory, (2) whether the results held in patients with MTS only, and (3) whether demographic and clinical information contributed additional variance to verbal memory in patients with MTS.
Post-hoc causal mediation analyses38 were then performed based on the “mediation” package in R software to determine whether left hippocampal volume mediated the association between imaging measures and verbal memory performance, testing only those imaging measures that were significant predictors (p < .05) in the initial stepwise regression analysis. A nonparametric bootstrap sampling procedure (1,000 bootstrapped samples) was used to test significance of the mediation effect38.
3. Results
Descriptive and inferential statistics for neuropsychological measures of each group are presented in Table 2. Both TLE groups exhibited significant impairment across all neuropsychological measures of verbal memory compared to controls. ANCOVA, Wilcoxon-Mann-Whitney, and Fisher’s Exact tests demonstrated that there were no systematic differences in the derived imaging measures, neuropsychological scores, and clinical variables between patients recruited at UC San Diego and UC San Francisco (see Supplementary Table 3).
Table 2.
Descriptive and inferential statistics for the neuropsychological measures.
| Z (p) |
||||||
|---|---|---|---|---|---|---|
| Task | Control | LTLE | RTLE | LTLE vs Control |
RTLE vs Control |
LTLE vs RTLE |
| CVLT | ||||||
| Learning | 12.76±2.84 | 8.29±3.09 | 9.73±1.98 | 4.45 (<.001*) | 3.24 (.001*) | 1.24 (.214) |
| Short-Delayed | 11.79±3.53 | 7.33±3.81 | 8.69±3.12 | 4.24 (<.001*) | 3.04 (.002*) | 0.84 (.403) |
| Long-Delayed | 11.17±3.39 | 6.52±4.01 | 8.23±2.77 | 4.02 (<.001*) | 3.15 (.002*) | 1.37 (.017*) |
| LM | ||||||
| Immediate | 12.13±2.96 | 7.23±2.75 | 7.88±3.06 | 5.22 (<.001*) | 3.76 (<.001*) | 0.64 (.524) |
| Delayed | 12.87±3.29 | 7.54±2.93 | 8.18±2.83 | 4.98 (<.001*) | 4.16 (<.001*) | 0.78 (.436) |
Note: Mean ± SDs are presented for the scaled scores obtained on each neuropsychological measure for each group. The Z andp values are the statistical results of Wilcoxon-Mann- Whitney tests, which were performed to compare the group differences among three groups.
p< .05.
3.1. Surface-Based Analysis
The results of the surface maps across the three imaging modalities (i.e., SWM FA/MD, cortical thickness, and rsBOLD) are presented in Figure 2A, 2B, and 2C, respectively.
Figure 2.
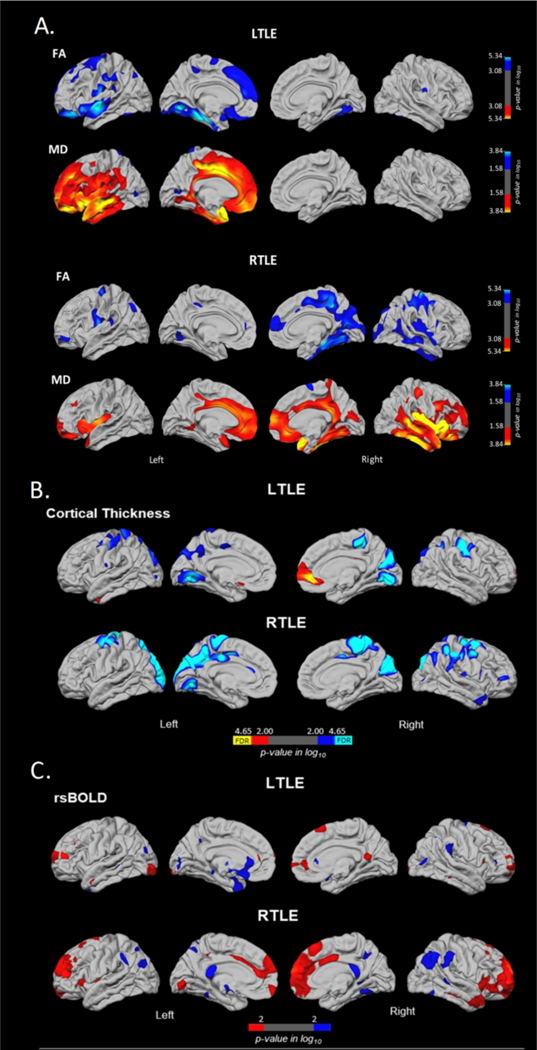
Surface-based mapping of SWM diffusion (Fig. 2A), cortical thickness (Fig. 2B), and rsBOLD magnitude (Fig. 2C) differences in patients with LTLE and RTLE relative to controls. Blue and cyan represent regions where patients showed lower values relative to controls, whereas red and yellow represent regions where patients showed greater values relative to controls. A: Correcting for multiple comparisons, PFDR < 05. B: Blue and red represent group differences with a liberal threshold of p < .01, whereas cyan and yellow represent the regions that are survived after FDR-correction, PFDR < .05. C: Blue and red represent group differences with a liberal threshold of p < .01.
3.1.1. SWM
Both TLE groups showed extensive SWM compromise within the ipsilateral hemisphere compared to controls with reduced FA and increased MD in lateral and medial temporal, and medial superior frontal regions (see Figure 2A). Specifically, patients with LTLE showed reduced FA in ipsilateral superior frontal and post-central, lateral and medial orbitofrontal, supramarginal, and superior and medial temporal regions, while patients with RTLE showed reduced FA in ipsilateral lateral and medial temporal lobe, medial frontal, and medial parieto-occipital regions. In addition, patients with LTLE showed increased MD restricted to the ipsilateral hemisphere, including both lateral and medial frontal, temporal and cingulate regions, while patients with RTLE showed increased MD in lateral and medial frontal and temporal lobe in the ipsilateral hemisphere, and both lateral and medial orbitofrontal and cingulate in the contralateral hemisphere.
3.1.2. Cortical Thickness
Patients with LTLE showed cortical thinning in bilateral posterior parietal, paracentral and precentral regions compared to controls. Patients with RTLE showed a highly similar pattern (see Figure 2B). Unlike SWM changes, areas of cortical thinning were bilaterally distributed in both LTLE and RTLE. However, after FDR-correction, patients with LTLE demonstrated a more restricted pattern of cortical thinning in bilateral medial temporo-occipital and contralateral posterior parietal, paracentral, and post-central regions only.
3.1.3. rs-fMRI
Patients with LTLE showed a higher magnitude of the rsBOLD response within restricted regions of the bilateral lateral frontal, paracentral and posterior cingulate cortex, as well as reduced magnitude within left medial anterior temporal and posterior orbitofrontal cortex (see Figure 2C). Patients with RTLE showed higher rsBOLD magnitude in bilateral lateral and medial frontal regions, as well as lower magnitude in isolated regions of bilateral posterior parietal regions. However, these differences were only significant using a liberal threshold of p < .01, and did not survive after FDR correction.
3.2. Correlations Among the Imaging Measures
Spearman’s rank order correlations among the various imaging measures are shown in Supplementary Table 4. As expected, FA and MD within the same ROIs were highly correlated (i.e., rs = −.57 in LMT/PC and −.64 in LLT), as were FA/MD correlations across the LLT and LMT/PC ROIs. Cortical thickness showed a modest association with MD in LMT/PC, whereas the rsBOLD showed no association with the other imaging measures.
3.3. The Relationship between Imaging Measures and Verbal Memory Performances
Results of the forward stepwise regression models are presented in Table 3 (see Supplementary Table 5 for details). The model that best predicted CVLT performances (i.e., with minimum corrected AIC value) included both FA and rsBOLD within the LMT/PC region, accounting for 17% of the variance in learning, 13% of the variance in short-delayed free recall, and 9% of the variance in long-delayed free recall. Specifically, both lower FA and lower rsBOLD magnitude were associated with poorer performance on all three CVLT measures based on the minimum corrected AIC. However, lower rsBOLD was only associated with poorer CVLT Learning with p < .05. In addition, higher MD in LMT/PC region was the only predictor of poorer LM performance, accounting for 13% of the variance in immediate recall, and 19% of the variance in delayed recall. The results of a backward stepwise regression were consistent and identical to those of the forward stepwise regression.
Table 3.
Results of the multiple linear forward stepwise regression. The variables presented ii the table were selected based on Akaike Information Criterion (AIC).
| Variable | B | SE | P | 95% CI | t | P |
|---|---|---|---|---|---|---|
| CVLT Learning FA in LMT/PC rsBOLD in LMT/PC |
0.70 0.66 |
0.26 0.25 |
0.32 0.32 |
[0.17, 1.23] [0.16, 1.16] |
2.66 2.63 |
.010* .011* |
| R2 = 0.20, Radj2 = 0.17, AICs = 299 | ||||||
| CVLT Short-Delayed FA in LMT/PC rsBOLD in LMT/PC |
0.88 0.53 |
0.33 0.31 |
0.34 0.22 |
[0.22, 1.55] [−0.09, 1.15] |
2.68 1.70 |
.010* .095 |
| R2 = 0.16, Radj2 = 0.13, AICs = 311 | ||||||
| CVLT Long-Delayed FA in LMT/PC rsBOLD in LMT/PC |
0.69 0.56 |
0.33 0.31 |
0.27 0.23 |
[0.02, 1.36] [−0.08, 1.19] |
2.07 1.76 |
.043* .084 |
| R2 = 0.12, Radj2 = 0.09, AICs = 307 | ||||||
| LM Immediate MD in LMT/PC |
−0.69 | 0.21 | −0.38 | [−1.12, −0.27] | −3.25 | .002* |
| R2 = 0.15, Radj2 = 0.13, AICs = 318 | ||||||
| LM Delayed MD in LMT/PC |
−0.88 | 0.22 | −0.45 | [−1.32, −0.44] | −3.98 | <.001* |
| R2 = 0.20, Radj2 = 0.19, AICs = 323 | ||||||
Note: SE = standard error, CI = confidence interval for B
p < .05
When demographic variables (i.e., age, sex, years of education, and handedness) were added, an additional 16–34% of the variance in verbal memory scores was explained by the full combination of variables. The adjusted R2 in the first block (i.e., imaging measures) to the second block (i.e., imaging measures and demographic variables) were from 0.17 (F = 6.72; p = .003) to 0.35 (F = 5.36;p < .001) in CVLT Learning, from 0.13 (F = 4.97;p = .011) to 0.29 (F = 4.12; p = .001) in CVLT Short-Delayed, from 0.09 (F = 3.64; p = .033) to 0.29 (F = 4.04; p = .002) in CVLT Long-Delayed, from 0.13 (F = 10.53; p = .002) to 0.44 (F = 9.12;p < .001) in LM Immediate, and from 0.19 (F = 15.87;p < .001) to 0.53 (F = 12.85;p < .001) in LM Delayed. Years of education contributed to CVLT (p < .05), whereas age, years of education, sex and handedness contributed to LM (p < .05). Detailed results of each hierarchical regression model are presented in Supplementary Table 6.
In the patients with MTS only, lower rsBOLD in LMT/PC (β = 0.61, p = .01) was associated with poorer CVLT Learning (F = 4.22, p = .033). Of the clinical/demographic variables, only years of education and age of seizure onset added to CVLT Learning (F = 3.29, p = .044) (see Supplementary Table 7). Side of seizure onset did not contribute to any of the verbal memory scores. Overall, the model with FA and rsBOLD in LMT/PC, and the full set of demographic and clinical variables explained 55% of the variance in CVLT Learning.
3.4. The Mediation Analysis
The imaging variables that showed an association with verbal memory in the stepwise regression analyses with p < .05 were selected and entered into separate post-hoc mediation analyses. As shown in Table 3, a total of six mediation analyses was performed, included FA in the LMT/PC region and CVLT learning, short-delayed and long-delayed free recall, magnitude of the rsBOLD response in LMT/PC region and CVLT learning, MD in LMT/PC and LM immediate and delayed recall. Left hippocampal volume was significantly associated with SWM (FA and MD), but not with rsBOLD in LMT/PC region. The results of bootstrapped unstandardized causal mediation effect showed that left hippocampal volume did not significantly mediate the effects of FA, MD, or rsBOLD within the LMT/PC region and verbal memory performance (see Figure 3). However, there was a trend for left hippocampal volume to mediate the relationship between FA within the LMT/PC region and CVLT Learning (p = .092). In patients with MTS, left hippocampal volume did not mediate the effects of FA, MD, or rsBOLD within the LMT/PC region and verbal memory performance.
Figure 3.
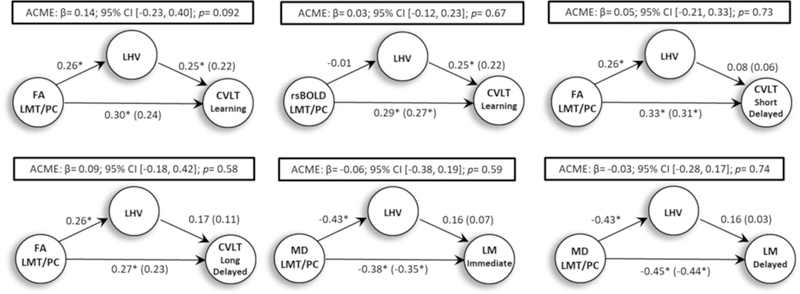
The results of the post-hoc causal mediation analysis. The average causal mediation effects (ACME) of left hippocampal volume (LHV) on the association between imaging measures and verbal memory performance.
4. Discussion
This study represents one of the first to evaluate the extent of surface-based microstructural, structural, and functional abnormalities in the same set of patients with TLE and provides novel data demonstrating the relative sensitivity of each imaging measure for predicting verbal memory performance. Furthermore, we focus on microstructural alterations within the SWM since this measure has been infrequently studied in TLE4,15, but may be important to cognition18,19.
4.1. Whole Brain Differences in SWM among Controls, LTLE, and RTLE
We found that patients with LTLE and RTLE show pronounced microstructural changes within the SWM compared to controls that are highly lateralized to the ipsilateral hemisphere. In particular, decreased FA and increased MD were observed in ipsilateral inferior frontal, medial frontal, lateral and medial temporal regions in both TLE groups. These findings are particularly striking for MD, which showed a broader distribution of alterations relative to FA in both lateral and medial temporal regions, as well as the anterior and posterior cingulate cortex. These alterations in FA and MD in frontotemporal regions are consistent with the patterns described by Liu et al. (2016)4. However, in our study this pattern appears somewhat broader and more restricted to the ipsilateral hemisphere. These results suggest that loss of tissue microstructure directly beneath the cortex may provide a more sensitive measure of brain pathology in TLE compared to cortical thickness and rsBOLD.
4.2. SWM and rsBOLD in LMT/PC is Associated with Verbal Memory
A second key finding is that both microstructural and functional abnormalities within the LMT/PC regions contributed to verbal memory performance, whereas cortical thinning in these regions did not. Notably, reduced FA and increased MD within the SWM explained the most variance in verbal memory performance of the various imaging measures. The SWM connects adjacent gyri in the form of U-fibers and/or longer intralobar fibers39 and may play a critical role in cognition by facilitating communication across neighboring cortex. In addition, the SWM within the LMT region includes fibers terminations from the perforant path, which serves as a major afferent pathway from LMT cortex to all subfields of the hippocampus. This pathway has been implicated in multiple aspects of memory performance, including verbal and spatial mnemonic discrimination15,40,41. Our findings suggest a greater role for the LMT/PC SWM in verbal memory performance relative to cortical thinning of adjacent cortex. Although the LLT region is often implicated in verbal memory tasks that are replete with semantic context and complex syntactical structure16, this region did not contribute uniquely to recall of contextual information (i.e., LM) in our study. Post-hoc correlation analyses revealed a trend for higher MD in the LLT SWM to be associated with lower LM immediate and delayed recall [r(64) = −0.23, p = .069 and r(64) = −0.24, p = .059, respectively]. However, these relationships were weaker than those between the LMT/PC and verbal memory. Second, this marginal relationship may reflect the fact that pathological changes within the LLT region were more modest than those in the LMT/PC region (see Supplementary Table 5), which may have attenuated a relationship with the former. Thus, our findings do not necessarily indicate that the LLT region is not important for verbal memory, but rather, it shows that neither structural nor functional changes within this region appeared to contribute unique variance to contextual recall beyond those contributed by the LMT/PC.
We also found that functional changes within the LMT/PC region contributed to verbal memory impairment in TLE13. Similar to previous studies42, our study demonstrated that lower rs-fMRI response within the LMT region was associated with poorer verbal memory performance. However, the contribution of rsBOLD was weaker and more restricted (i.e., CVLT) in the full group analysis relative to those observed with FA/MD. Although the reason for a lesser role of functional relative to microstructural changes to memory is not clear, this finding is in line with previous studies suggesting that DTI and rs-fMRI provide unique information for predicting cognitive performances in TLE, and that DTI measures may be more predictive of cognitive decline43. One possibility is that rs-fMRI and DTI have different measurement errors, both between and within participants, which may have resulted in more noise in the rs-fMRI signal. In this current study, the standard deviations of the rs-fMRI signal in LLT and LMT/PC regions were 1.3 and 1.27, respectively, whereas the standard deviations of the FA and MD values in LLT and LMT/PC ranged from 0.96 and 1.23. In addition, previous studies have demonstrated that the stability in the rsBOLD signal within a participant (i.e., intra-class correlation coefficient; ICC) is lower for rsBOLD measurements than for those obtained from DTI44,45. Despite the potential for greater noise in the rsBOLD signal, rsBOLD in the LMT/PC region was the only imaging predictor of verbal memory in patients with MTS. Thus, our findings suggest that both integrity of the SWM and functionality of the overlying cortex contribute to verbal memory, but that the rsBOLD response may be particularly important for patients with MTS.
4.3. Association between SWM and Verbal Memory is Independent of Left Hippocampal Volume
Left hippocampal atrophy is known to be a strong predictor of verbal memory impairment. However, hippocampal volume loss can only partially explain verbal memory impairment in TLE. Although we demonstrate that loss of SWM microstructure within the LMT/PC region is a stronger predictor of verbal memory than thinning within the adjacent cortex, this does not rule out the possibility that left hippocampal volume may play a role in the association between SWM integrity and verbal memory performance. According to Liu et al.’s (2016)4 study, hippocampal volume mediates the effect of group (TLE vs. controls) differences on SWM microstructure. Specifically, patients with greater ipsilateral hippocampal atrophy displayed a higher load of SWM abnormalities. Consistent with their study, we also found that greater left hippocampal volume loss was associated with lower FA and higher MD within the SWM. However, our findings demonstrate that left hippocampal volume has little to no influence (i.e., mediation effect) on the association between SWM integrity and verbal memory. Thus, integrity of LMT/PC SWM appears to be a strong predictor of verbal memory performance, independent of left hippocampal atrophy.
Although the primary purpose of this paper was to examine the sensitivity of different imaging modalities to verbal memory, we also note the importance of key demographic and clinical variables in explaining additional variance. Our results are consistent with literature showed that patients with epilepsy had fewer years of education than controls, and that education was a strong predictor of verbal memory performance in most of our models, explaining up to 39% of the variance in performance17. Although our study cannot determine causality, the most likely explanation is that poor verbal memory leads to lower educational attainment, as it is well known that in addition to MTL damage, seizures affect alertness both in children and adults, interfering with short-term memory and impacting the ability to learn46. In addition, age, sex, handedness, and age of seizure onset contributed to verbal memory performance beyond our imaging measures. Therefore, models that include demographic, imaging, and clinical information appear to provide the best prediction. However, many of these variables are highly co-linear and a larger, longitudinal study would be needed to better understand causality and/or to explore how interactions among these variables lead to impairments in memory performance.
Despite our novel finding of the important role of SWM integrity in verbal memory performances in TLE, there are several limitations of this study that should be addressed. First, although both cortical thickness and rs-fMRI data in our study show similar patterns compared to those reported in previous studies4,47–49, our group differences were modest and many did not survive FDR correction. Although the reason for our more modest findings is unclear, this could reflect variations in post-processing across studies, which has been shown to influence surface-based group difference estimates50. Nevertheless, our SWM findings are robust, suggesting that we were well-powered to detect group differences. A second limitation of this study is that we only measured intrinsic fluctuations in the rsBOLD signal rather than activations produced by a memory task. It is possible that we would have found stronger associations between our functional LMT/PC measure and verbal memory with other measures (e.g., connectivity of rs-fMRI and/or task-based fMRI with a memory-related task) or with a more robust sample size of patients. Third, although we selected LMT/PC and LLT ROIs based on a meta-analysis of regions most frequently shown to contribute to verbal memory, other regions that we did not include in our analyses (including right medial temporal lobe and extratemporal regions) may contribute additional variance to verbal memory performance. In addition, whether our findings on the relative sensitivity of different imaging signals hold when smaller LMT/PC subregions are studied should be studied in a larger patient sample. Fourth, we did not test the degree to which SWM versus deep white matter tract FA/MD measurements contribute to verbal memory performance. Although this analysis would also be of interest, we wished to compare across imaging measures within the same ROIs—an analysis that could not be performed using tract-based measures. Another limitation of our study is that we included a well-characterized, but potentially heterogeneous group of patients with refractory TLE. Although we perform post-hoc analyses on patients with MTS only, we were not powered to break down the group into further subgroups or to evaluate how a full spectrum of clinical factors (e.g., type of AED) influence verbal memory scores. Furthermore, there were differences in the sample sizes of each TLE group, which might have led to slight power different in the surface-based group analyses. In addition, our study demonstrated that education played a critical and intriguing role in our imaging-memory models. Future research may benefit from exploring how education and imaging measures interact with each other to predict different aspects of cognitive functions in the patients with TLE and other epilepsy syndromes. Finally, although we demonstrated strong associations between SWM integrity and pre-operative verbal memory performance, the clinical importance of these findings may be hinge on the degree to which the pre-operative integrity of our multimodal imaging measures, including SWM, predict post-operative verbal memory decline.
Supplementary Material
Significance.
These findings suggest that microstructural loss within the SWM is pronounced in patients with TLE, and injury to the SWM within the LMT/PC region plays a critical role in verbal memory impairment.
Key Point Box
DTI can detect subtle changes to the superficial white matter of patients with TLE that may help to lateralize the seizure focus.
Injury to the superficial white matter within the left medial temporal lobe/posterior cingulate contributes to verbal memory impairment.
The association between superficial white matter and verbal memory in patient with TLE was independent of left hippocampal volume.
Acknowledegements
This work was supported by the National Institutes of Health [R01 NS065838 to C.R.M.].
Footnotes
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of Conflicts of Interest
Neither of the authors has any conflict of interest to disclose.
References
- 1.Otte WM, van Eijsden P, Sander JW, et al. A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia. 2012; 53(4):659–67. [DOI] [PubMed] [Google Scholar]
- 2.Bernasconi N, Duchesne S, Janke A, et al. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. NeuroImage. 2004; 23(2):717–23. [DOI] [PubMed] [Google Scholar]
- 3.Haneef Z, Lenartowicz A, Yeh HJ, et al. Effect of lateralized temporal lobe epilepsy on the default mode network. Epilepsy Behav. 2012; 25(3):350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu M, Bernhardt BC, Hong S-J, et al. The superficial white matter in temporal lobe epilepsy: a key link between structural and functional network disruptions. Brain J Neurol. 2016; 139(Pt 9):2431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson D, Go C, Rutka JT, et al. Bilateral diffusion tensor abnormalities of temporal lobe and cingulate gyrus white matter in children with temporal lobe epilepsy. Epilepsy Res. 2008; 81(2–3):128–35. [DOI] [PubMed] [Google Scholar]
- 6.Oishi K, Zilles K, Amunts K, et al. Human brain white matter atlas: Identification and assignment of common anatomical structures in superficial white matter. NeuroImage. 2008; 43(3):447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helmstaedter C Neuropsychological complaints, deficits, and difficulties in everyday life. In: Comprehensive care for people with epilepsy. East Lyngh: John Libbey; 2001. p. 293–306. [Google Scholar]
- 8.Jones-Gotman M, Zatorre RJ, Olivier A, et al. Learning and retention of words and designs following excision from medial or lateral temporal-lobe structures. Neuropsychologia. 1997; 35(7):963–73. [DOI] [PubMed] [Google Scholar]
- 9.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957; 20(1): 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermann BP, Seidenberg M, Dow C, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006; 60(1):80–7. [DOI] [PubMed] [Google Scholar]
- 11.Alessio A, Damasceno BP, Camargo CHP, et al. Differences in memory performance and other clinical characteristics in patients with mesial temporal lobe epilepsy with and without hippocampal atrophy. Epilepsy Behav. 2004; 5(1):22–7. [DOI] [PubMed] [Google Scholar]
- 12.Alessio A, Bonilha L, Rorden C, et al. Memory and language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy Behav. 2006; 8(3):593–600. [DOI] [PubMed] [Google Scholar]
- 13.Doucet G, Osipowicz K, Sharan A, et al. Extratemporal functional connectivity impairments at rest are related to memory performance in mesial temporal epilepsy. Hum Brain Mapp. 2013; 34(9):2202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diehl B, Busch RM, Duncan JS, et al. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008; 49(8):1409–18. [DOI] [PubMed] [Google Scholar]
- 15.McDonald CR, Leyden KM, Hagler DJ, et al. White matter microstructure complements morphometry for predicting verbal memory in epilepsy. Cortex. 2014; 58:139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saling MM. Verbal memory in mesial temporal lobe epilepsy: Beyond material specificity. Brain. 2009; 132(3):570–82. [DOI] [PubMed] [Google Scholar]
- 17.Kim H Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011; 54(3):2446–61. [DOI] [PubMed] [Google Scholar]
- 18.Fornari E, Maeder P, Meuli R, et al. Demyelination of superficial white matter in early Alzheimer’s disease: a magnetization transfer imaging study. Neurobiol Aging. 2012; 33(2):428.e7–428.e19. [DOI] [PubMed] [Google Scholar]
- 19.Nazeri A, Chakravarty MM, Felsky D, et al. Alterations of superficial white matter in schizophrenia and relationship to cognitive performance. Neuropsychopharmacology. 2013; 38(10):1954–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010; 51(6):1069–77. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 22.Wechsler D Wechsler Memory Scale–Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 23.Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. NeuroImage. 2006; 30(2):436–43. [DOI] [PubMed] [Google Scholar]
- 24.Holland D, Kuperman JM, Dale AM. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. Neuroimage. 2010; 50(1):175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuang J, Hrabe J, Kangarlu A, et al. Correction of Eddy-Current Distortions in Diffusion Tensor Images Using the Known Directions and Strengths of Diffusion Gradients. J Magn Reson Imaging JMRI. 2006; 24(5):1188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagler DJ, Ahmadi ME, Kuperman J, et al. Automated white-matter tractography using a probabilistic diffusion tensor atlas: Application to temporal lobe epilepsy. Hum Brain Mapp. 2009; 30(5):1535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996; 29(3):162–73. [DOI] [PubMed] [Google Scholar]
- 28.Jernigan TL, Brown TT, Hagler DJ, et al. The Pediatric Imaging, Neurocognition, and Genetics (PING) Data Repository. NeuroImage. 2016; 124:1149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012; 59(3):2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satterthwaite TD, Wolf DH, Loughead J, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. NeuroImage. 2012; 60(1):623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou Q-H, Zhu C-Z, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J Neurosci Methods. 2008; 172(1):137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elman JA, Panizzon MS, Hagler DJ, et al. Task-evoked pupil dilation and BOLD variance as indicators of locus coeruleus dysfunction. Cortex J Devoted Study Nerv Syst Behav. 2017; 97:60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999; 9(2):179–94. [DOI] [PubMed] [Google Scholar]
- 34.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. NeuroImage. 2005; 24(1):163–73. [DOI] [PubMed] [Google Scholar]
- 35.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999; 9(2):195–207. [DOI] [PubMed] [Google Scholar]
- 36.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006; 31(3):968–80. [DOI] [PubMed] [Google Scholar]
- 37.Kucukboyaci NE, Kemmotsu N, Leyden KM, et al. Integration of multimodal MRI data via PCA to explain language performance. NeuroImage Clin. 2014; 5:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tingley D, Yamamoto T, Hirose K, et al. Mediation: R package for causal mediation analysis. UCLA Stat Stat Assoc [Internet]. 2014. [cited 2017]; . Available from: http://dspace.mit.edu/handle/1721.1791154 [Google Scholar]
- 39.Schüz A, Braitenberg V. The human cortical white matter: Quantitative aspects of cortico-cortical long-range connectivity In: Cortical Areas: Unity and Diversity. London: Taylor and Francis; 2002. p. 377–85. [Google Scholar]
- 40.Vago DR, Bevan A, Kesner RP. The role of the direct perforant path input to the CA1 subregion of the dorsal hippocampus in memory retention and retrieval. Hippocampus. 2007; 17(10):977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett IJ, Stark CEL. Mnemonic discrimination relates to perforant path integrity: An ultra-high resolution diffusion tensor imaging study. Neurobiol Learn Mem. 2016; 129(Supplement C):107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Dam WO, Decker S, Durbin JS, et al. Resting state signatures of domain and demand-specific memory performance. NeuroImage. 2015; 118:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osipowicz K, Sperling MR, Sharan AD, et al. Functional MRI, resting state fMRI, and DTI for predicting verbal fluency outcome following resective surgery for temporal lobe epilepsy. J Neurosurg. 2015; 124(4):929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marenco S, Rawlings R, Rohde GK, et al. Regional distribution of measurement error in diffusion tensor imaging. Psychiatry Res. 2006; 147(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caceres A, Hall DL, Zelaya FO, et al. Measuring fMRI reliability with the intra-class correlation coefficient. NeuroImage. 2009; 45(3):758–68. [DOI] [PubMed] [Google Scholar]
- 46.McCagh J, Fisk JE, Baker GA. Epilepsy, psychosocial and cognitive functioning. Epilepsy Res. 2009; 86(1):1–14. [DOI] [PubMed] [Google Scholar]
- 47.Bernhardt BC, Bernasconi N, Concha L, et al. Cortical thickness analysis in temporal lobe epilepsy: Reproducibility and relation to outcome. Neurology. 2010; 74(22):1776–84. [DOI] [PubMed] [Google Scholar]
- 48.Lin JJ, Salamon N, Lee AD, et al. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex. 2007; 17(9):2007–18. [DOI] [PubMed] [Google Scholar]
- 49.McDonald CR, Hagler DJ, Ahmadi ME, et al. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia. 2008; 49(5):794–803. [DOI] [PubMed] [Google Scholar]
- 50.Senjem ML, Gunter JL, Shiung MM, et al. Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. NeuroImage. 2005; 26(2):600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


